Robbins Basic Pathology chap 1,2,3
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
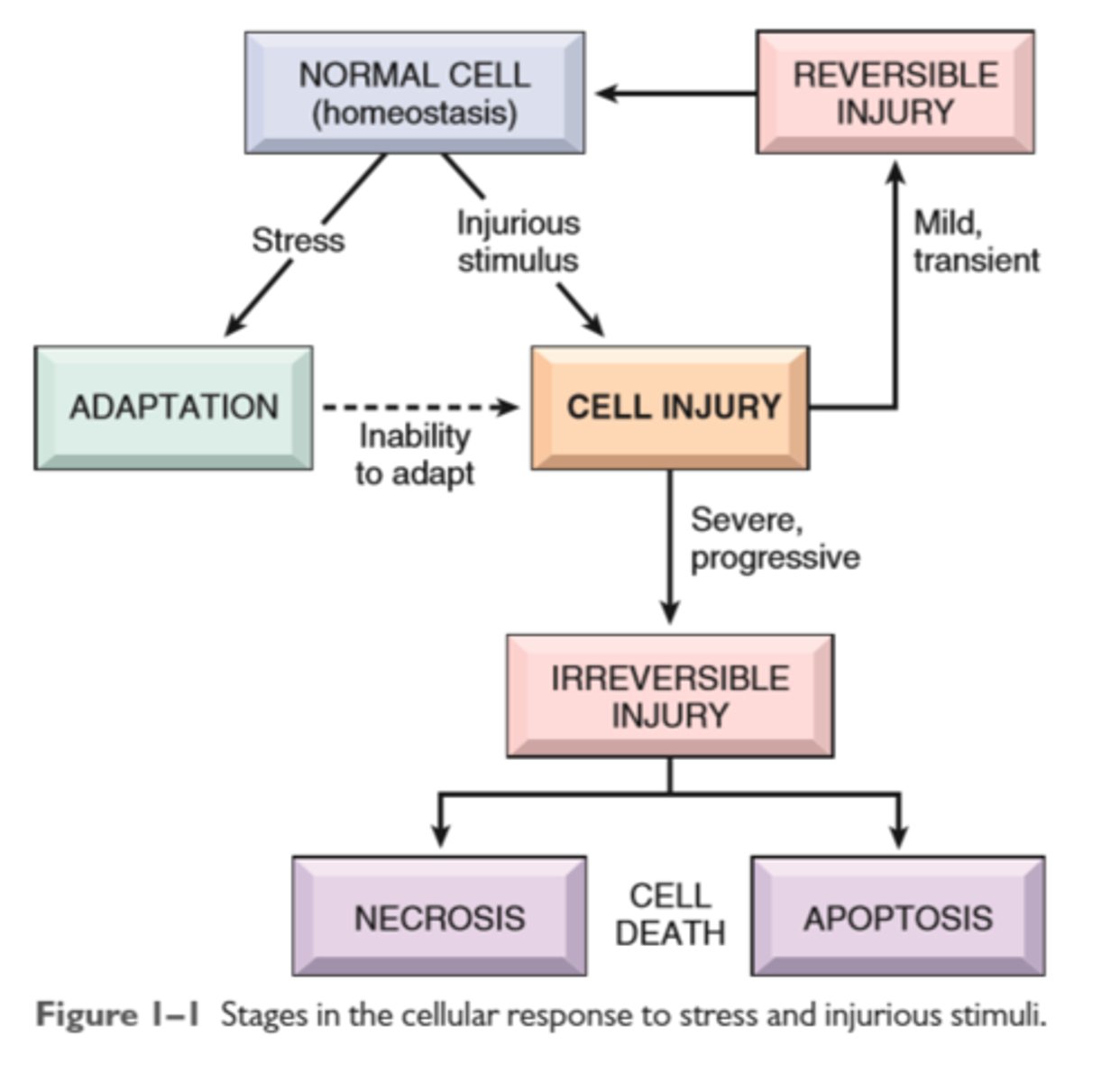
Stages in cellular response to stress and injurious stimulus
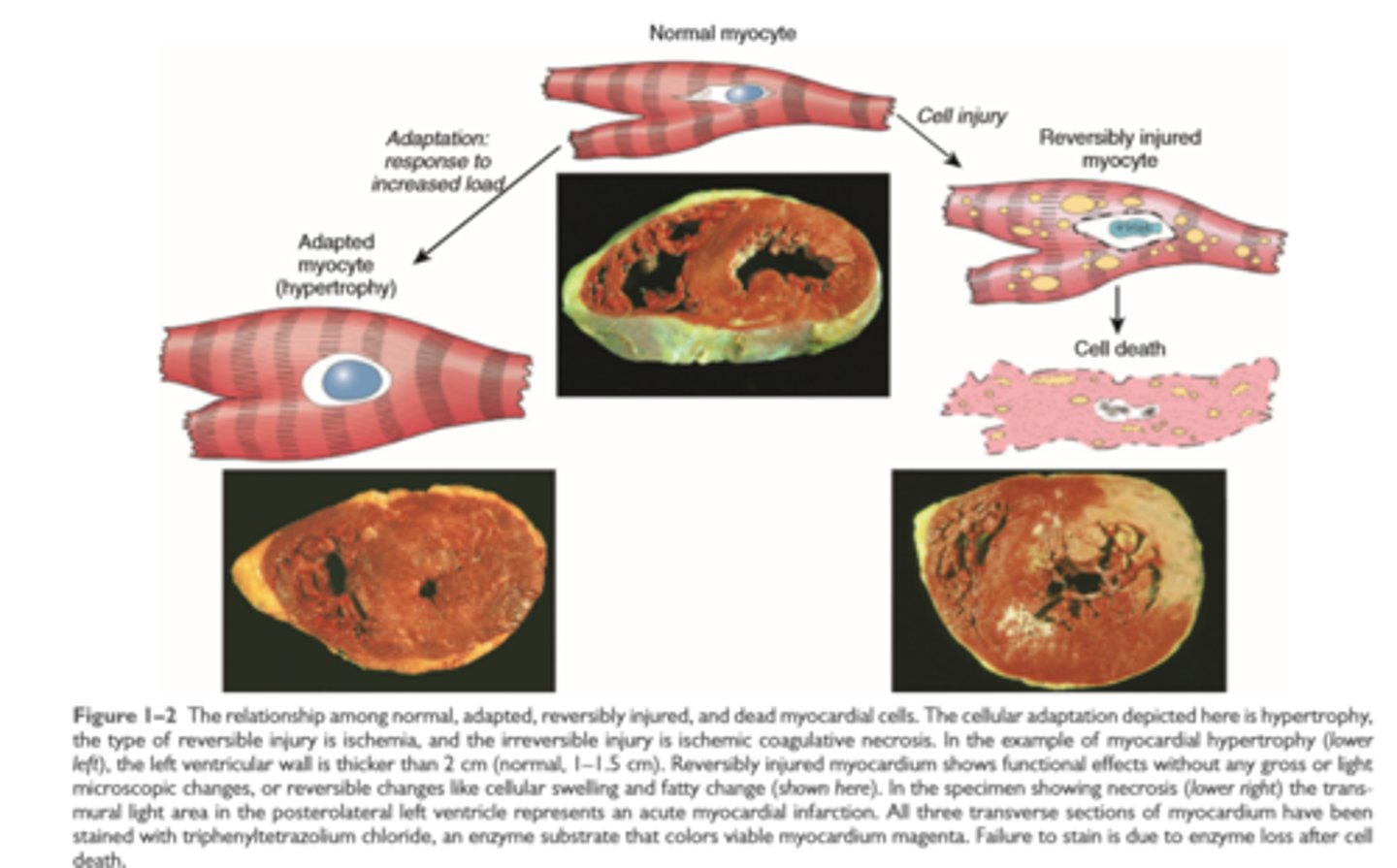
The relationship among normal, adapted, reversibly injured and dead myocardial cells
Hypertrophy
Increase in cell size often in response to increase in workload induced by growth factors in response to mechanical stress or other stimuli; occurs in tissues incapable of cell division
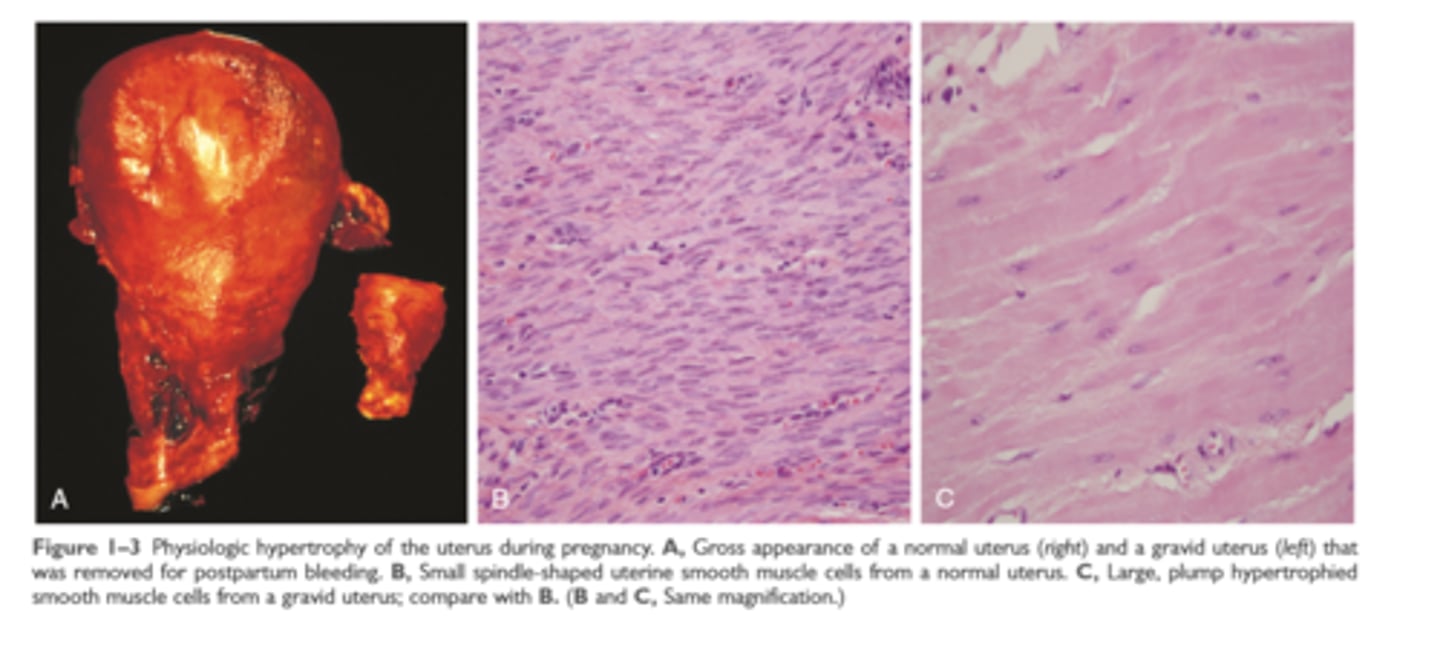
Physiologic hypertrophy of uterus during pregnancy
Hyperplasia
Increase in cell number in response to hormones and other growth factors; occurs in tissues that are capable of dividing or that contain abundant stem cells
Atrophy
decrease in cell and organ size as a result of decreased nutrient supply or disuse; associated with decreased synthesis of cellular building blocks and increased breakdown of cellular organelles
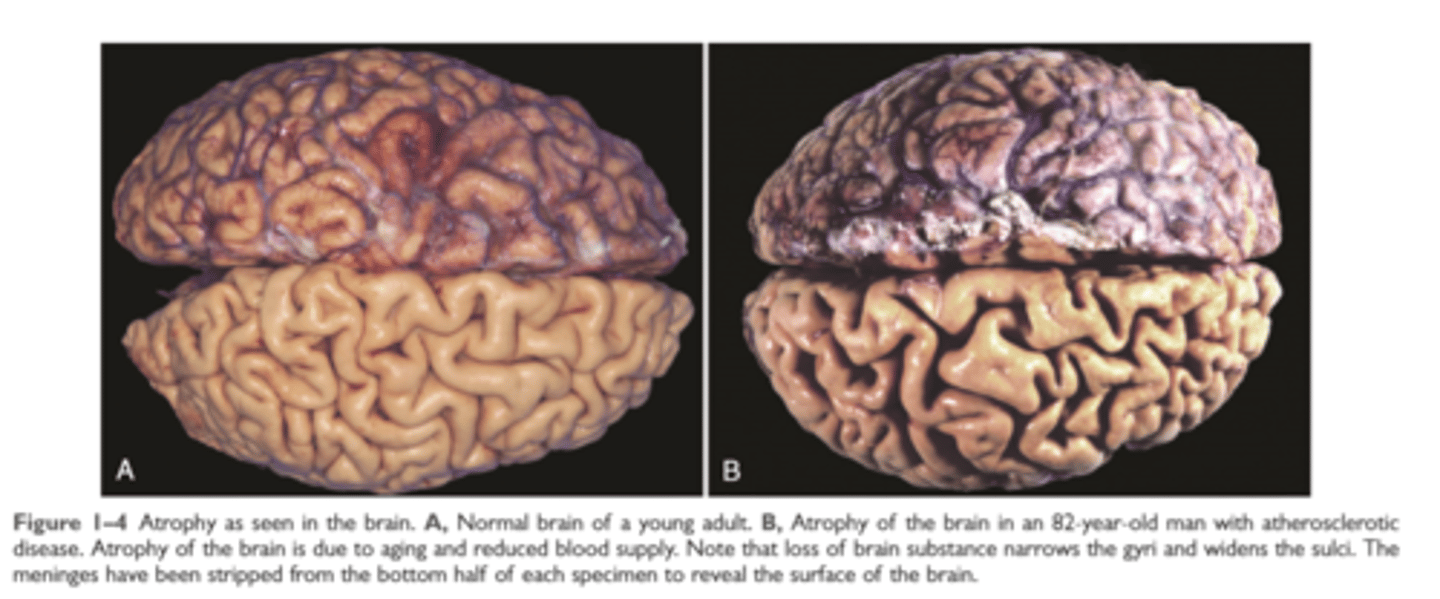
Atrophy of atherosclerotic brain
Metaplasia
Change in phenotype of differentiated cells; often in response to chronic irritation that makes cells better able to withstand the stress; usually induced by altered differentiation pathway of tissue stem cells; may result in reduced functions or increased propensity for malignant transformation
Metaplasia of normal columnar epithelium to squamous epithelium in bronchus
Cellular adaptations to stress
Hypertrophy: increased cell and organ size, often in response to increased workload; induced by growth factors produced in response to mechanical stress or other stimuli; occurs in tissues incapable of cell division
Hyperplasia: increased cell numbers in response to hormones and other growth factors; occurs in tissues whose cells are able to divide or contain abundant tissue stem cells
Atrophy: decreased cell and organ size, as a result of decreased nutrient supply or disuse; associated with decreased synthesis of cellular building blocks and increased breakdown of cellular organelles
Metaplasia: change in phenotype of differentiated cells, often in response to chronic irritation, that makes cells better able to withstand the stress; usually induced by altered differentiation pathway of tissue stem cells; may result in reduced functions or increased propensity for malignant transformation
Reversible cell injury
cell swelling, fatty change, plasma membrane blebbing, loss of microvilli, mitochondrial swelling, dilation of the ER, eosinophilia
Morphologic changes in reversible and irreversible cell injury
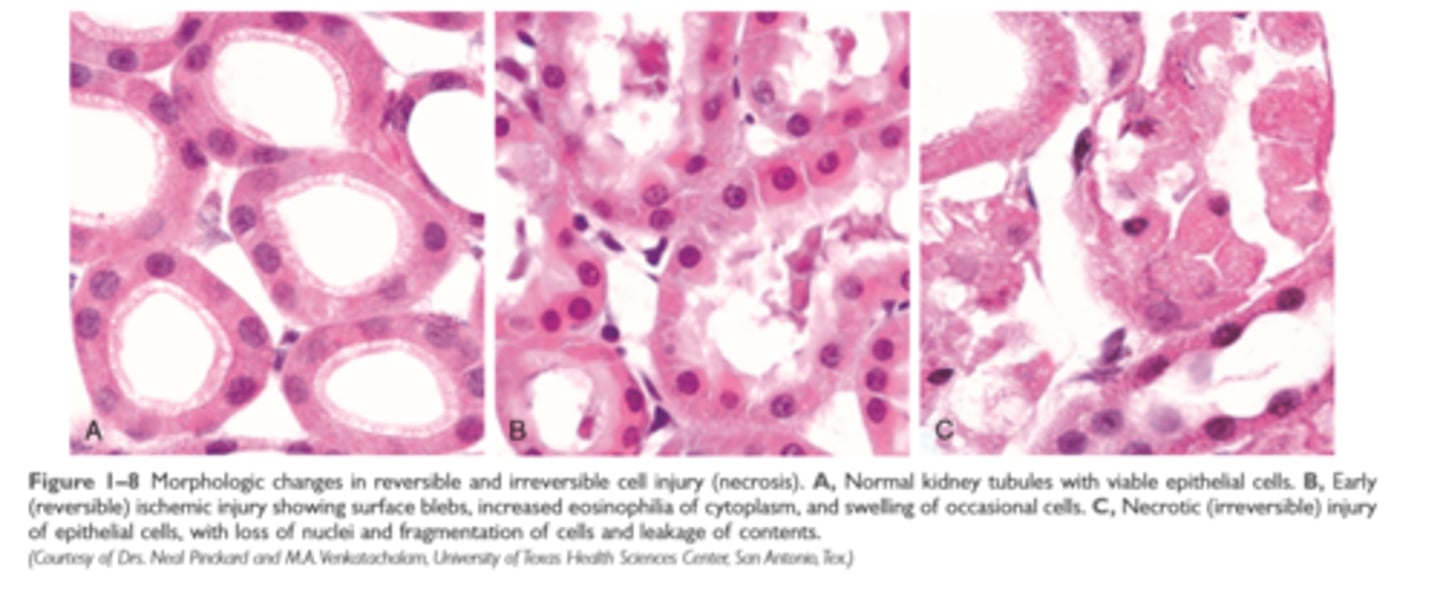
Necrosis
increased eosinophilia, nuclear shrinkage, fragmentation, and dissolution of plasma membrane and organelle membranes, abundant myelin figures, leakage and enzymatic digestion of cellular contents
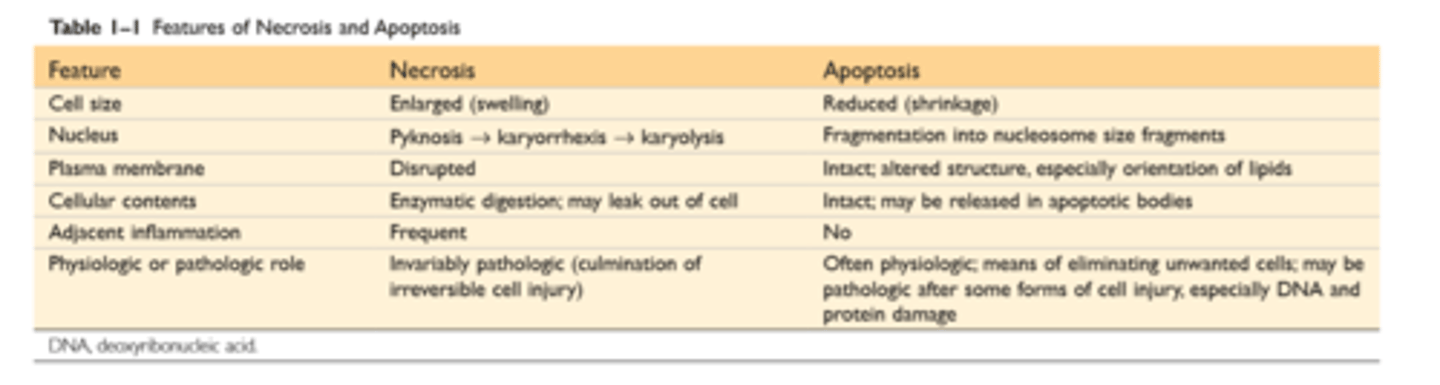
Features of necrosis and apoptosis
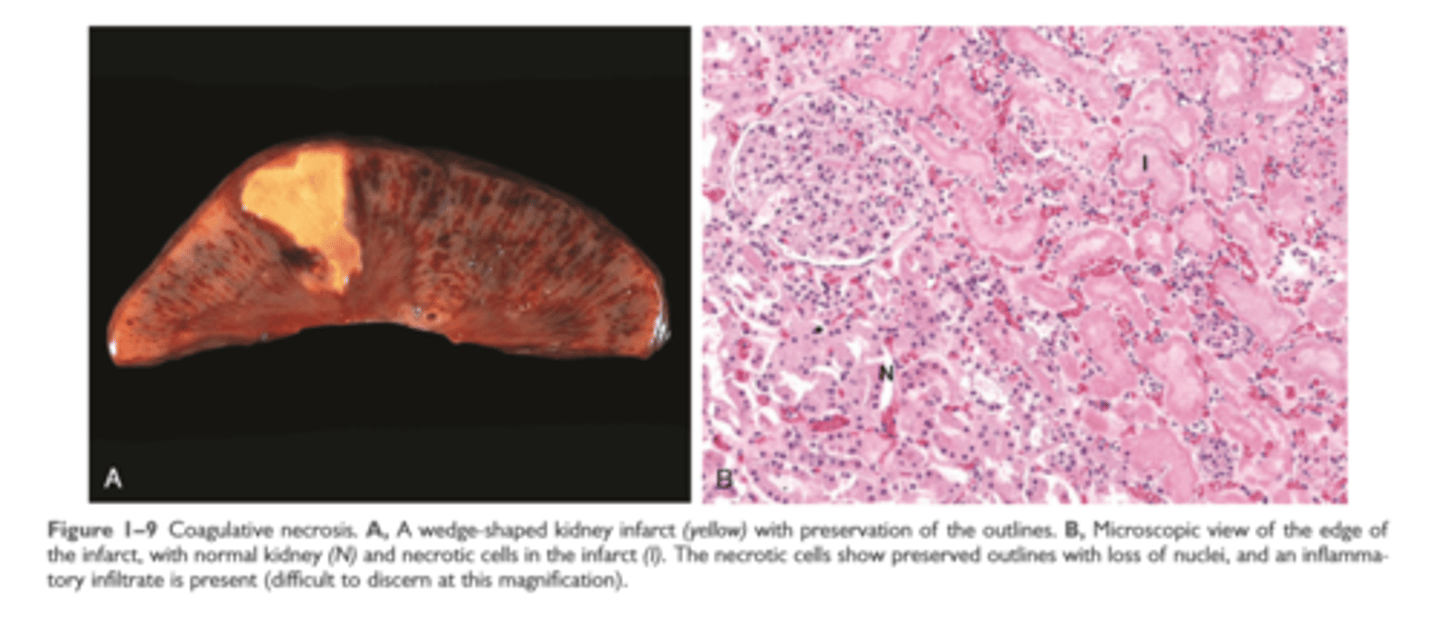
Coagulative necrosis
injury denatures structural proteins and protelytic enzymes preserving microscopic architecture
Coagulative necrosis
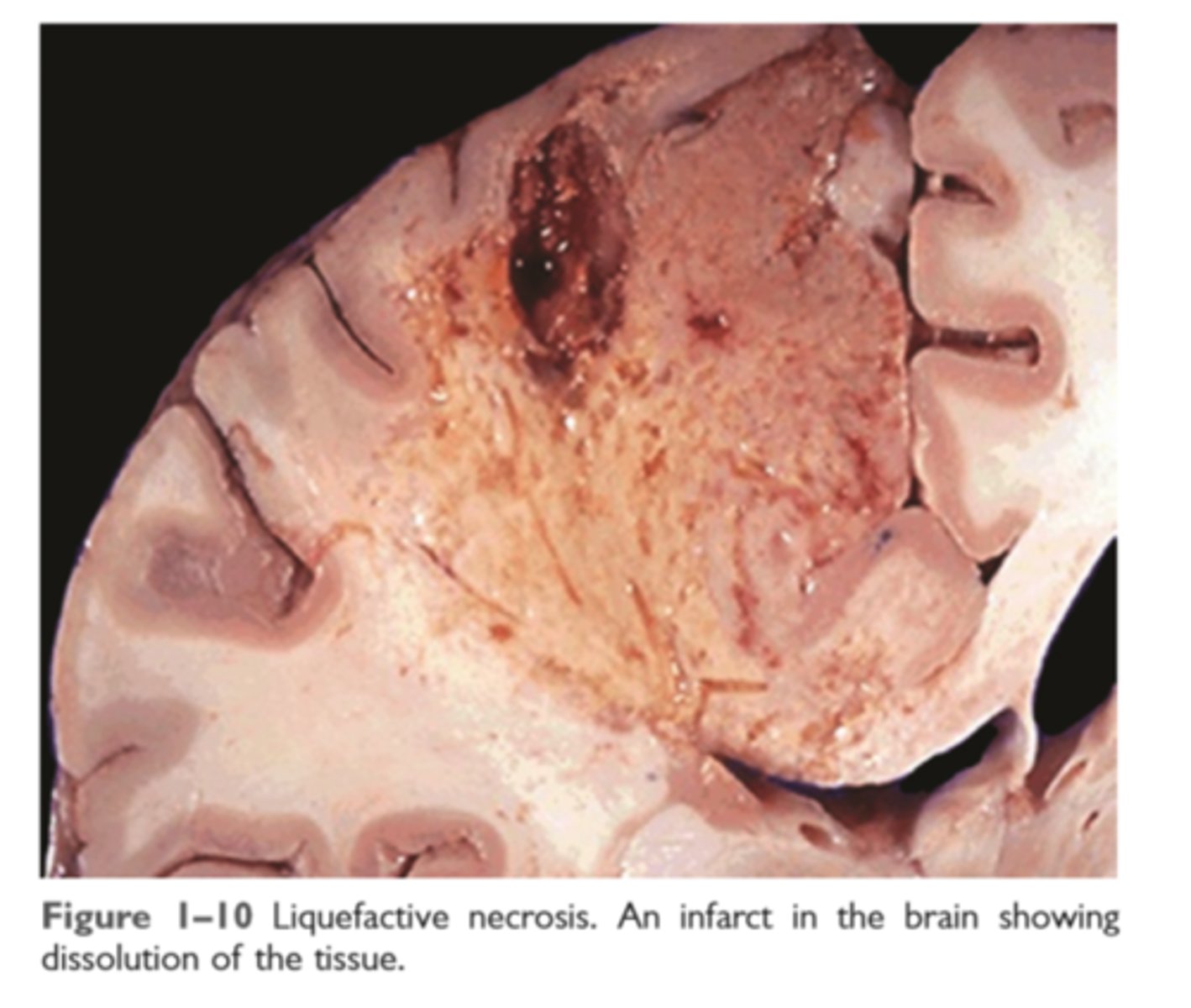
Liquefactive necrosis
seen in focal bacterial or fungal infections
Liquefactive necrosis
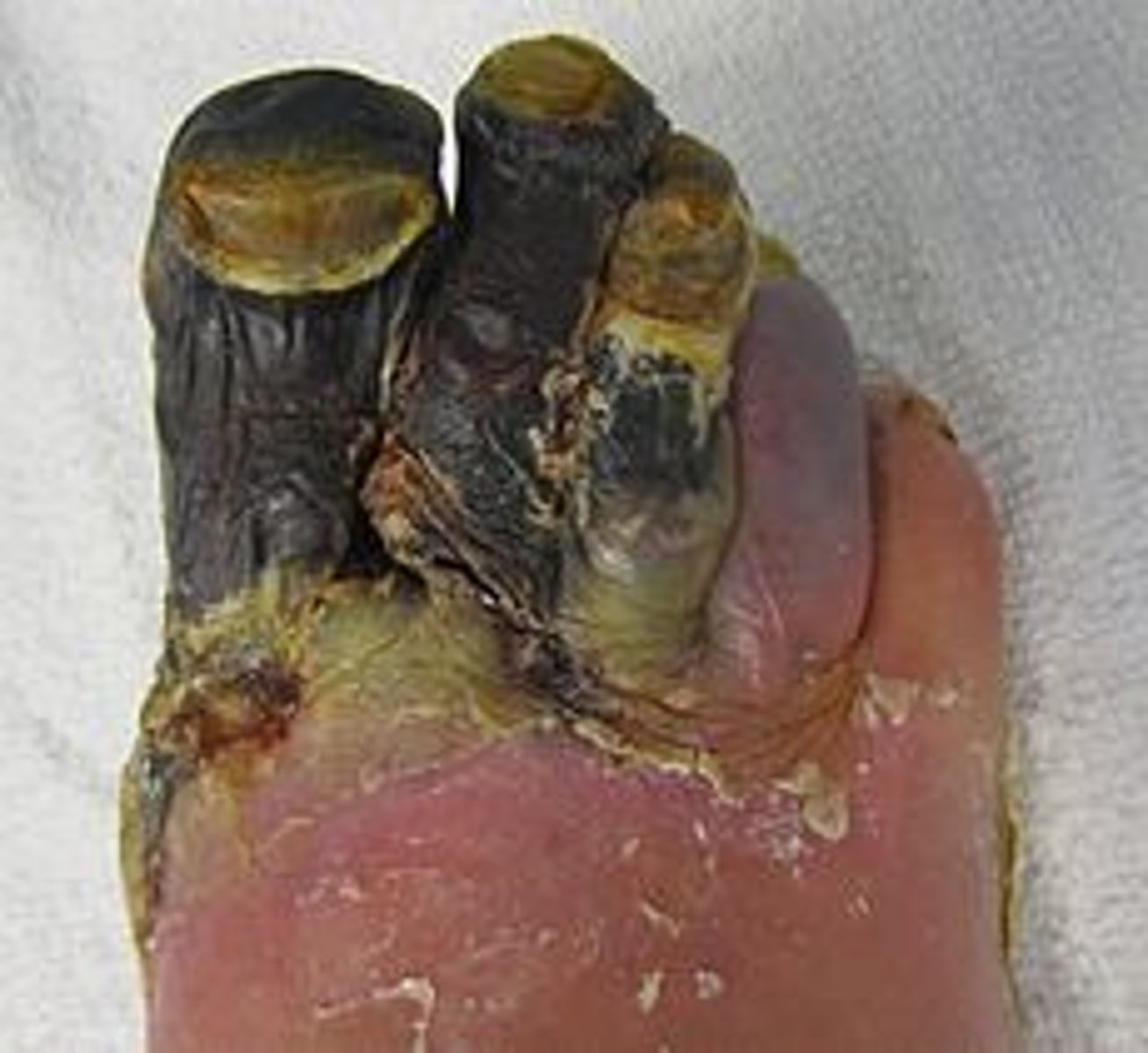
Gangrenous necrosis
coagulative necrosis superimposed by bacterial infection and modified by the liquefactive action of bacteria and leukocytes (wet gangrene)
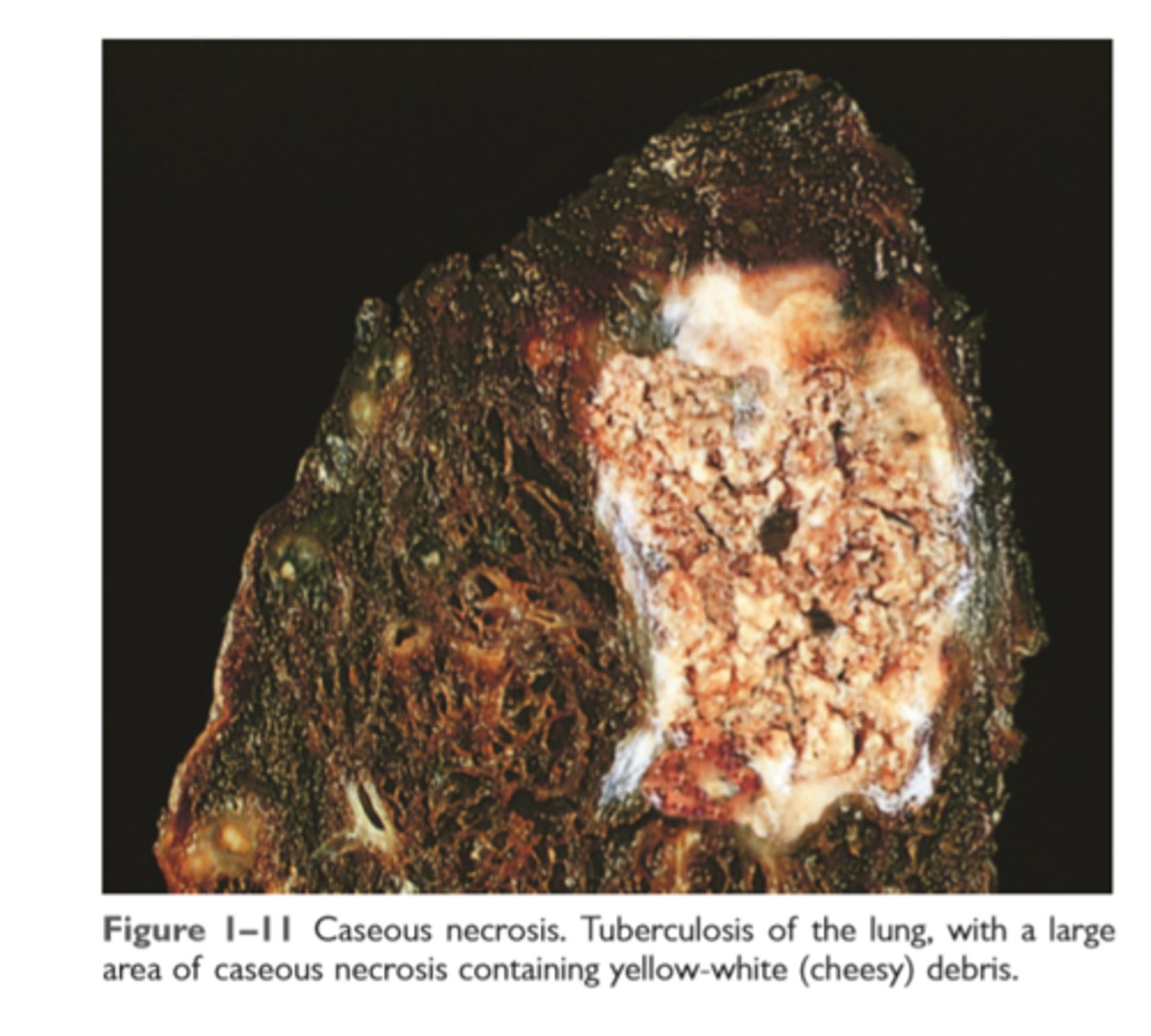
Caseous necrosis
Friable yellow white appearance
Microscopic archatecture obliterated and pink surrounded by inflammatory border (granuloma)
Caseous necrosis
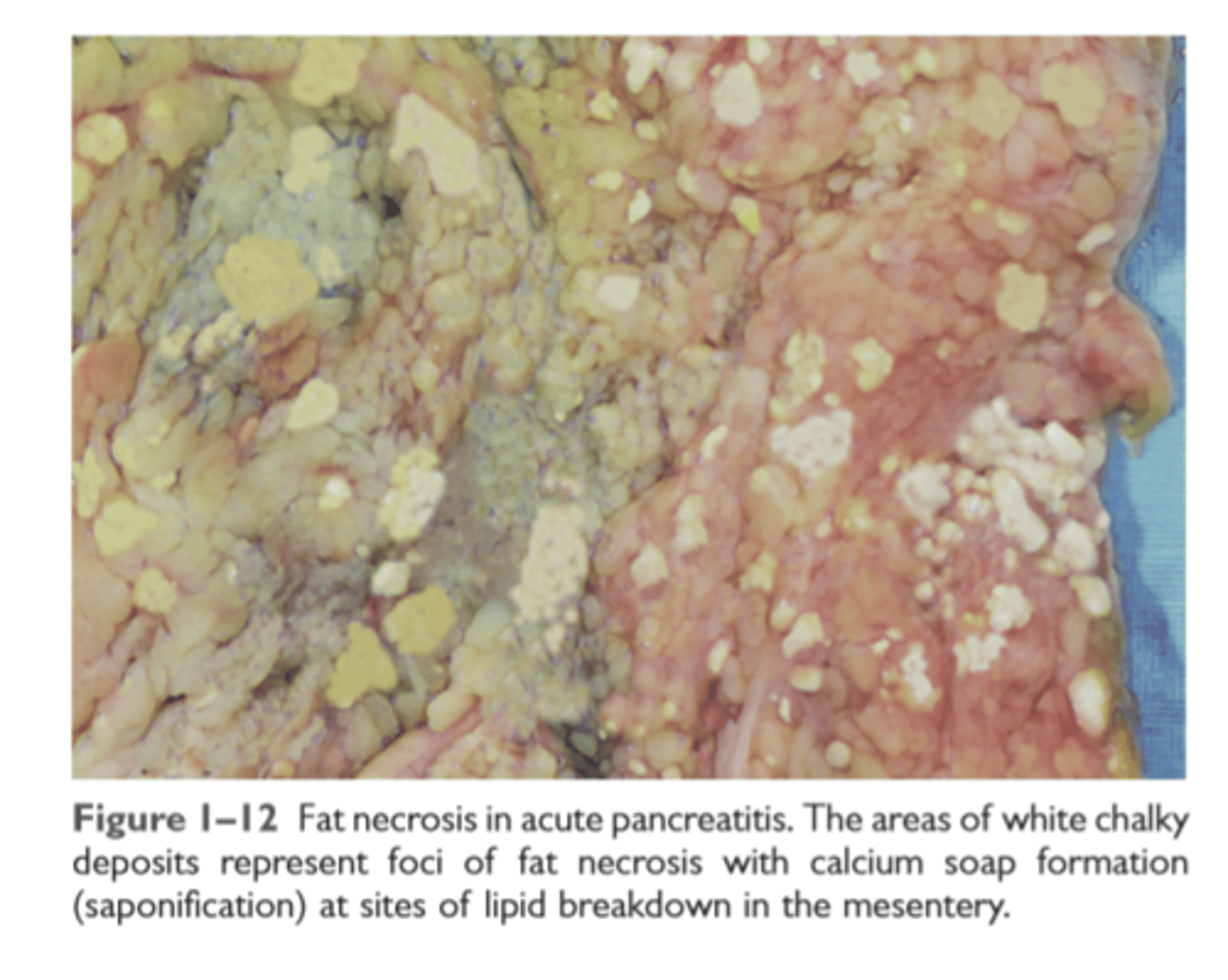
Fat necrosis
focal fat destruction from release of activated lipases. Fatty acids combine with calcium to produce chalky white areas
Fat necrosis in pancreatitis
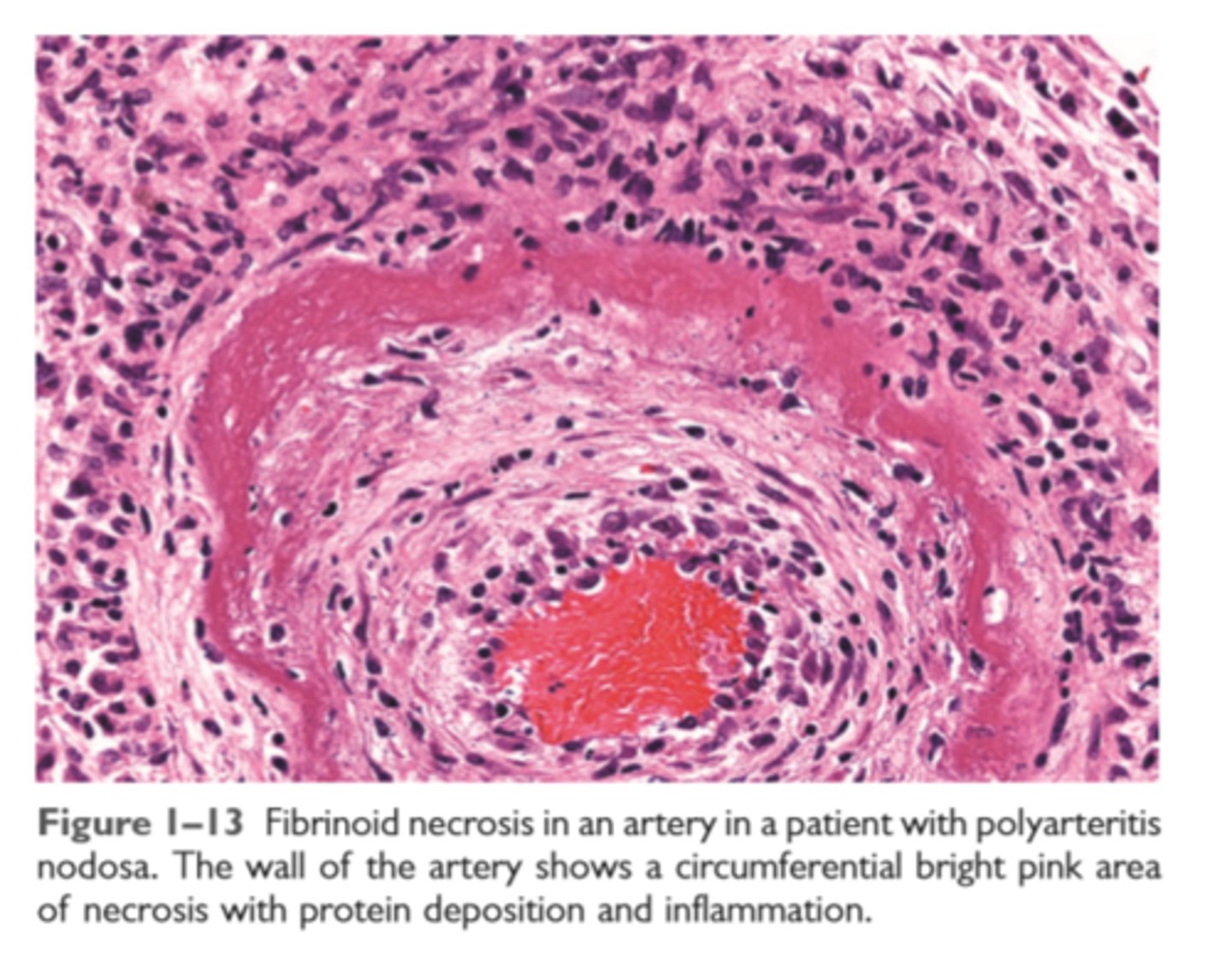
Fibrinoid necrosis
complex of antigens and antibodies deposit in walls of arteries. The deposited immune complexes combine with fibrin to produce bright pink amorphous appearance on H&E
Fibrinoid necrosis in an artery
Morphologic alterations in injured cells and tissue
Reversible cell injury: cell swelling, fatty change, plasma membrane blebbing and loss of microvilli, mitochondrial swelling, dilation of the ER, eosinophilia (due to decreased cytoplasmic RNA)
Necrosis: increased eosinophilia; nuclear shrinkage, fragmentation, and dissolution; breakdown of plasma membrane and organellar membranes; abundant myelin figures; leakage and enzymatic digestion of cellular contents
Patterns of tissue necrosis: Under different conditions, necrosis in tissues may assume specific patterns: coagulative, liquefactive, gangrenous, caseous, fat, and fibrinoid.
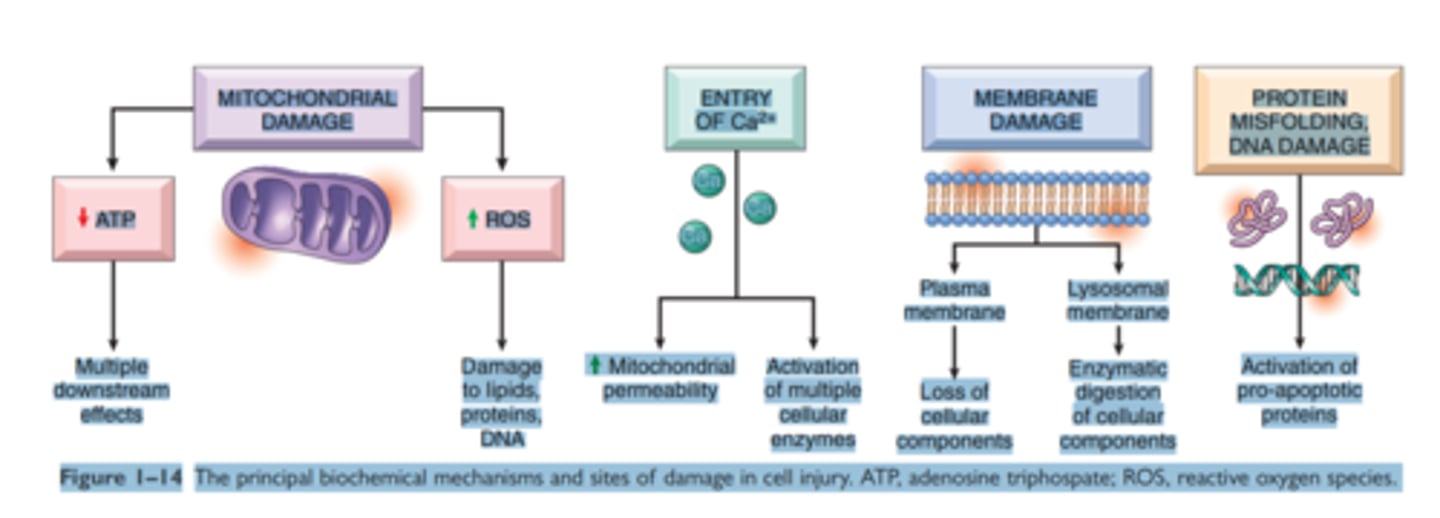
Mechanisms of Cell injury
ATP depletion
Mitochondrial damage
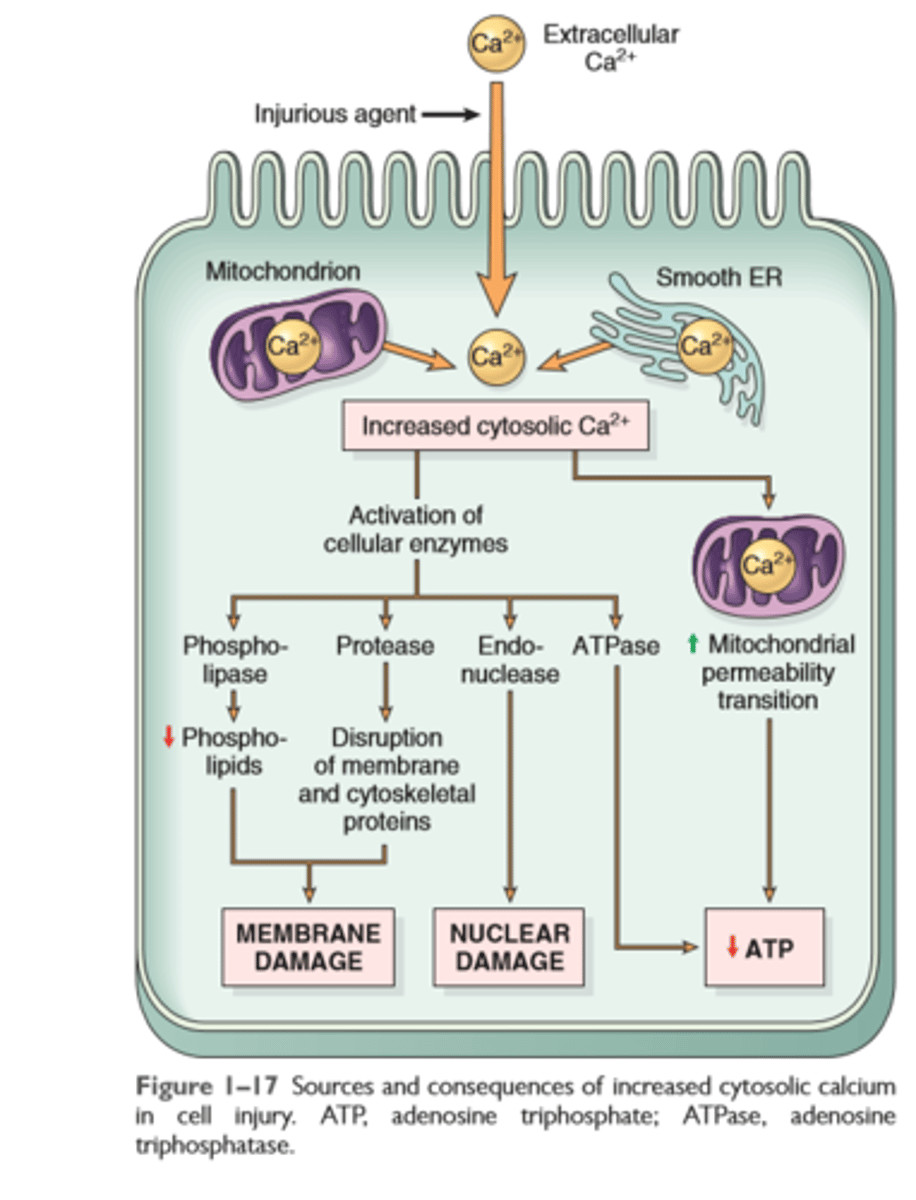
Influx of Calcium
Accumulation of Reactive Oxygen Species
Inc Permeability of cell membrane
Accumulation of DNA damage and misfolded proteins
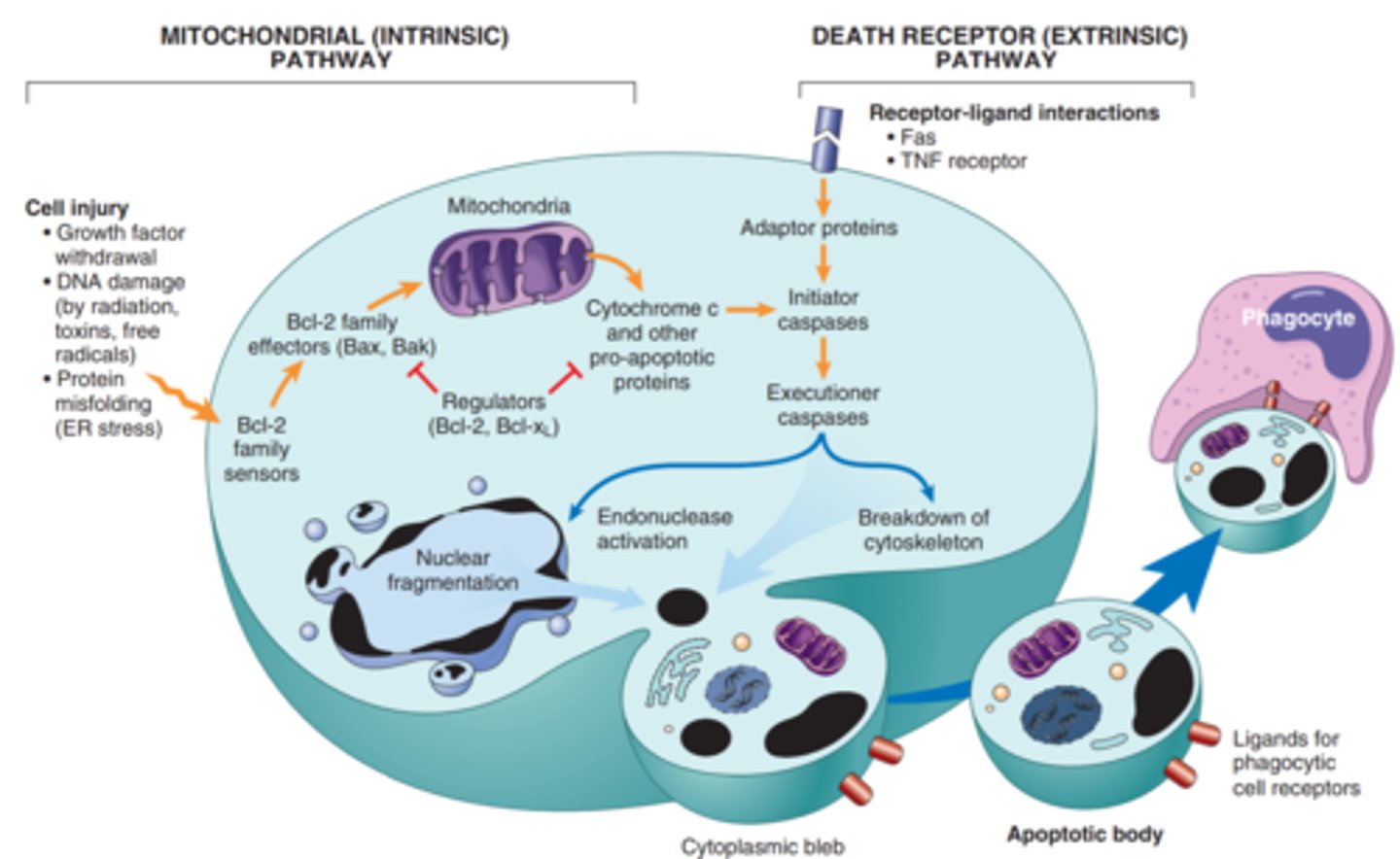
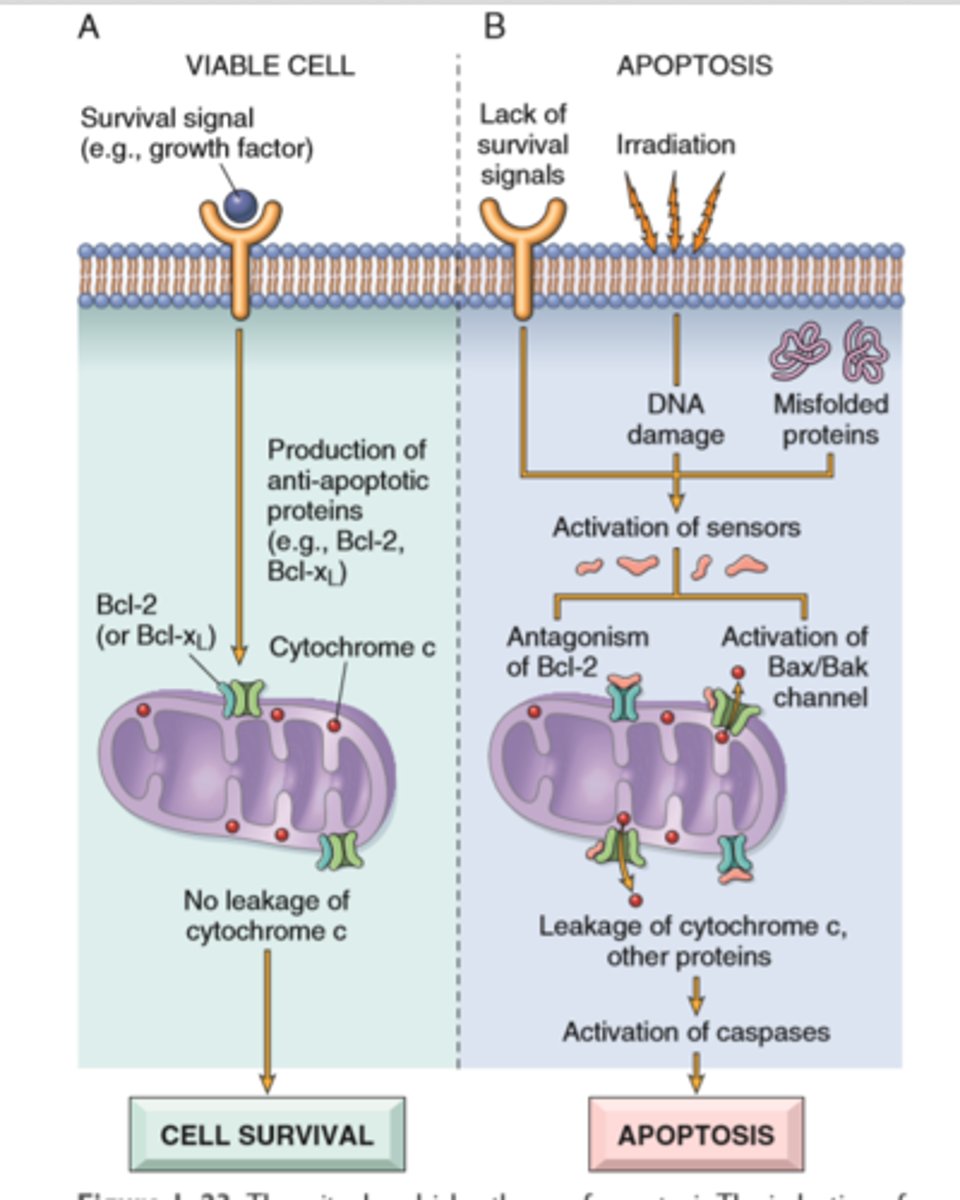
Mitochondrial (instrinsic) and Death Receptor (extrensic) pathway
Intrinsic-proteins of the Bcl-2 family, which regulate mitochondrial permeability, become imbalanced and leakage of various substances from mitochondria leads to caspase activation.
Extrinsic-y, signals from plasma membrane receptors lead to the assembly of adaptor proteins into a "death-inducing signaling complex," which activates caspases, and the end result is the same
Mitochondrial Damage and Dysfunction
Cytostolic influx of intracellular Ca2+
ROS/free radicals in aerobic respiration and leukocytes
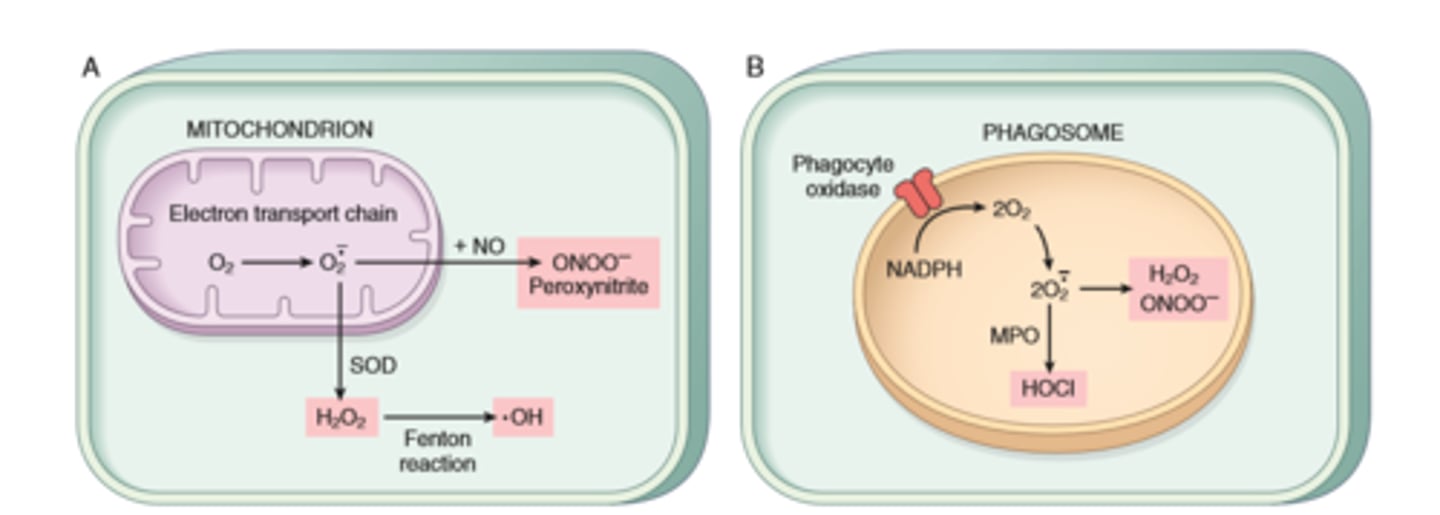
ROS/free radicals- removal vs pathogenesis
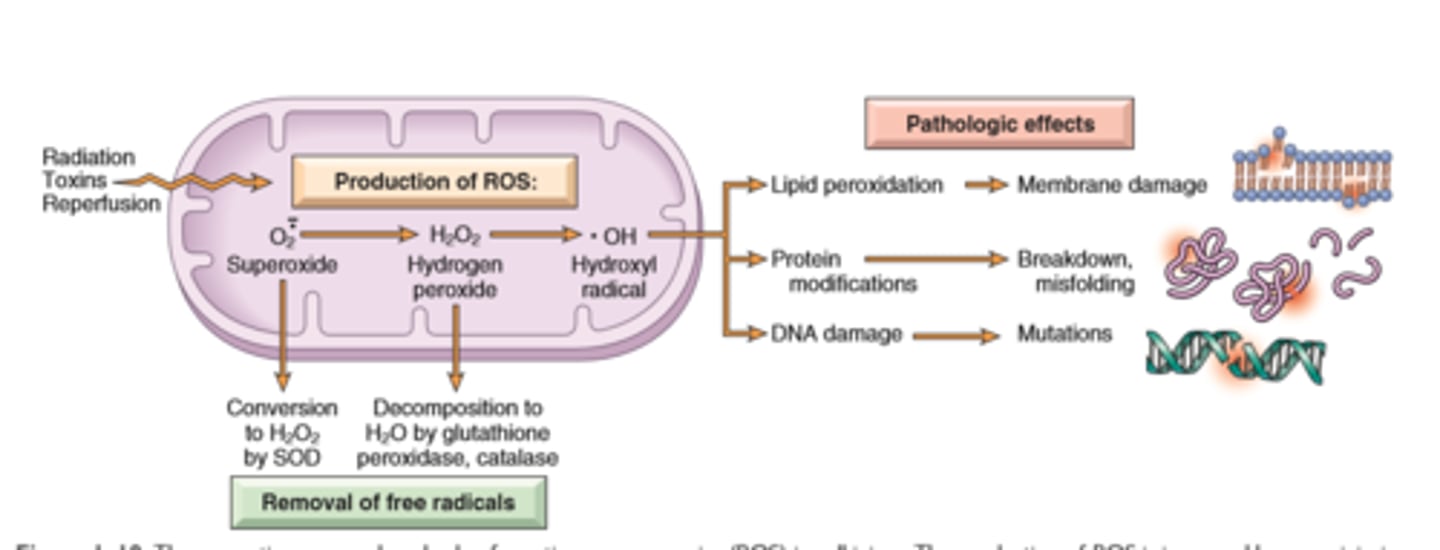
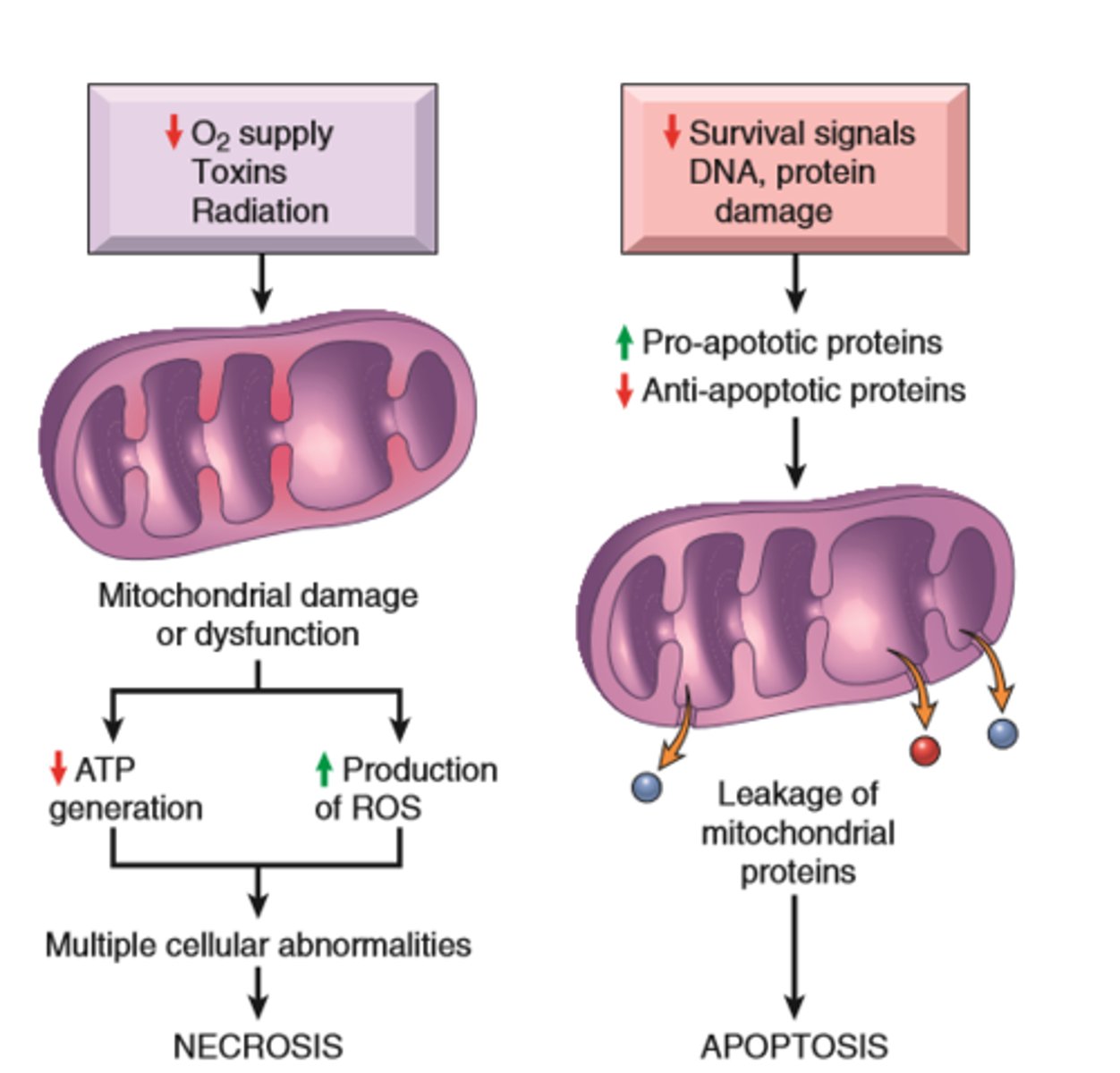
Mechanisms of cell injury
ATP depletion
Mitochondrial Damage
Influx of calcium
Accumulation of ROS
Increased permeability of cell membranes
Accumulation of damaged DNA and misfolded proteins
Apoptosis via mitochondrial pathway in DNA/protein damage
Unfolded protein response and ER stress
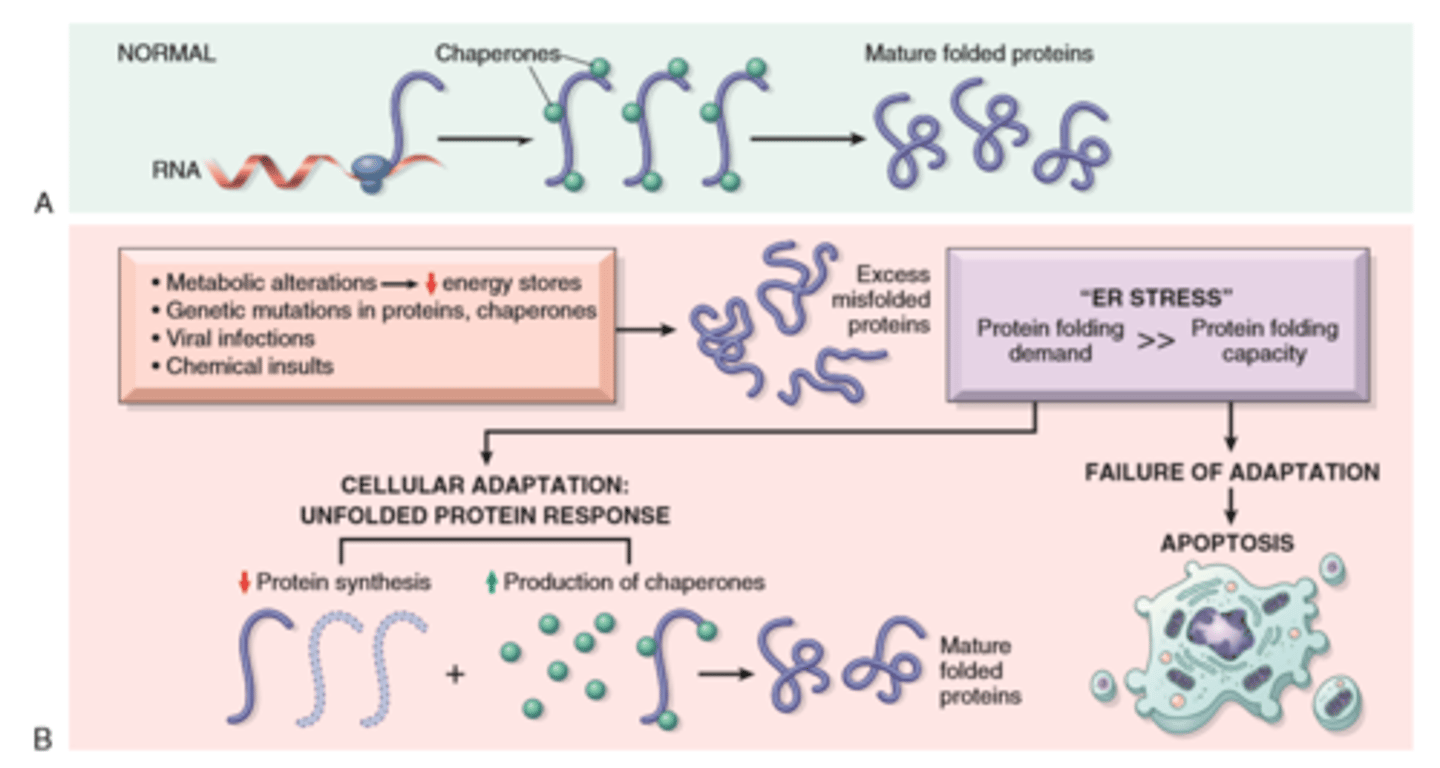
Apoptosis
Regulated mechanism of cell death the serves to eliminate unwanted and irreparable damage cells with lease possible host reaction
Characterized by enzymatic degradation of proteins and DNA, initiated by caspases; and by recognition and removal of dead cells by phagocytes
Initiated by 2 major pathways
Mitochondrial (intrinsic) pathways is triggered by loss of survival signals, DNA damage, and accumulation of misfolded proteins (ER stress); associated with leakage of pro-apoptotic proteins from mitochondrial membrane into the cytoplasm; where they trigger caspase activation (inhibited by anti-apoptotic members of the Bcl family which are induced by survival signals including growth factors)
Death receptor (extrinsic) pathway is responsible for elimination of self reactive lymphocytes and damage by cytotoxic t lymphocytes; in initiated by engagement of death receptors (members of TNF receptor family) by ligands on adjacent cells
Autophagy
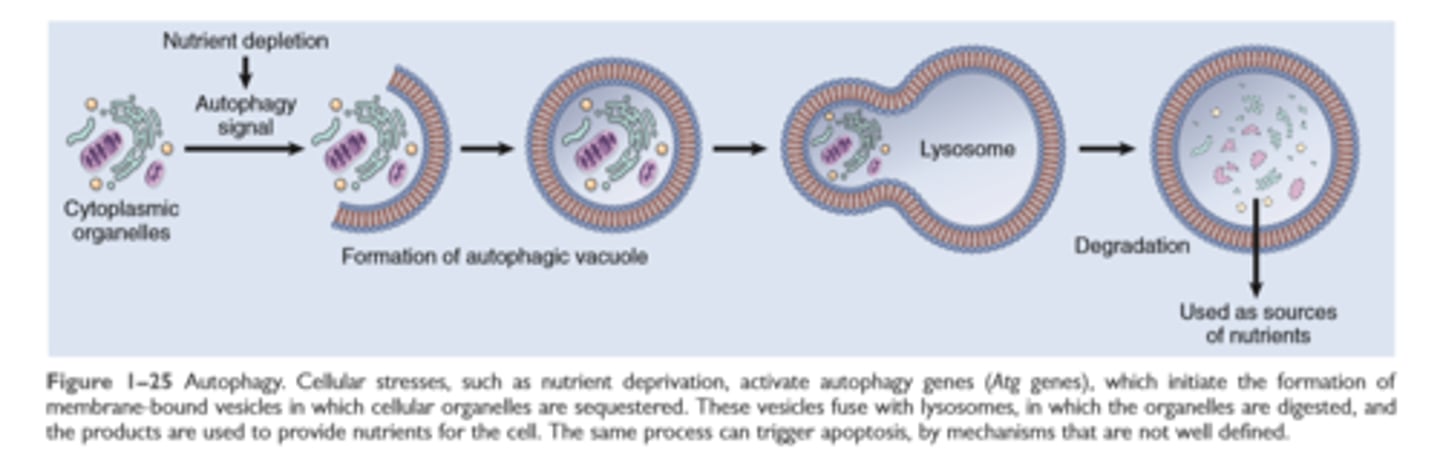
Intracellular accumulations
Fatty change
cholesterol and cholesterol esters
proteins
glycogen
pigments
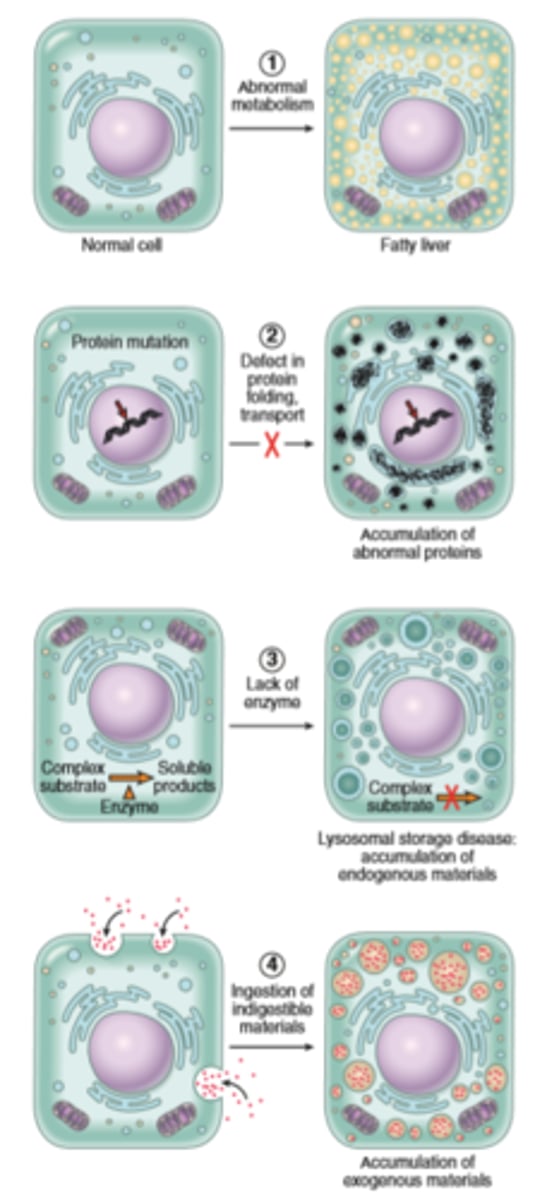
Abnormal intracellular depositions and calcification
result of excessive intake, defective transport or catabolism
Deposition of lipids
Fatty change- accumulation of free triglycerides in cells resulting from excessive intake or defective transport (often from effects of defective transport proteins) manifestation of reversible cell injury
Cholesterol deposition-result of defective catabolism and excessive intake; in macrophages and smooth muscle cells of vessel walls in atherosclerosis
Deposition of proteins- reabsorbed proteins in kidney tubules; immunoglobulins in plasma cells
Deposition of glycogen- in macrophages of patients with defects in lysosomal enzymes that break down glycogen (glycogen storage diseases)
Deposition of pigments-typically indigestible pigments such as carbon, lipofuscin (breakdown products of lipid peroxidation) or iron (usually due to overoad as in hemosiderosis)
Pathologic calcifications
Dystrophic calcifications-deposition of calcium at sites of cell injury and necrosis
Metastatic calcification- deposition of calcium in normal tissues, caused by hypercalcemia (usually a consequence of parathyroid hormone excess)
Cellular aging
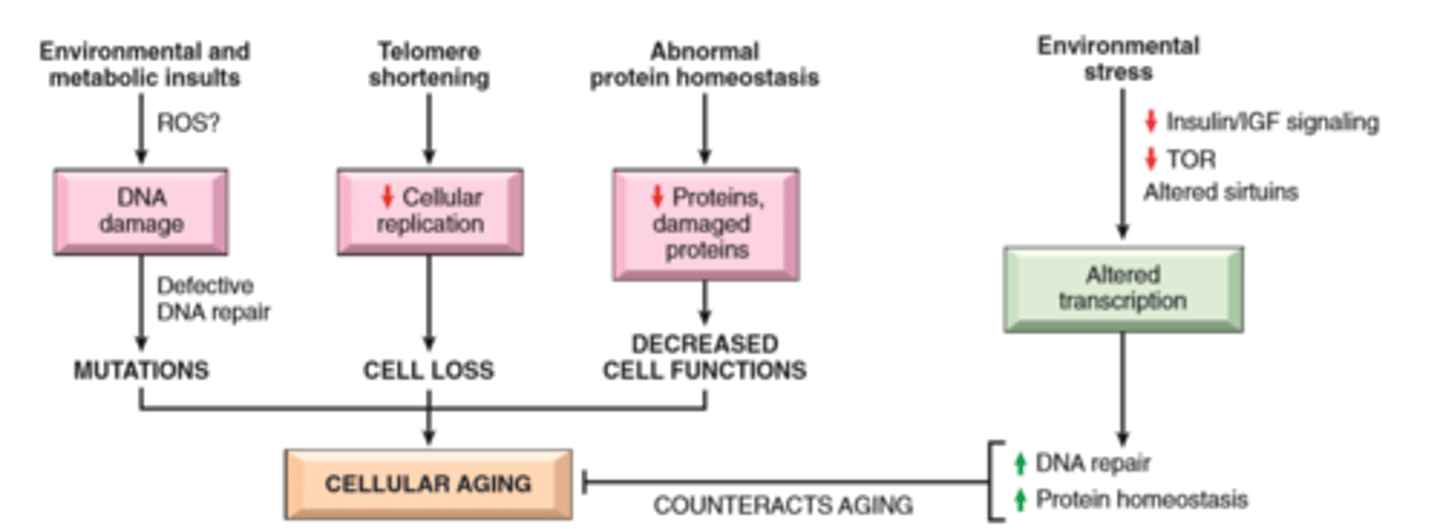
Role of telomerase in replicative senescence
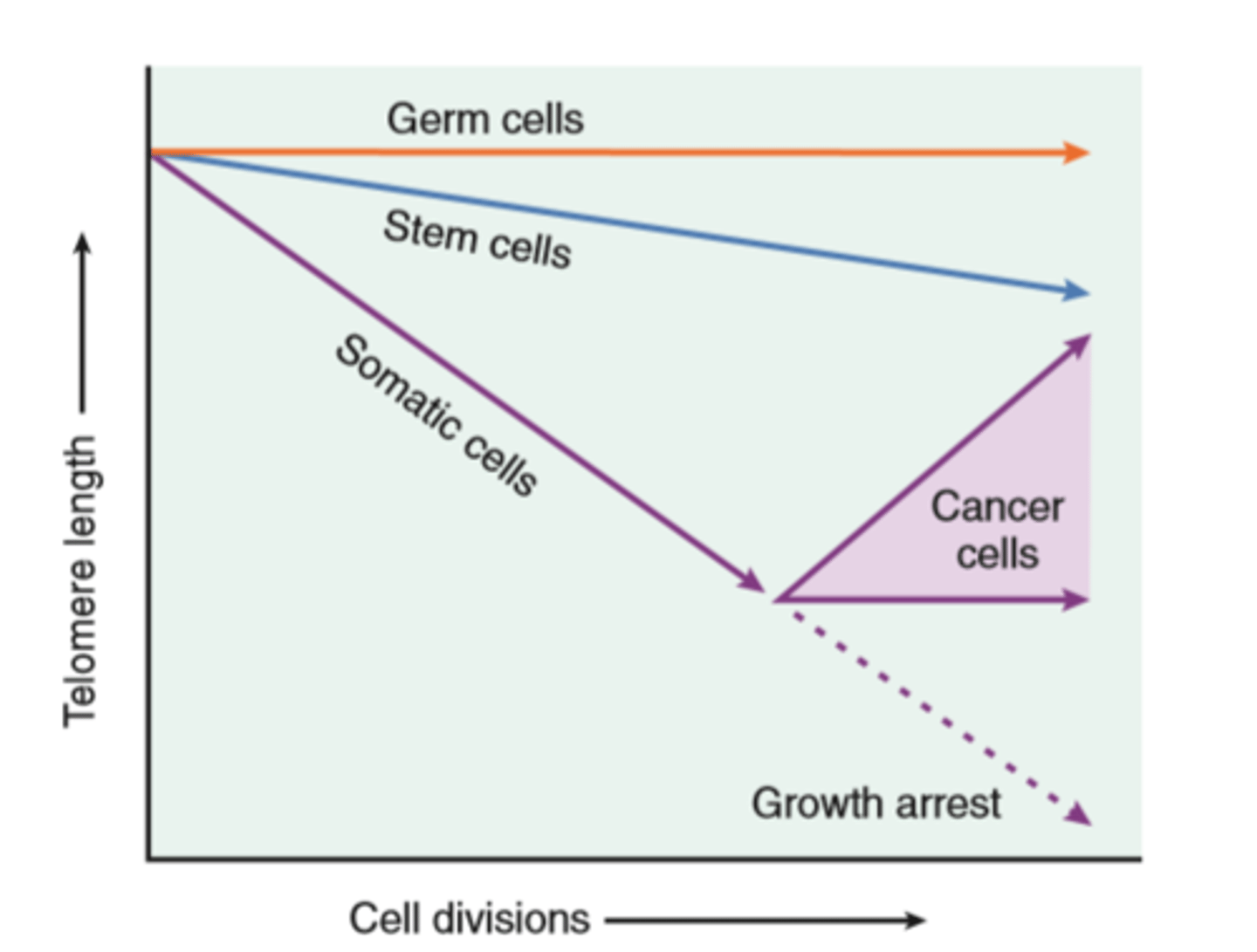
Cellular aging
Results from combination of accumulating cellular damage (e.g free radicals) reduced capacity to divide (replicative senescense), and reduced ability to repair damaged DNA
Accumulation of DNA damage: defective DNA repair mechanisms; conversely DNA repair may be activated by calorie restriction which is known to prolong aging in model organisms
Replicative senescense: reduced capacity of cells to divide secondary to progressive shortening of chromosomal ends (telomeres)
Other factors: progressive accumulation of metabolic damage; possible roles of growth factors promoting aging in simple model organisms
Chap 2 Inflammation and Repair
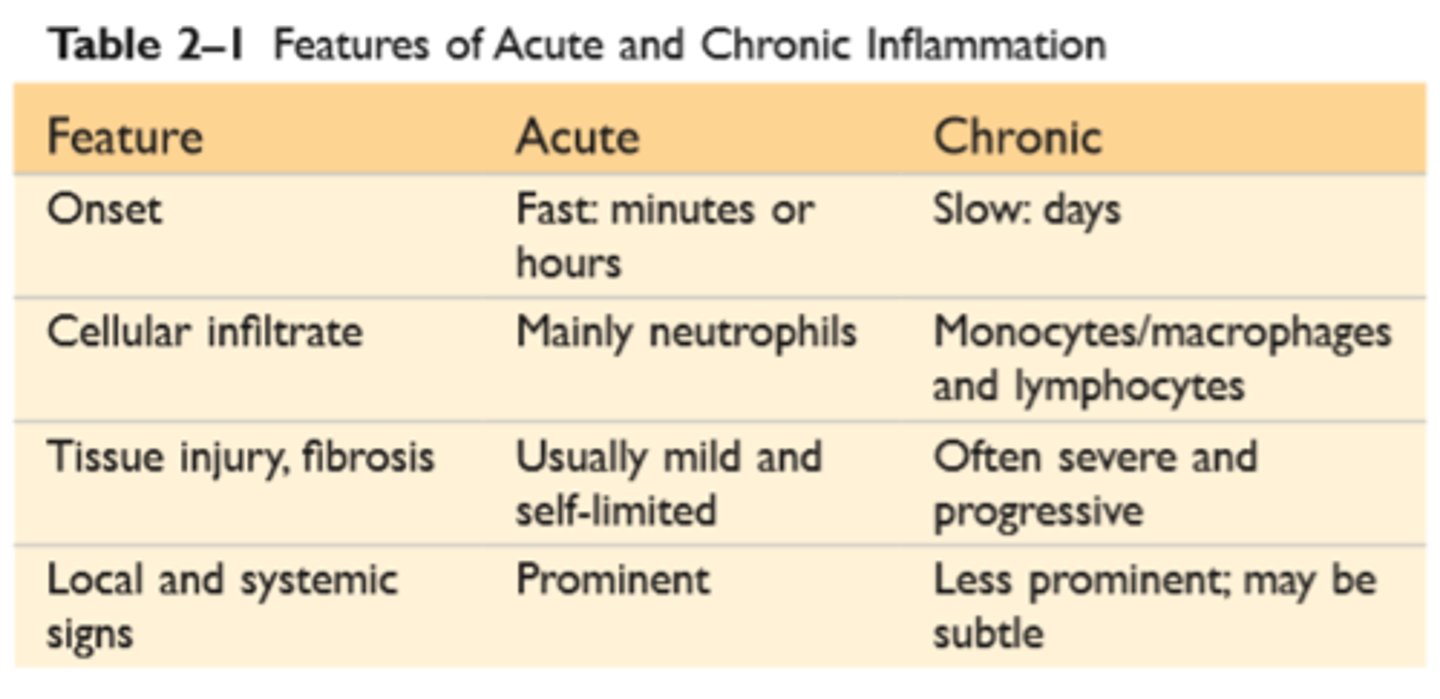
Acute and Chronic Inflammatory Response
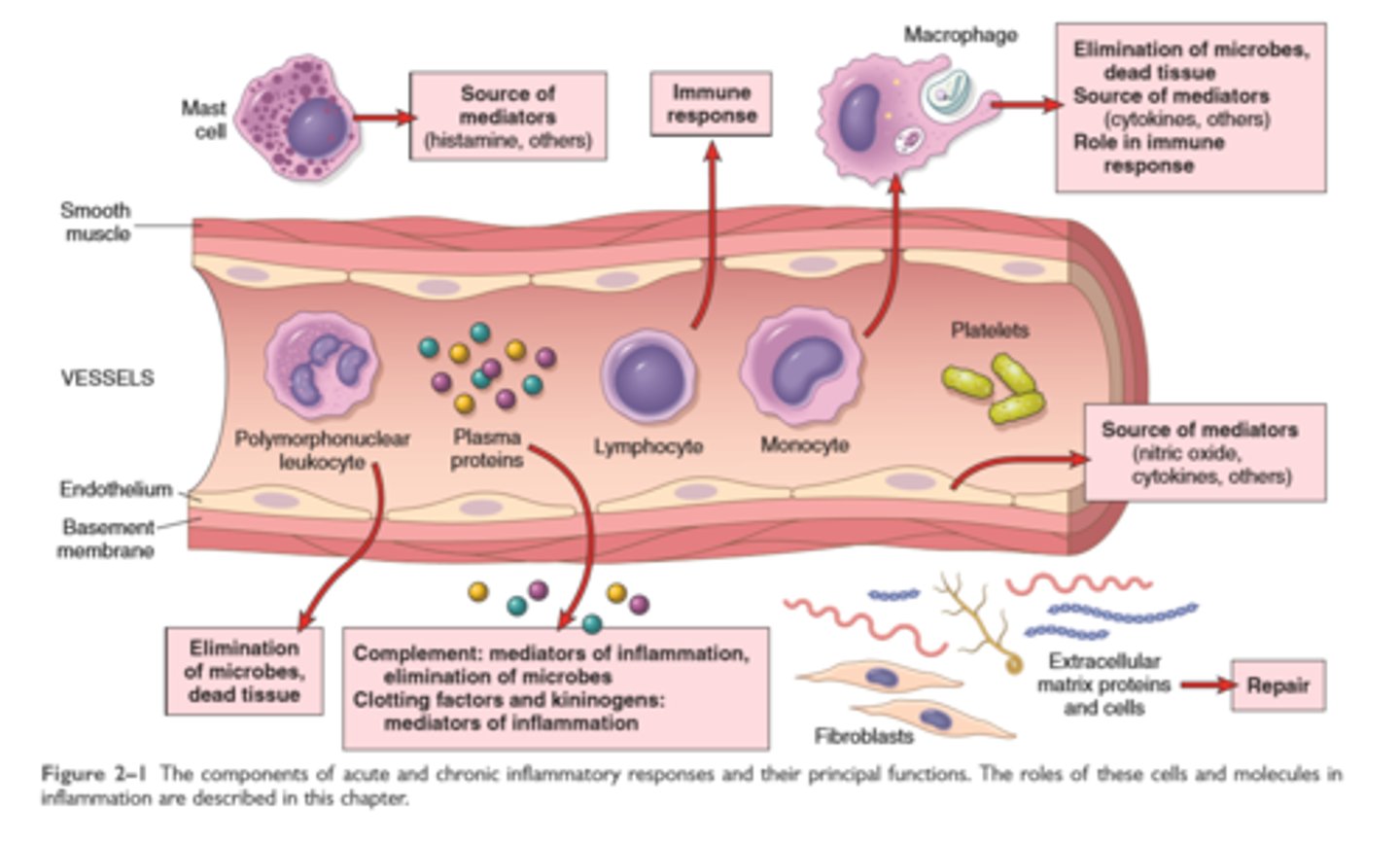
Features of Acute and chronic inflammation
General features of inflammation
Inflammation is a defensive host response to foreign invaders and necrotic tissue but it is itself capable of causing tissue damage
The main components of inflammation is are a vascular reaction and a cellular response; both are activated by mediators derived from plasma proteins and various cells
5 R's- recognition of injurious agent, recruitment of leukocytes, removal of the agent, regulation of response, resolution (repair)
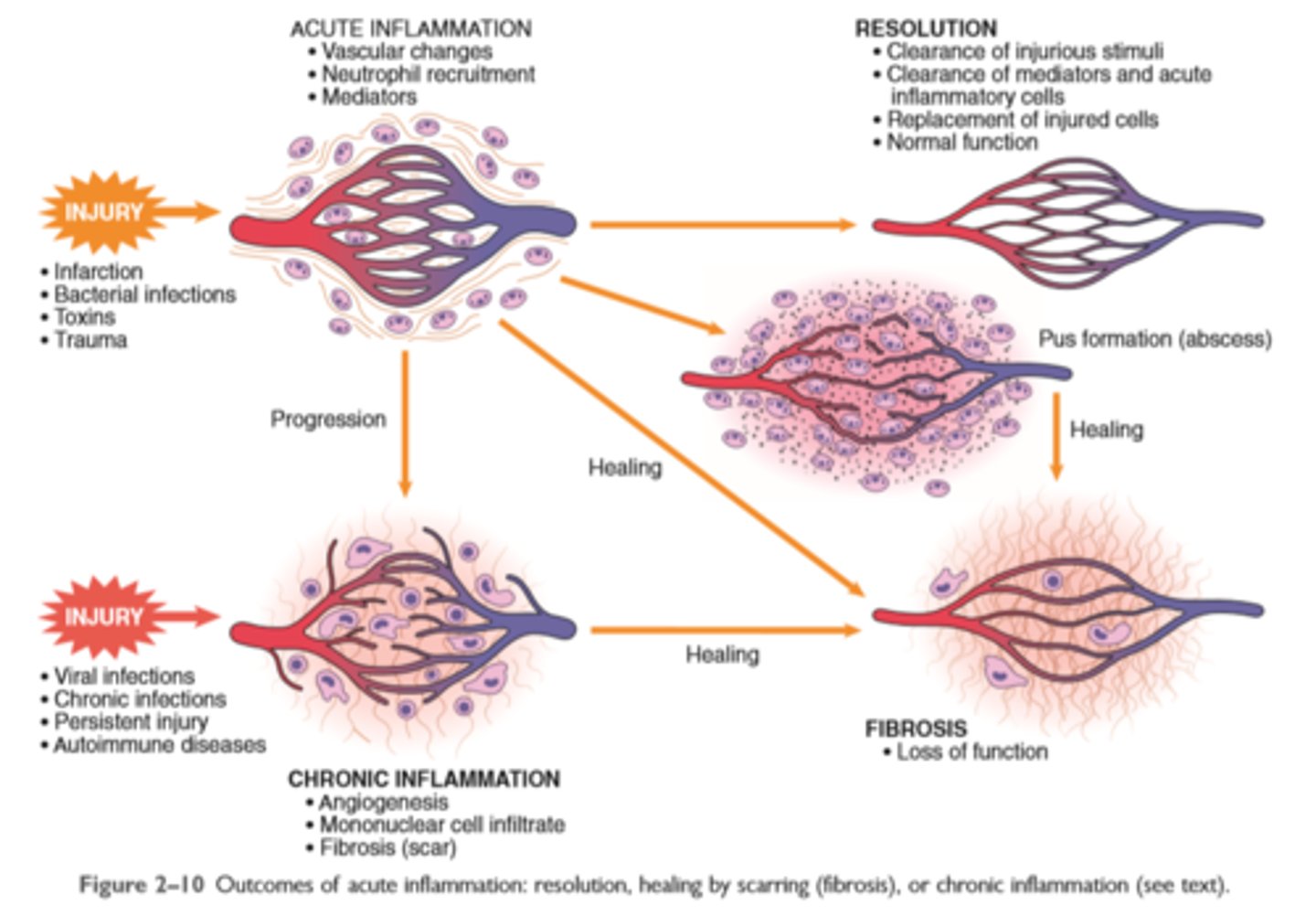
The outcome of acute inflammation is either elimination of the noxious stimulus, followed by decline of reaction and repair of damaged tissue, or persistent injury resulting in chronic inflammation
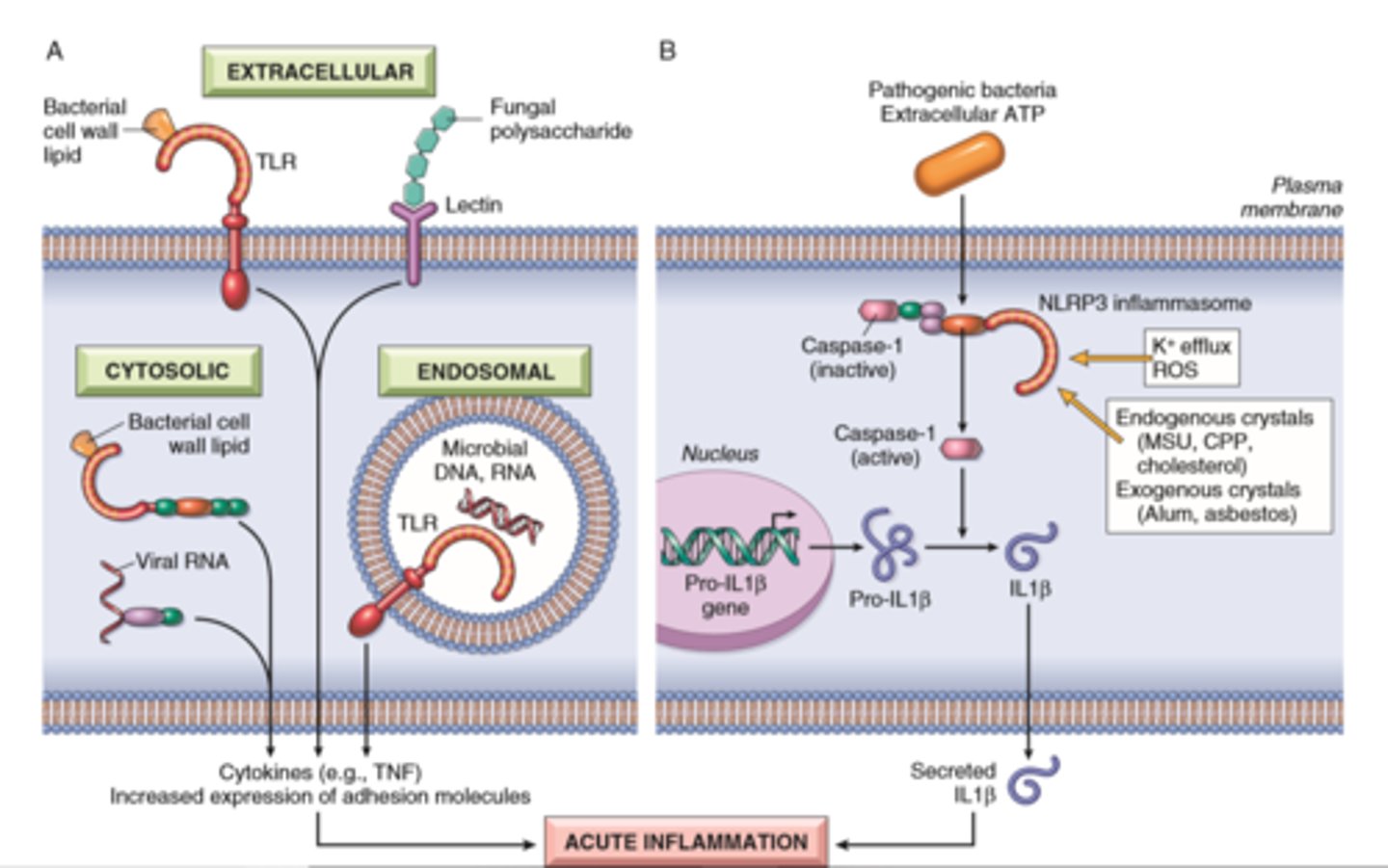
Vascular and cellular Inflammation
TLR's, Inflammasome
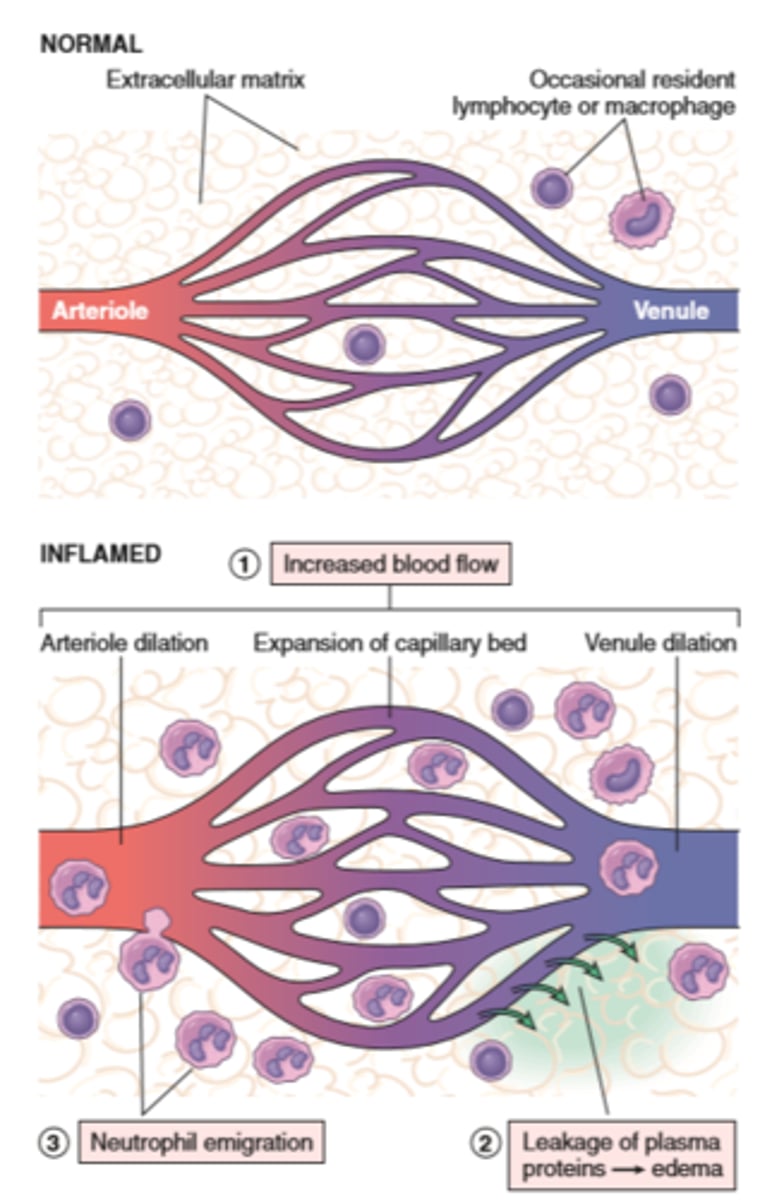
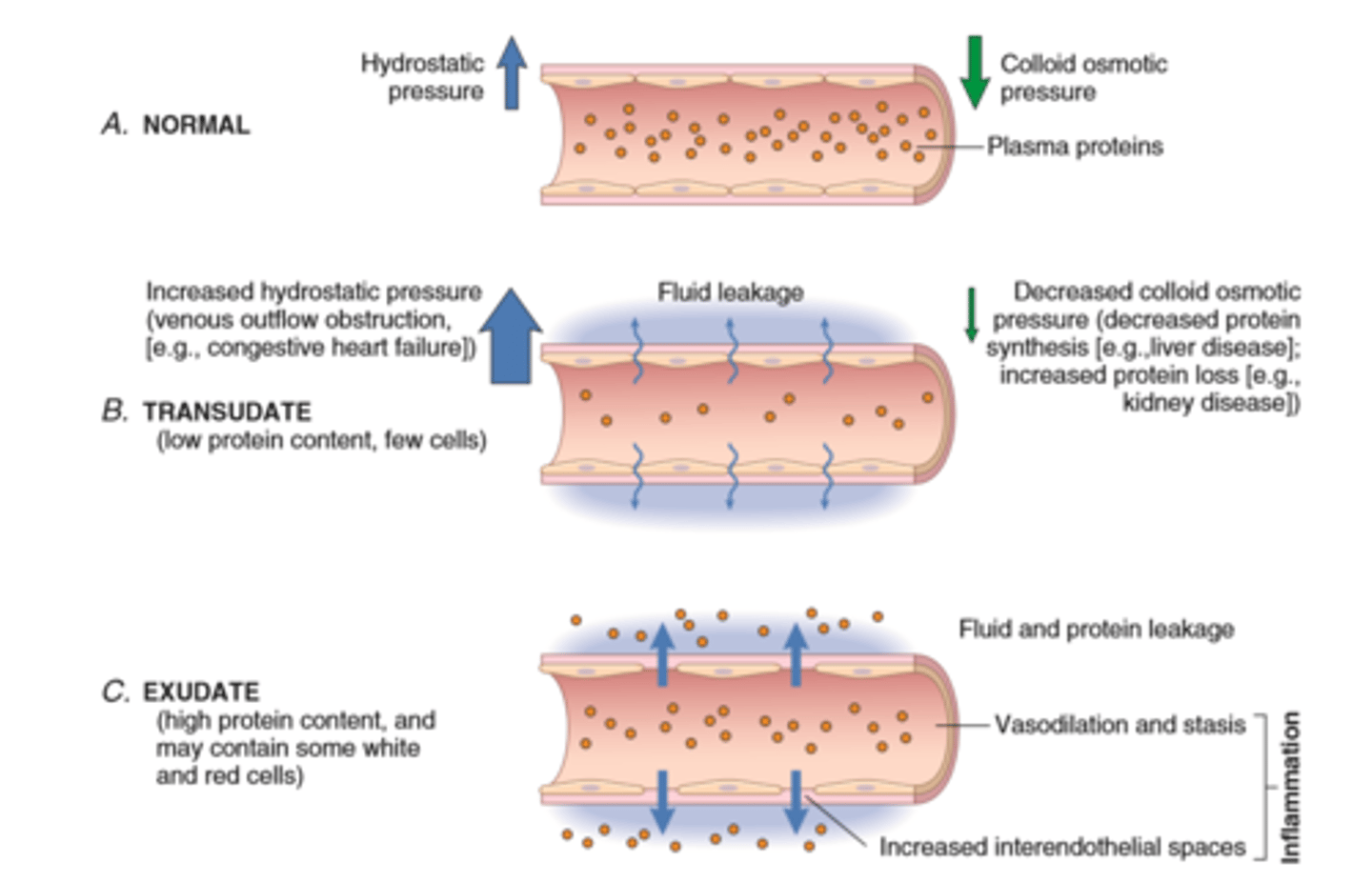
Vascular changes
Vascular Reactions in Acute Inflammation
Vasodilation is induced by chemical mediators such as histamine and is the cause of erethyma and stasis of blood flow
Increased vascular permeability is induced by histamine, kinins and other mediators that produce gaps between endothelial cells; by direct or leukocyte induced endothelial injury; and by increased passage of fluids through the endothelium
This increased permeability allows proteins and leukocytes to enter sites of infection or tissue damage; fluid leak through blood vessels results in edema
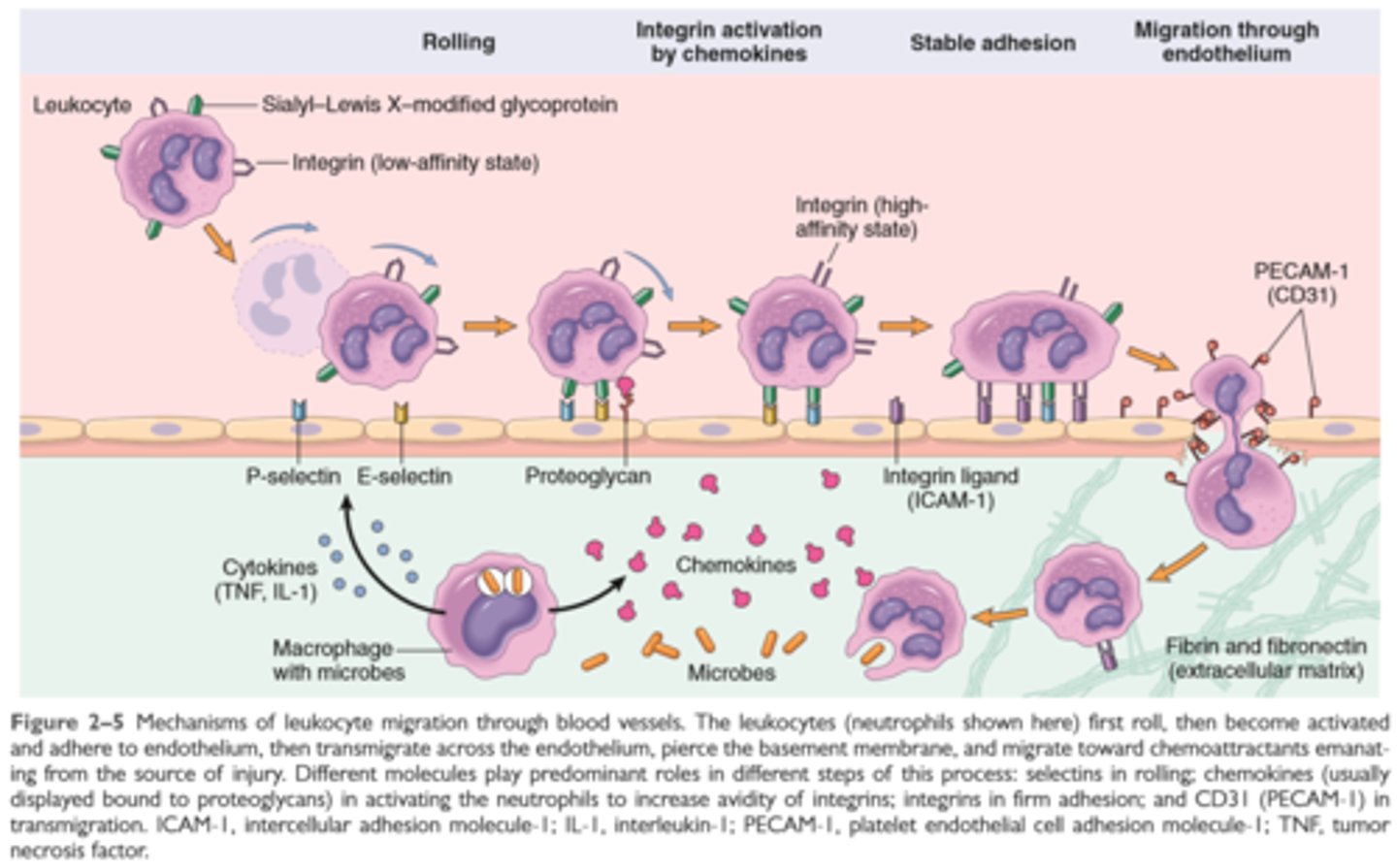
Leukocyte Recruitment
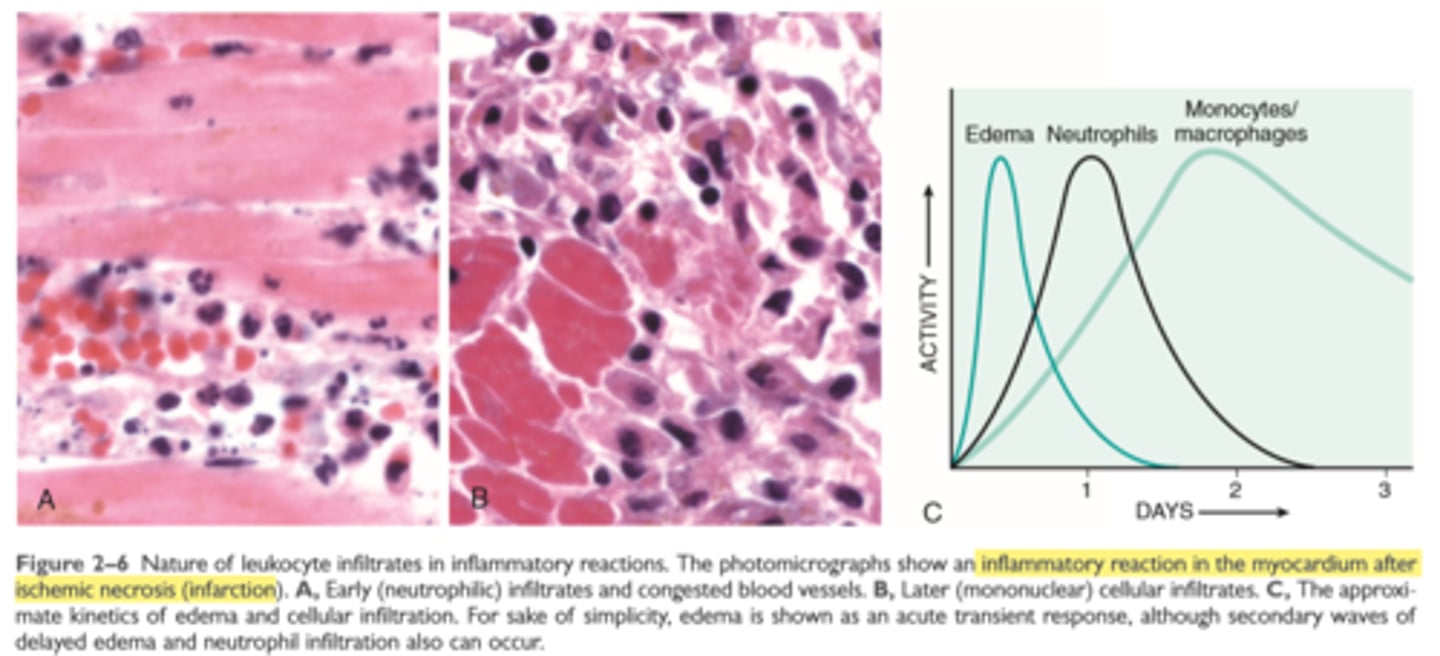
Neutrophils vs monocytes
Leukocyte recruitment to sites of inflammation
Leukocytes are recruited from the blood to the extravascular tissue where infectious pathogens or damaged tissues may be located and are activated to perform their functions
Leukocyte recruitment is a multistep process consisting of loose attachment to and rolling on endothelium (mediated by selectins) firm attachment to endothelium (mediated by integrins) and migration to interendothelial spaces
Various cytokines promote expression of selection of integrin ligands on endothelium (TNF, IL1); increase the avidity of integrins for their ligands (chemokines); and promote directional migration of leukocytes (also chemokines); many of these cytokines are produced by tissue macrophages and other cells responding to pathogens or damaged tissues
Neutrophils predominate in the early infiltrate and are later replaced by macrophages
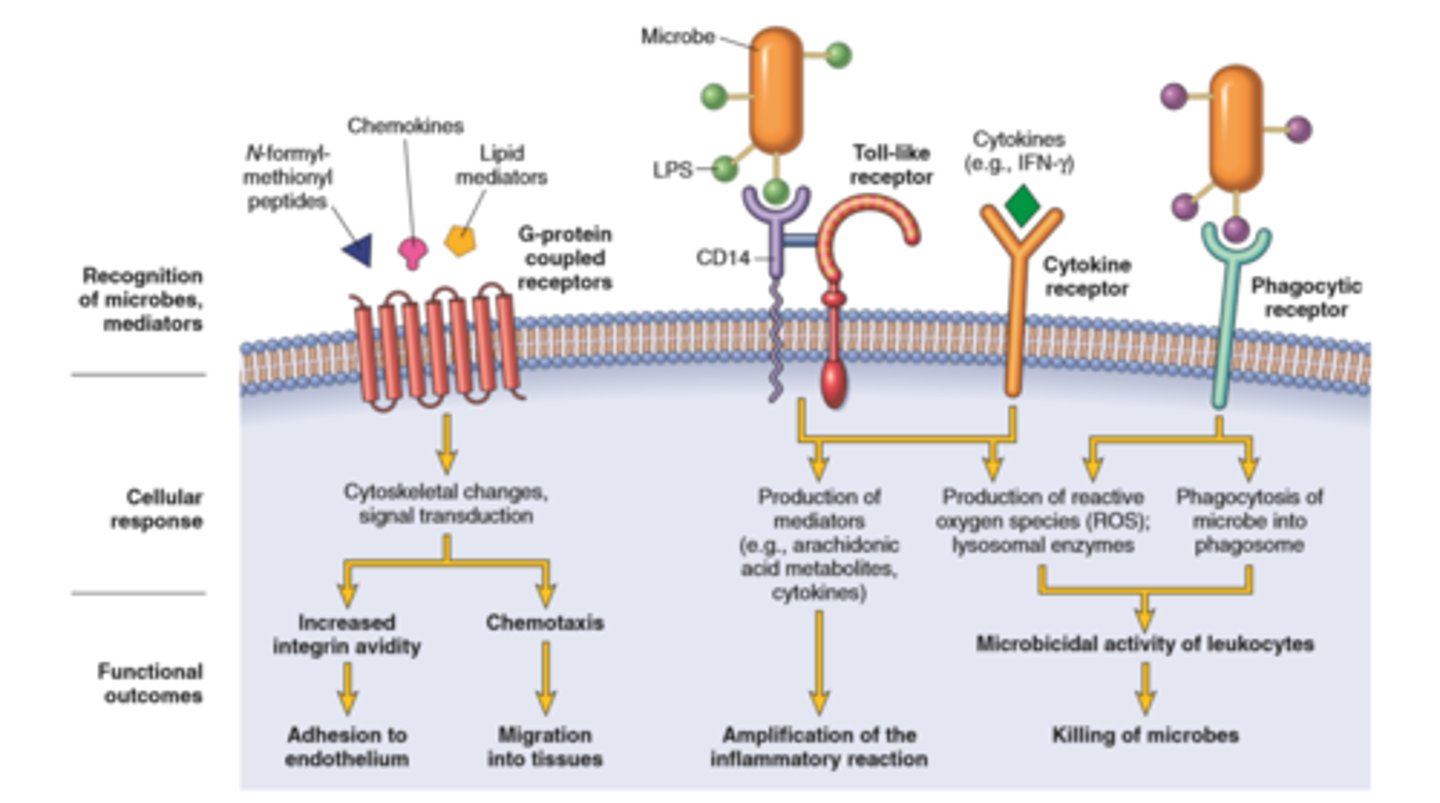
Leukocyte activation
Phagocytosis
Leukocyte effector mechanisms
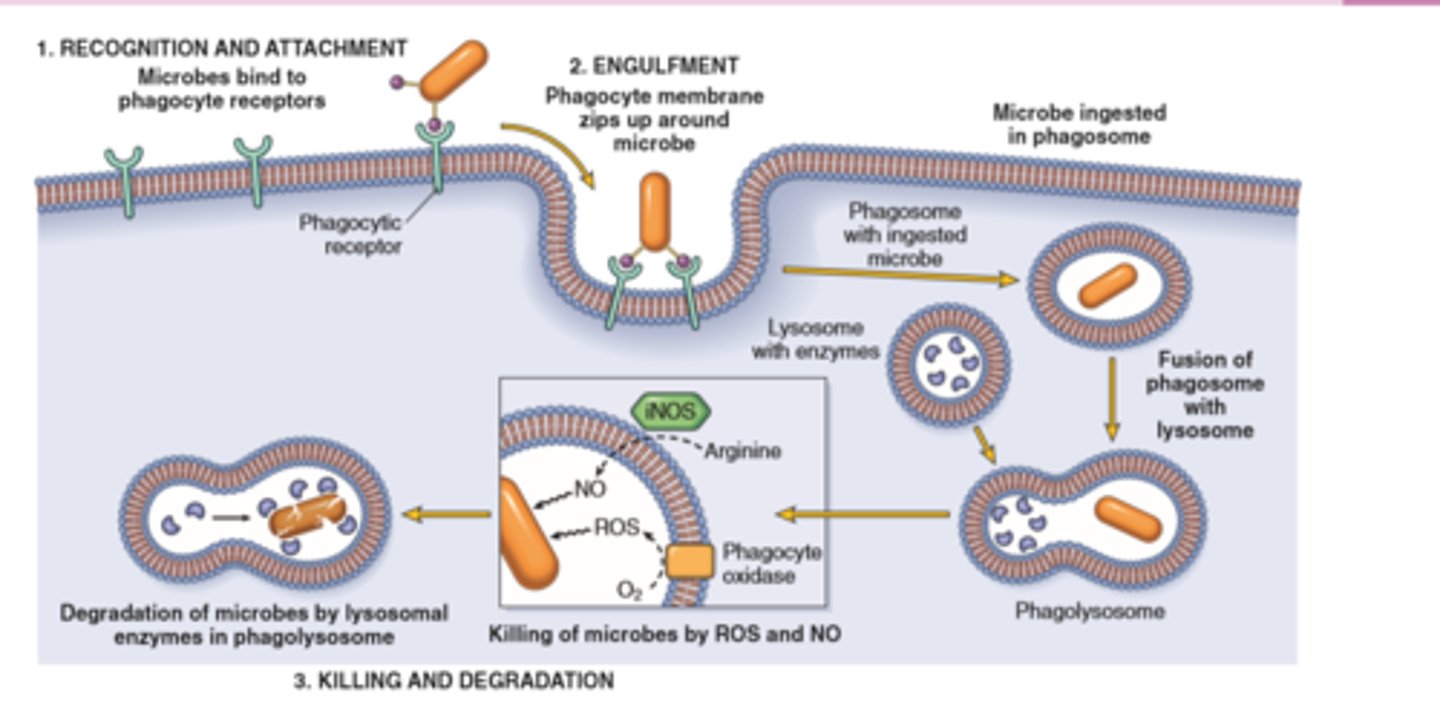
Leukocytes can eliminate microbes and dead cells by phagocytosis, followed by their destruction in phagolysosomes
Destruction is caused by free radicals (ROS, NO) generated in activated leukocytes and lysosomal enzymes
Enzymes and ROS may be released into the extracellular environment.
The mechanisms that function to eliminate microbes and dead cells (the physiologic role of inflammation) are also capable of damaging normal tissues (the pathologic consequences of inflammation).
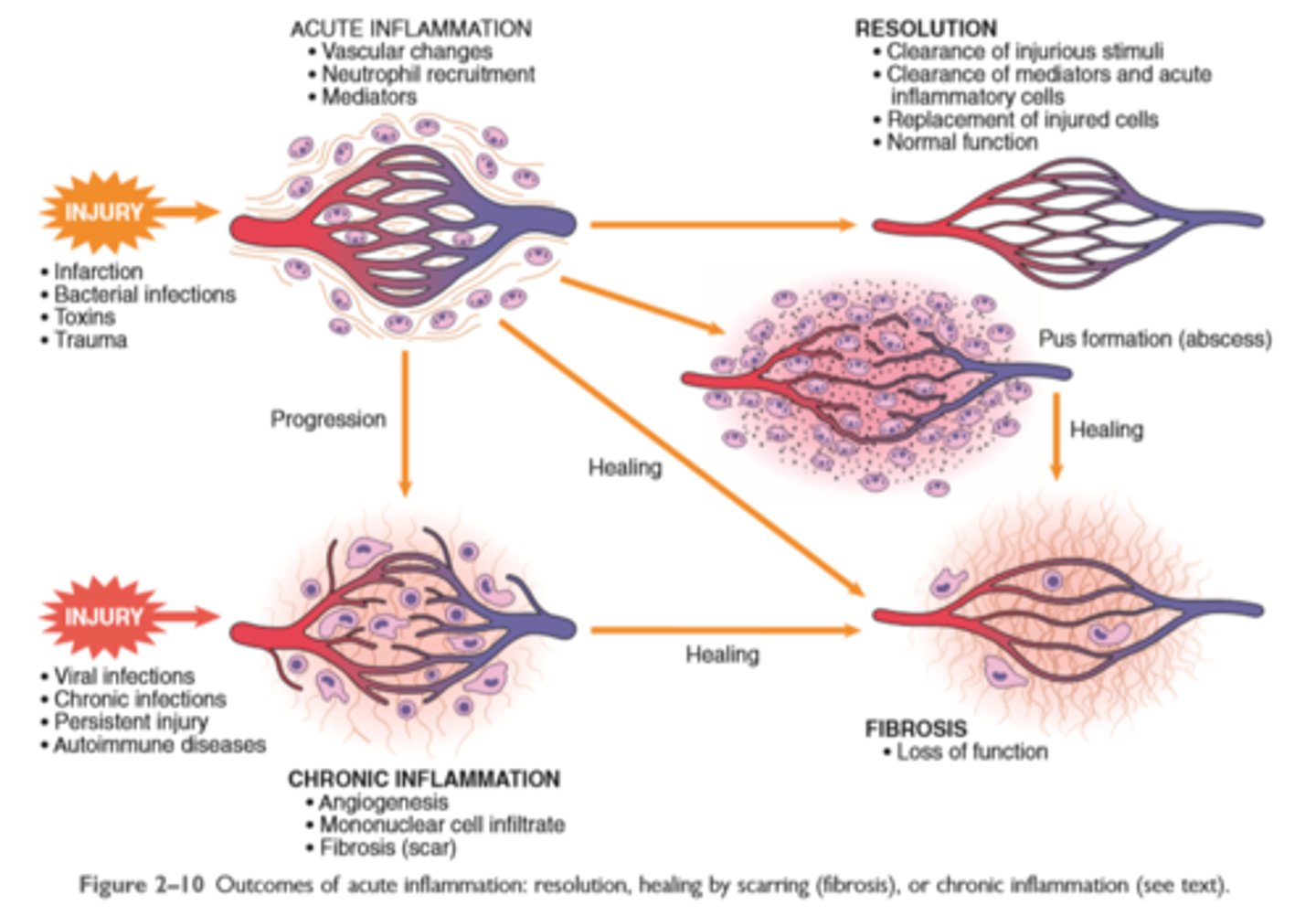
Outcomes of acute inflammation
Sequence of events in acute inflammation
The vascular changes in acute inflammation are characterized by increased blood flow secondary to arteriolar and capillary bed dilation (erythema and warmth)
Increased vascular permeability, as a consequence of either widening of interendothelial cell junctions of the venules or direct endothelial cell injury, results in an exudate of protein-rich extravascular fluid (tissue edema)
The leukocytes, initially predominantly neutrophils, adhere to the endothelium via adhesion molecules and then leave the microvasculature and migrate to the site of injury under the influence of chemotactic agents
Phagocytosis, killing, and degradation of the offending agent follow. • Genetic or acquired defects in leukocyte functions give rise to recurrent infections.
The outcome of acute inflammation may be removal of the exudate with restoration of normal tissue architecture (resolution); transition to chronic inflammation; or extensive destruction of the tissue resulting in scarring
Serous inflammation
outpouring of a watery, relatively protein-poor fluid either from the plasma or from the secretions of mesothelial cells lining the peritoneal, pleural, and pericardial cavities
Fluid in a serous cavity is called an effusion
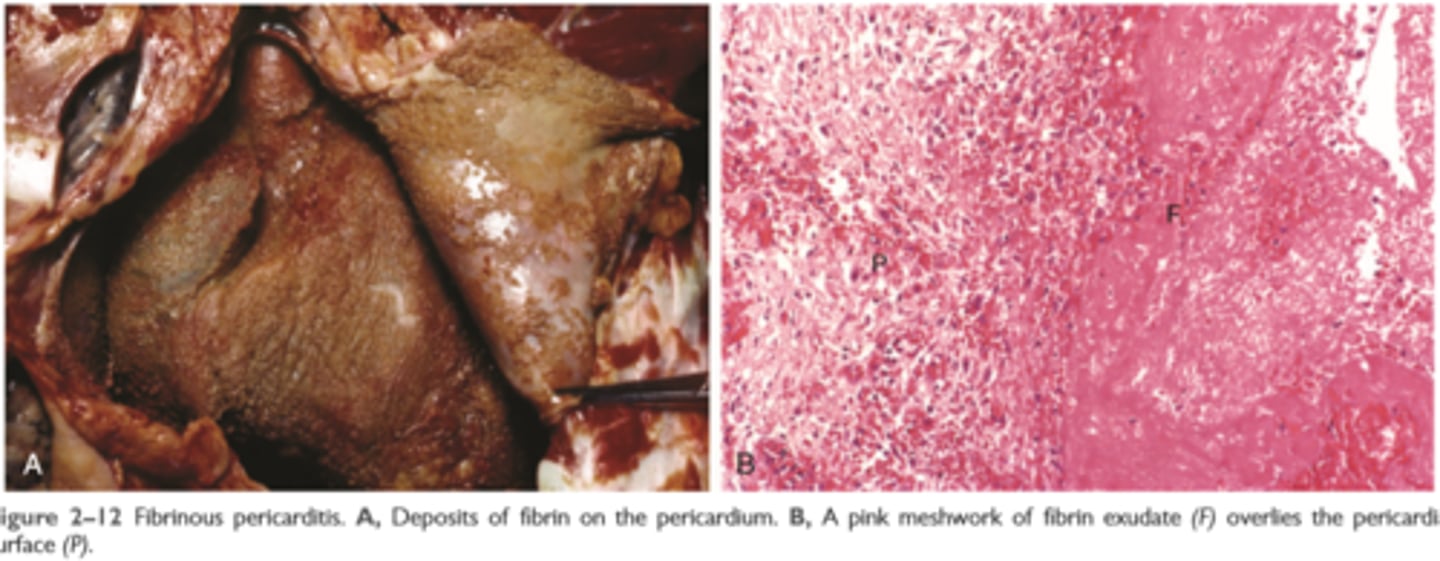
Fibrinous inflammation
occurs as a consequence of more severe injuries, resulting in greater vascular permeability that allows large molecules (such as fibrinogen) to pass the endothelial barrier
Histologically, the accumulated extravascular fibrin appears as an eosinophilic meshwork of threads or sometimes as an amorphous coagulum (Fig. 2-12)
A fibrinous exudate is characteristic of inflammation in the lining of body cavities, such as the meninges, pericardium, and pleura
Such exudates may be degraded by fibrinolysis, and the accumulated debris may be removed by macrophages, resulting in restoration of the normal tissue structure (resolution)
extensive fibrin-rich exudates may not be completely removed, and are replaced by an ingrowth of fibroblasts and blood vessels (organization), leading ultimately to scarring that may have significant clinical consequences
Suppurative (purulent) inflammation and abscess formation
These are manifested by the collection of large amounts of purulent exudate (pus) consisting of neutrophils, necrotic cells, and edema fluid
Certain organisms (e.g., staphylococci) are more likely to induce such localized suppuration and are therefore referred to as pyogenic (pus-forming)
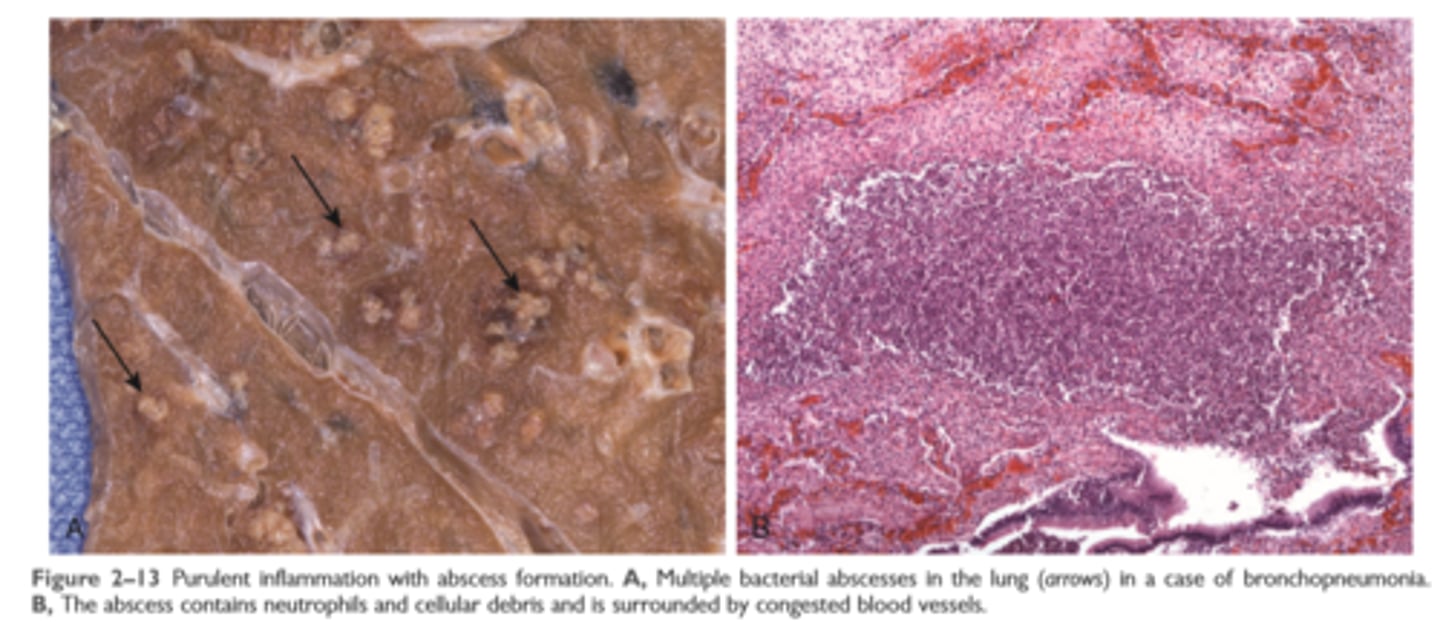
Abscesses
Abscesses are focal collections of pus that may be caused by seeding of pyogenic organisms into a tissue or by secondary infections of necrotic foci. Abscesses typically have a central, largely necrotic region rimmed by a layer of preserved neutrophils
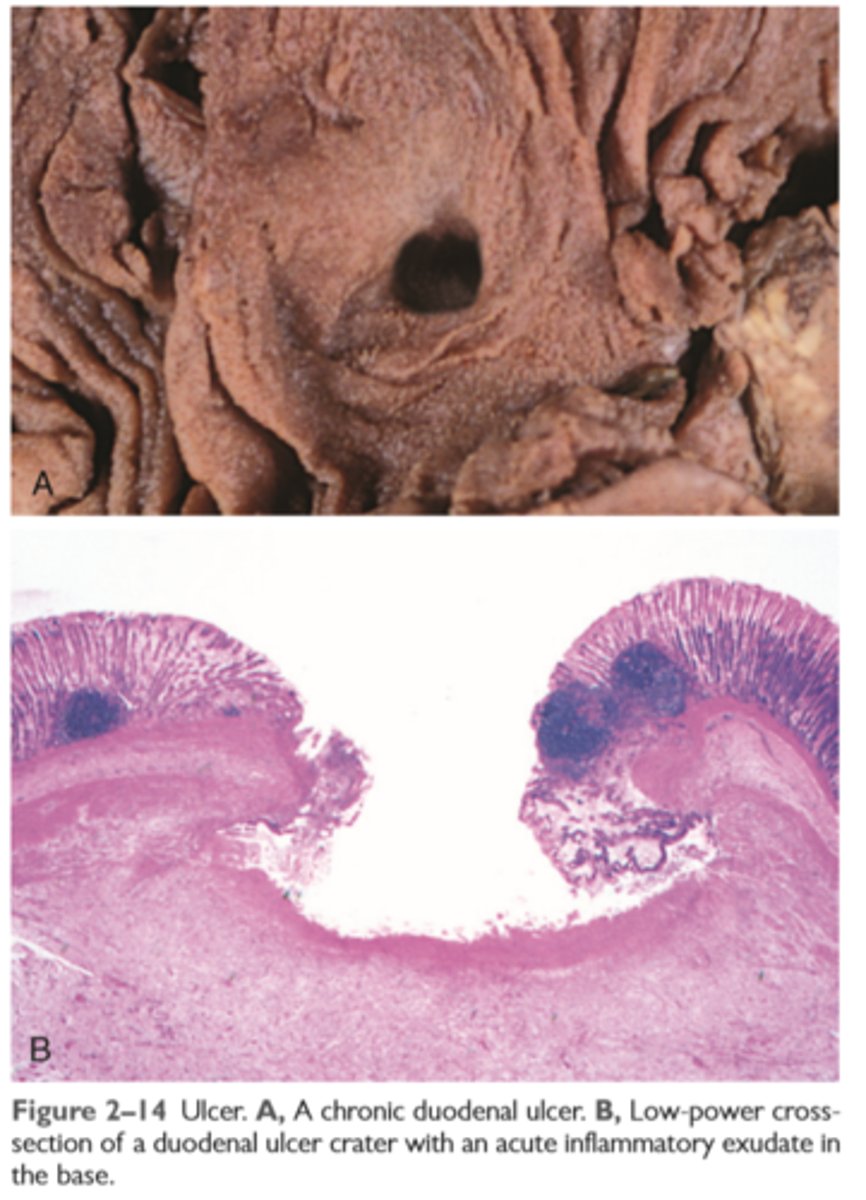
Ulcer
is a local defect, or excavation, of the surface of an organ or tissue that is produced by necrosis of cells and sloughing (shedding) of necrotic and inflammatory tissue
Ulceration can occur only when tissue necrosis and resultant inflammation exist on or near a
surface
Ulcers are most commonly encountered in (1) the mucosa of the mouth, stomach, intestines, or genitourinary tract and (2) in the subcutaneous tissues of the lower extremities in older persons who have circulatory disturbances predisposing affected tissue to extensive necrosis
Ulcerations are best exemplified by peptic ulcer of the stomach or duodenum, in which acute and chronic inflammation coexist. During the acute stage, there is intense polymorphonuclear infiltration and vascular dilation in the margins of the defect. With chronicity, the margins and base of the ulcer develop scarring with accumulation of lymphocytes, macrophages, and plasma cells
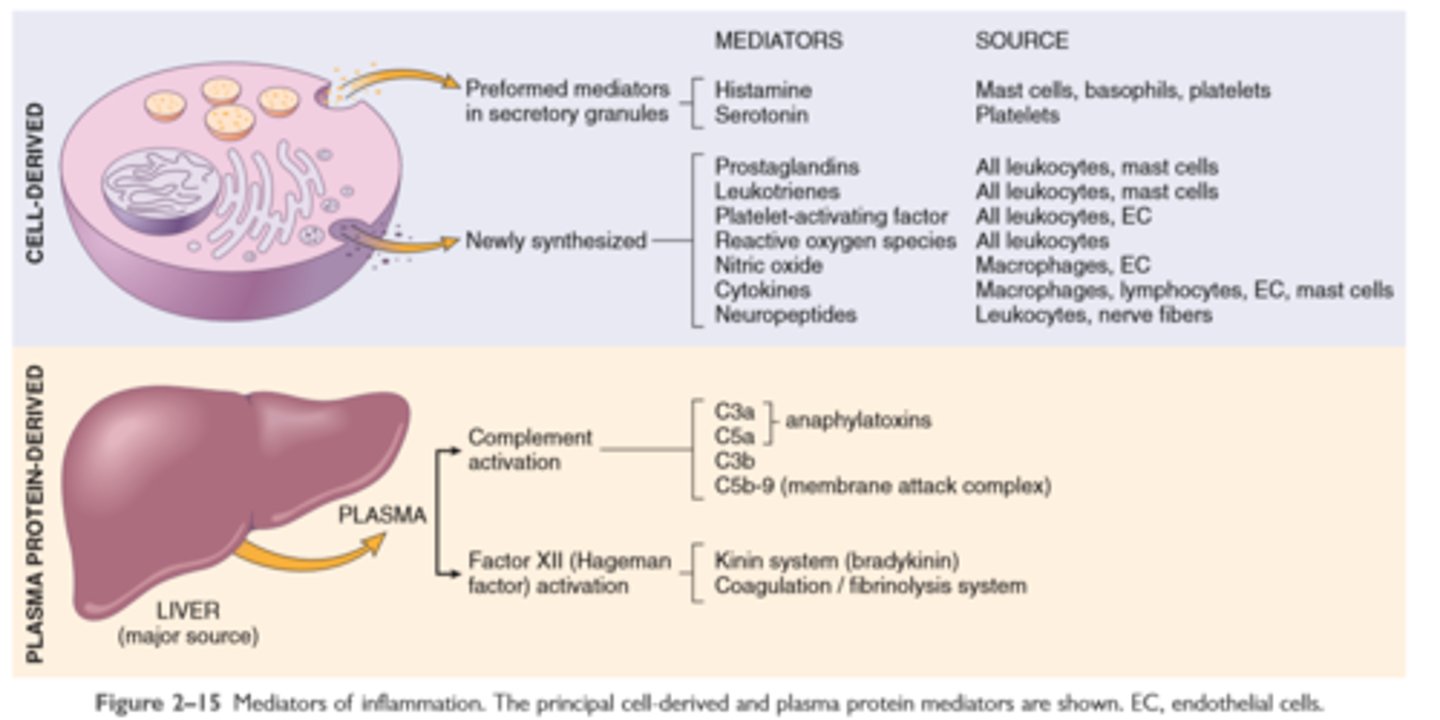
Mediators of Inflammation
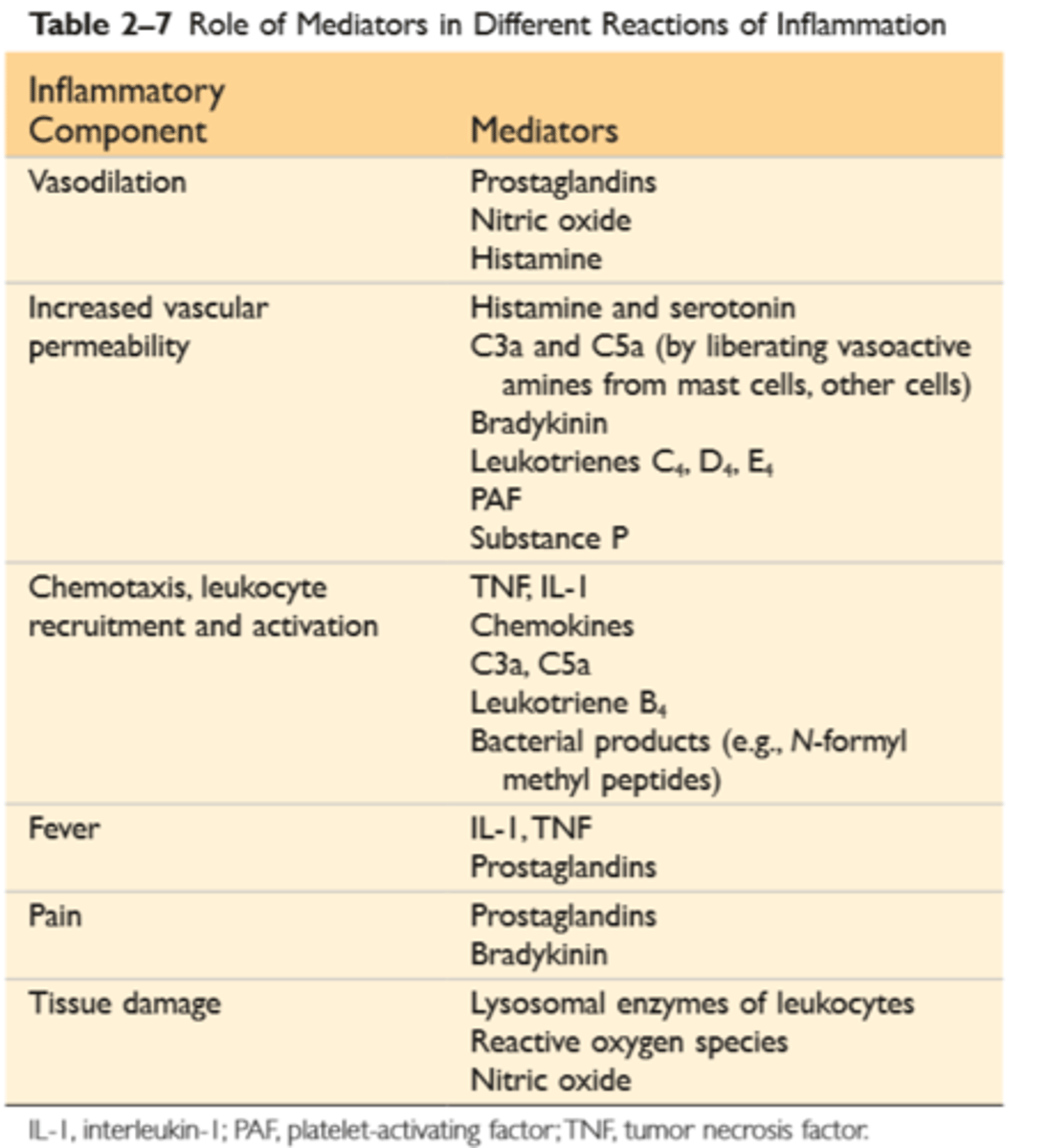
Actions of principle mediators of inflammation
Vasoactive amines
histamine and serotonin
Mast cell
a migrant cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte
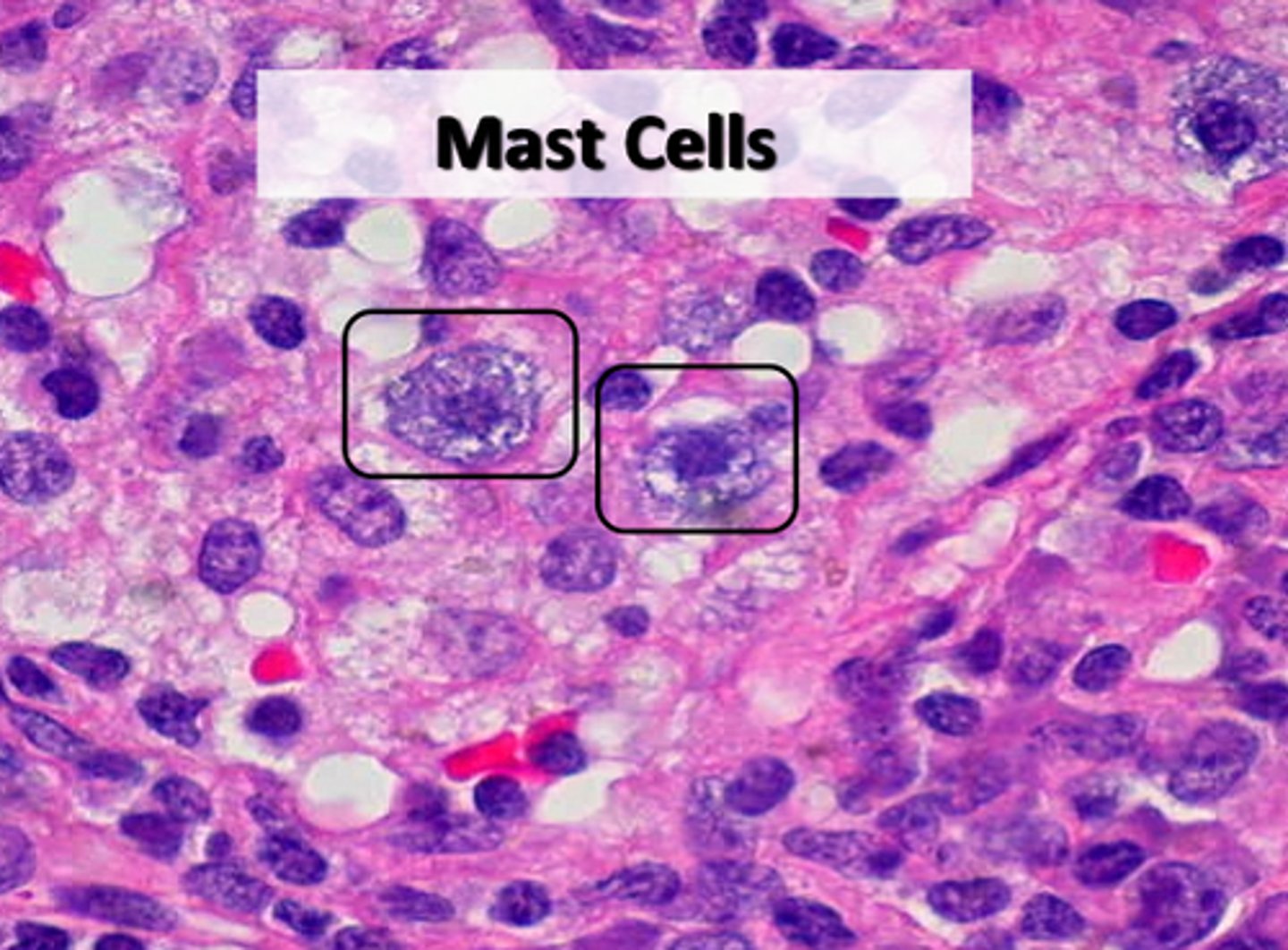
Histamine
causes arterial dilation which increases vascular permeability
Anti inflammatories block eicosanoid production
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin and ibuprofen, inhibit cyclooxygenase activity, thereby blocking all prostaglandin synthesis (hence their efficacy in treating pain and fever).
Eicosanoids
Prostaglandins, leukotrienes and thromboxane
Serotonin
vasoconstrictor
Production of arachidonic acid metabolites and their roles in inflammation
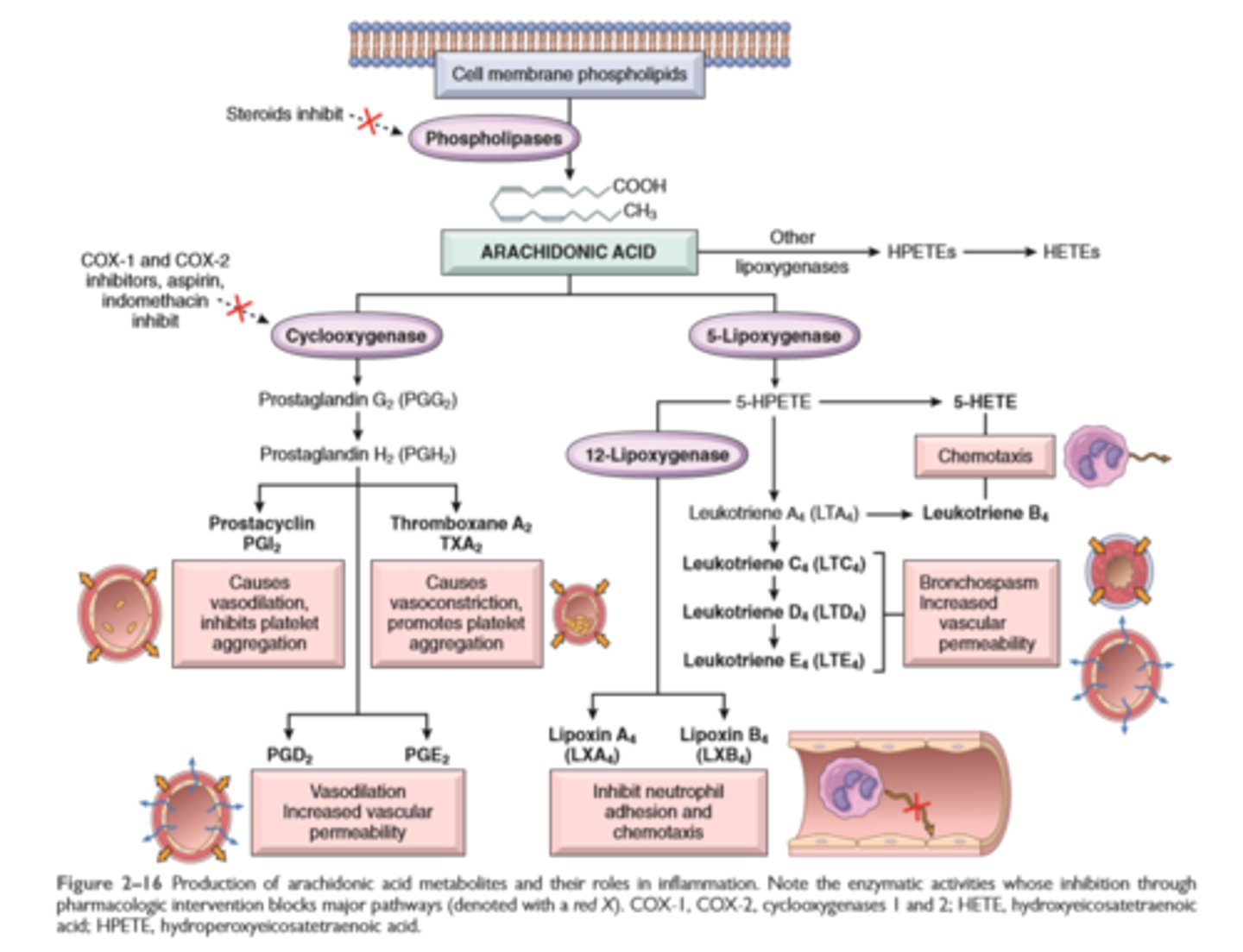
Glucocorticoids
powerful anti-inflammatory agents, act in part by inhibiting the activity of phospholipase A2 and thus the release of AA from membrane lipids
Roles of cytokines in inflammation
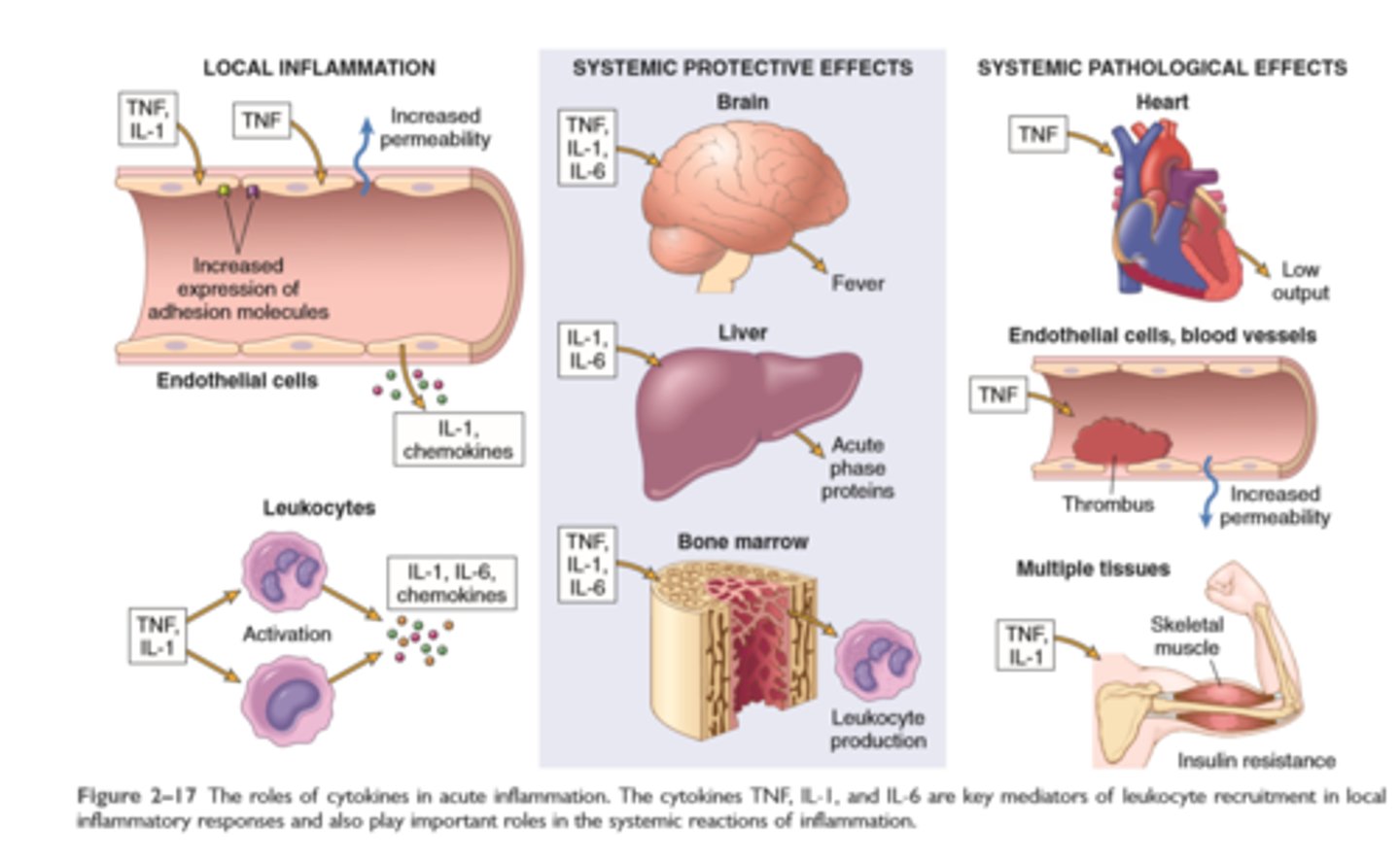
Major cell derived mediators of inflammation
Vasoactive amines—histamine, serotonin: Their main effects are vasodilation and increased vascular permeability
Arachidonic acid metabolites—prostaglandins and leukotrienes: Several forms exist and are involved in vascular reactions, leukocyte chemotaxis, and other reactions of inflammation; they are antagonized by lipoxins
Cytokines: These proteins, produced by many cell types, usually act at short range; they mediate multiple effects, mainly in leukocyte recruitment and migration; principal ones in acute inflammation are TNF, IL-1, IL-6, and chemokines
ROS: Roles include microbial killing and tissue injury
NO: Effects are vasodilation and microbial killing
Lysosomal enzymes: Roles include microbial killing and tissue injury.
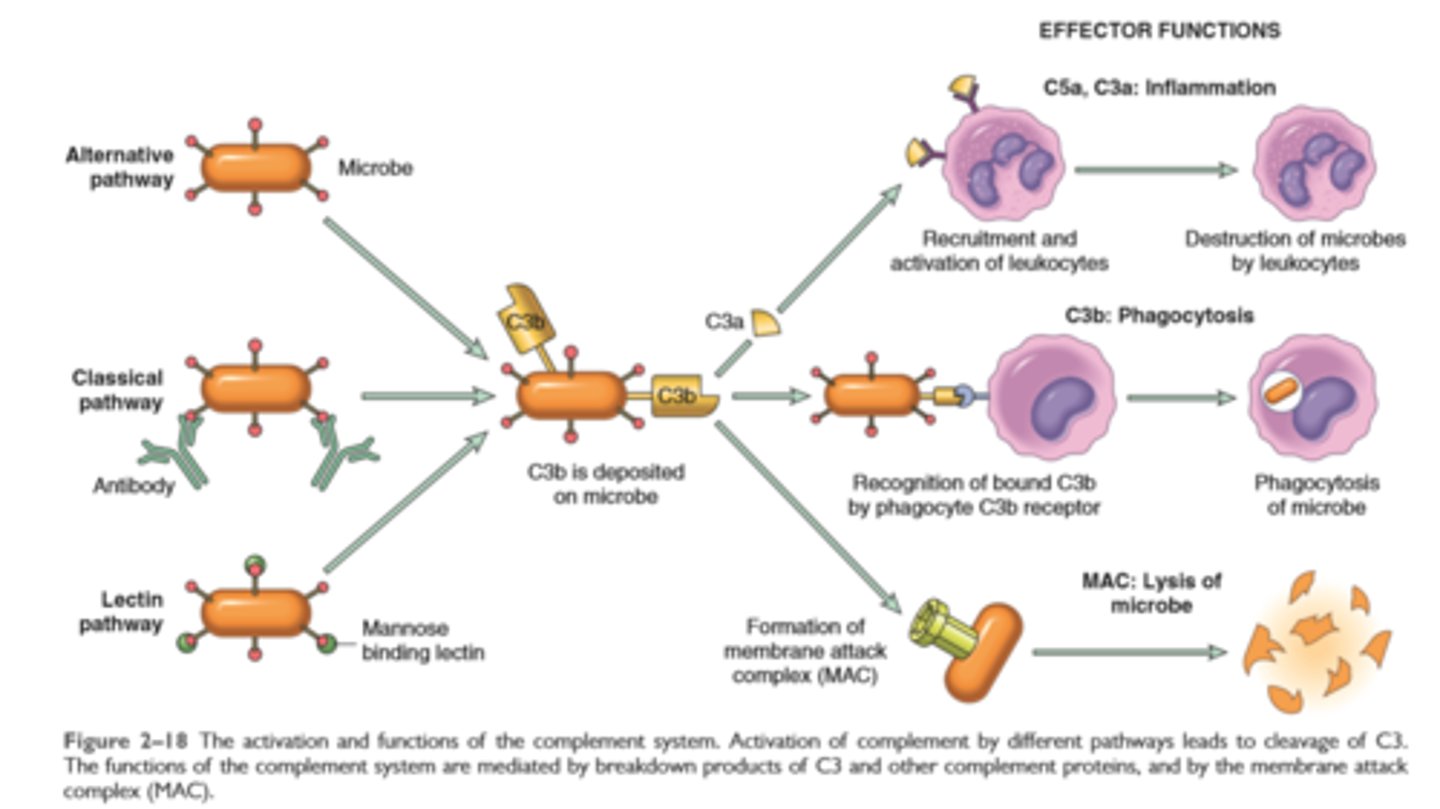
Activation and function of complement system
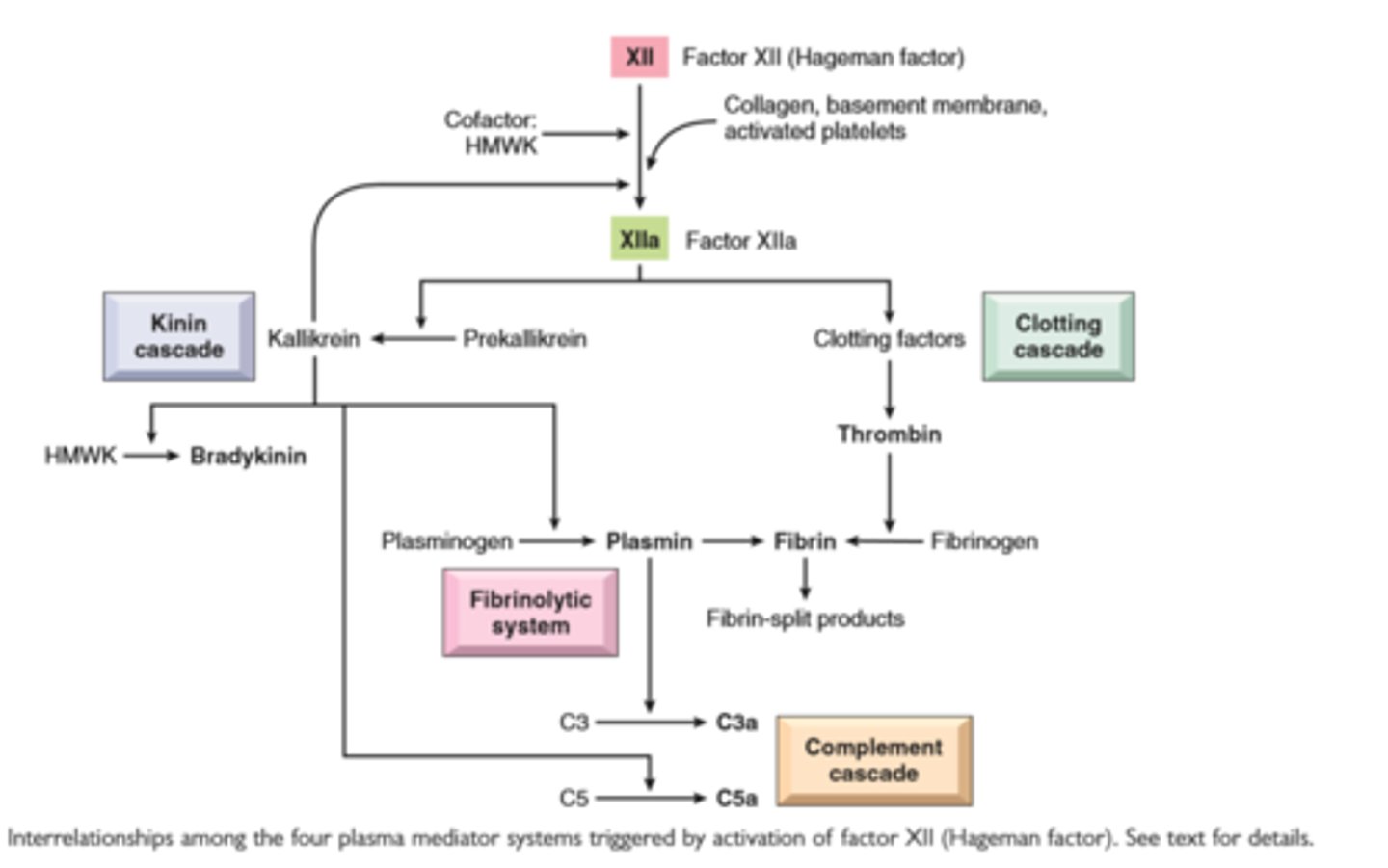
Interrelationships among the four plasma mediator systems triggered by activation of factor XII (Hageman factor)
Role of mediators in inflammatory reactions
Plasma protein mediators of inflammation
Complement proteins: Activation of the complement system by microbes or antibodies leads to the generation of multiple breakdown products, which are responsible for leukocyte chemotaxis, opsonization and phagocytosis of microbes and other particles, and cell killing
Coagulation proteins: Activated factor XII triggers the clotting, kinin, and complement cascades and activates the fibrinolytic system
Kinins: Produced by proteolytic cleavage of precursors, this group mediates vascular reaction and pain
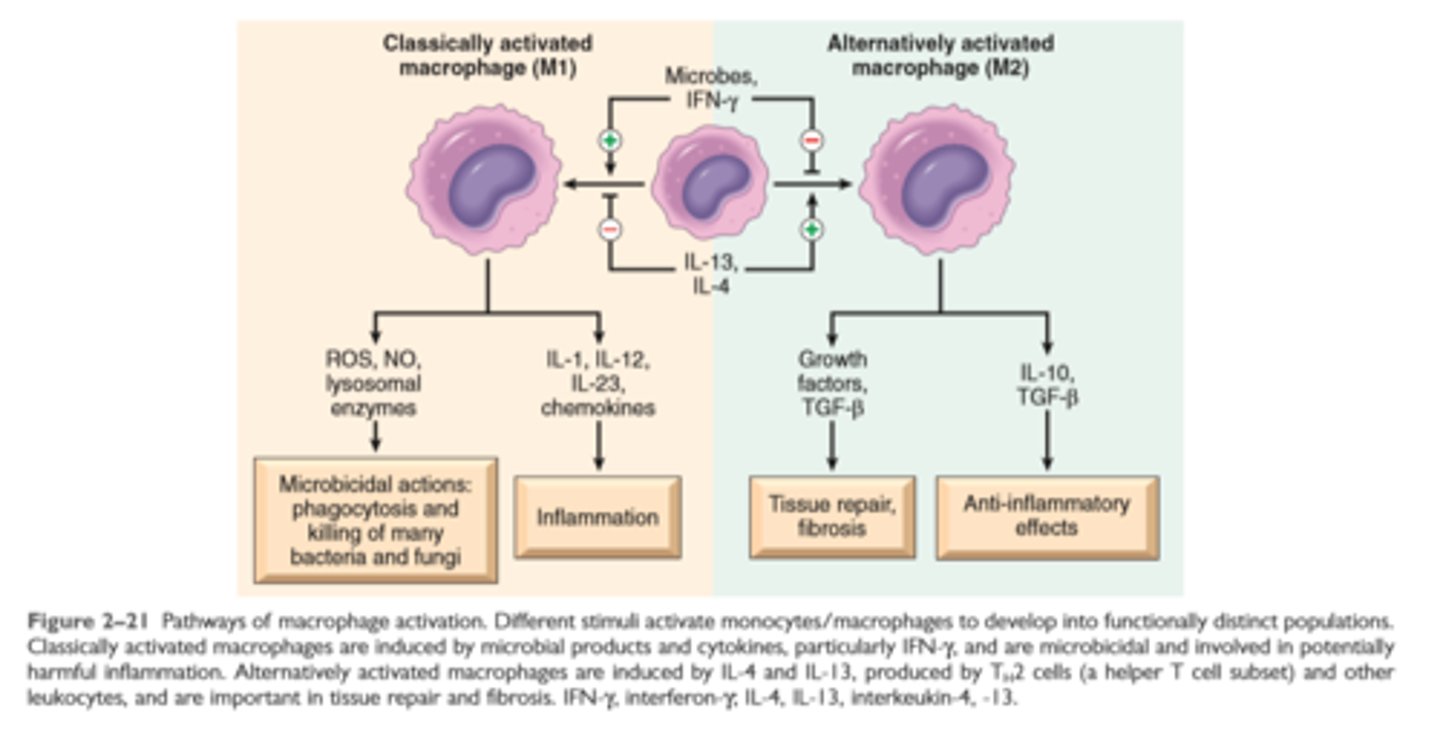
Pathways of macrophage activation
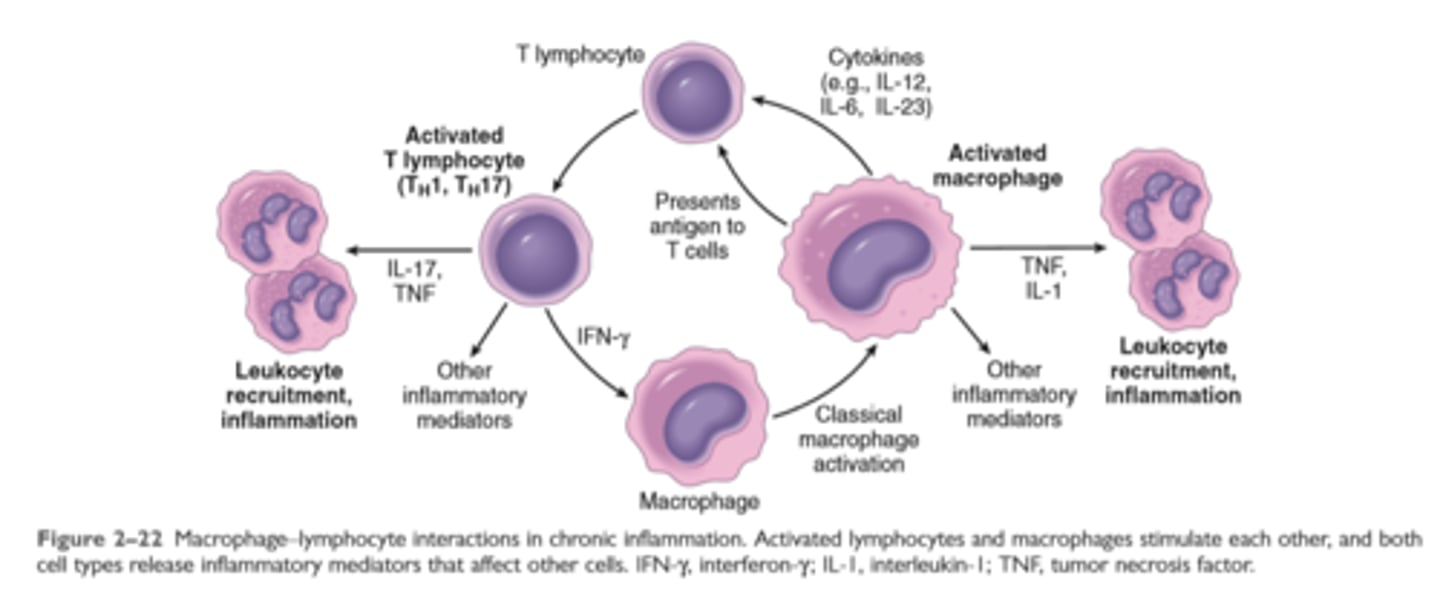
Macrophage Lymphocyte interaction in chronic inflammation
A typical granuloma resulting from infection with Mycobacterium tuberculosis showing central area of caseous necrosis, activated epithelioid macrophages, giant cells, and a peripheral accumulation of lymphocytes.
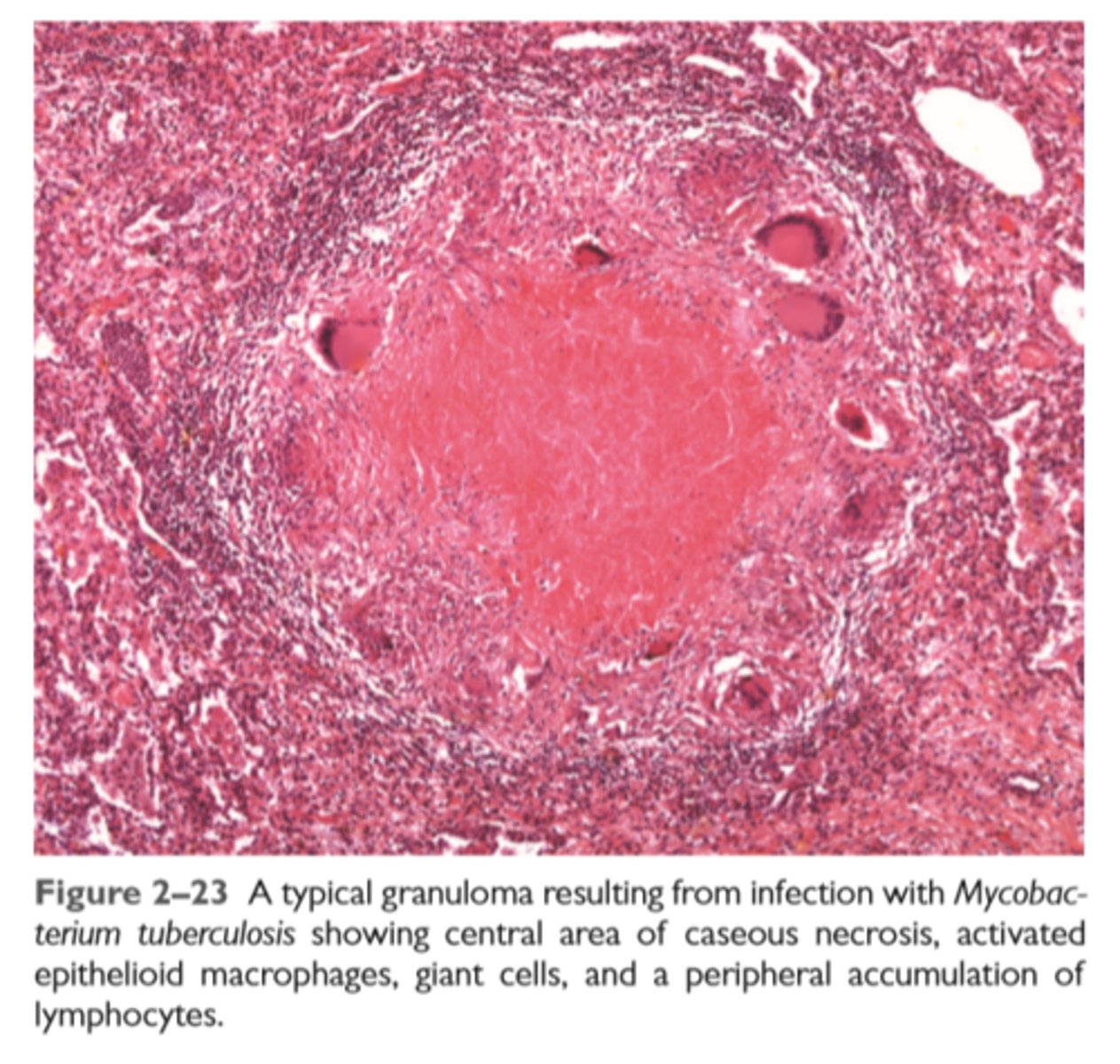
Features of chronic inflammation
Prolonged host response to persistent stimulus
Caused by microbes that resist elimination, immune responses against self and environmental antigens, and some toxic substances (e.g., silica); underlies many important diseases
Characterized by persistent inflammation, tissue injury, attempted repair by scarring, and immune response
Cellular infiltrate consisting of activated macrophages, lymphocytes, and plasma cells, often with prominent fibrosis
Mediated by cytokines produced by macrophages and lymphocytes (notably T lymphocytes), with a tendency to an amplified and prolonged inflammatory response owing to bidirectional interactions between these cells
Systemic effects of inflammation
Fever: cytokines (TNF, IL-1) stimulate production of prostaglandins in hypothalamus
Production of acute-phase proteins: C-reactive protein, others; synthesis stimulated by cytokines (IL-6, others) acting on liver cells
Leukocytosis: cytokines (CSFs) stimulate production of leukocytes from precursors in the bone marrow
In some severe infections, septic shock: fall in blood pressure, disseminated intravascular coagulation, metabolic abnormalities; induced by high levels of TNF
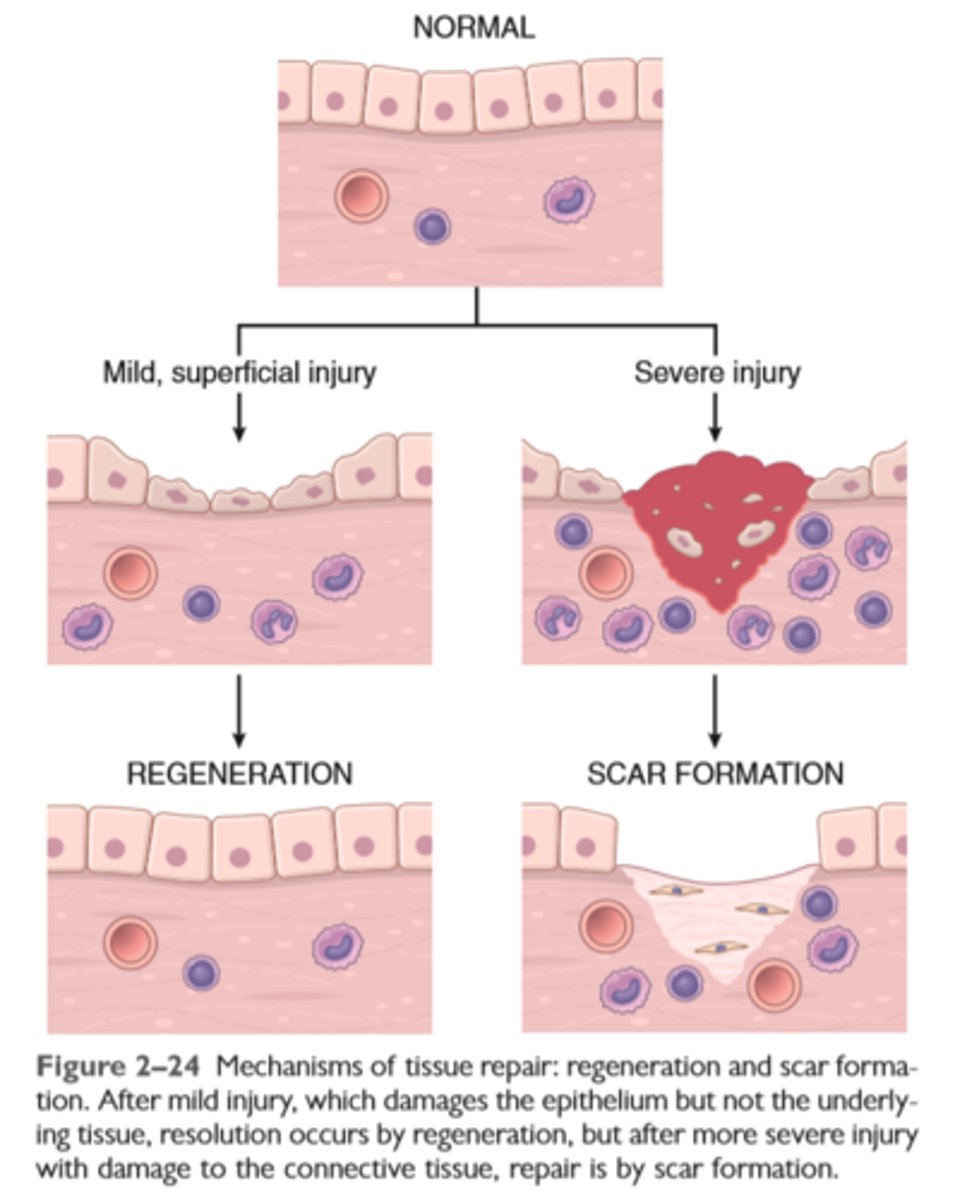
Mechanisms of tissue repair
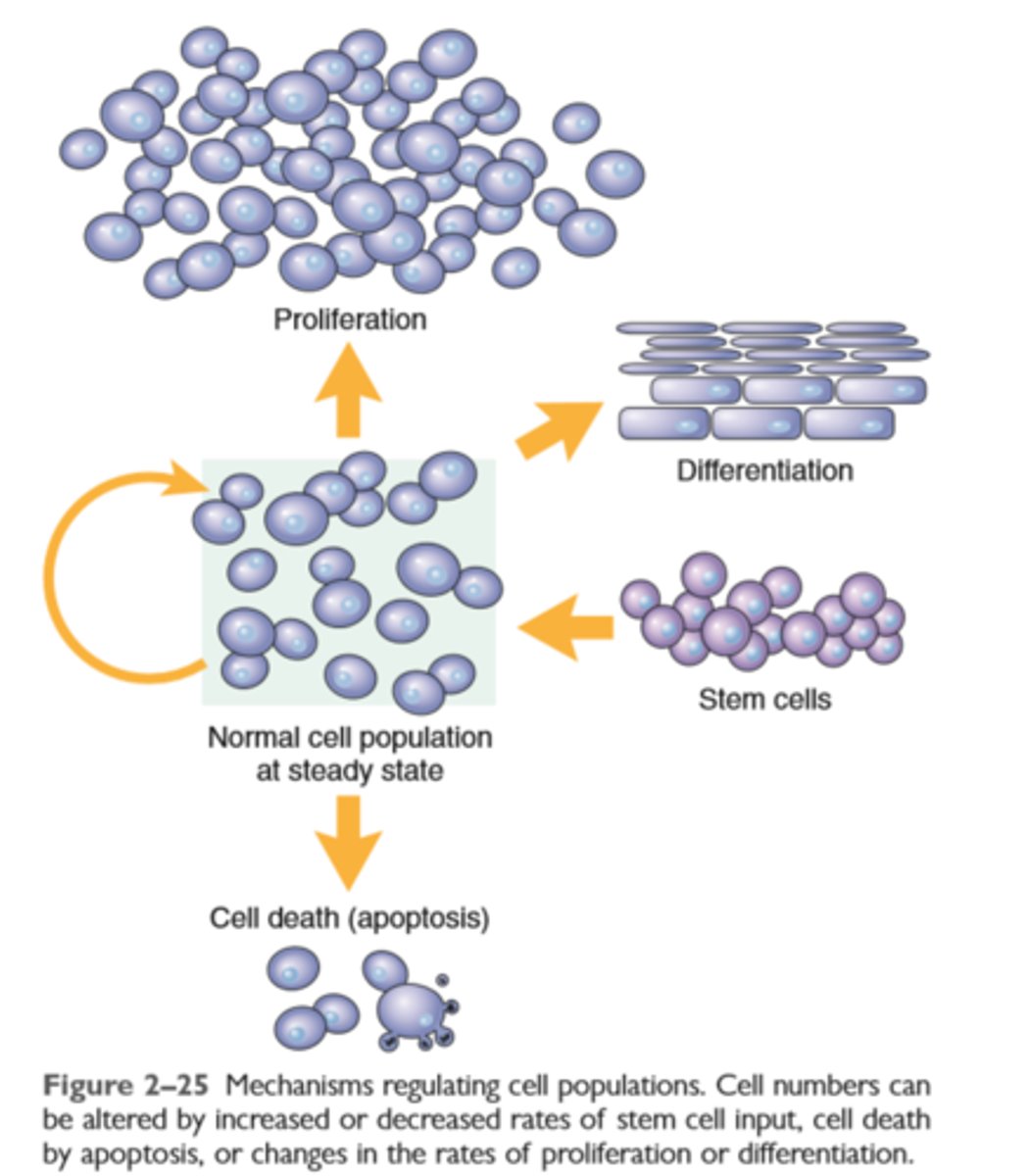
Mechanisms of regulating cell populations
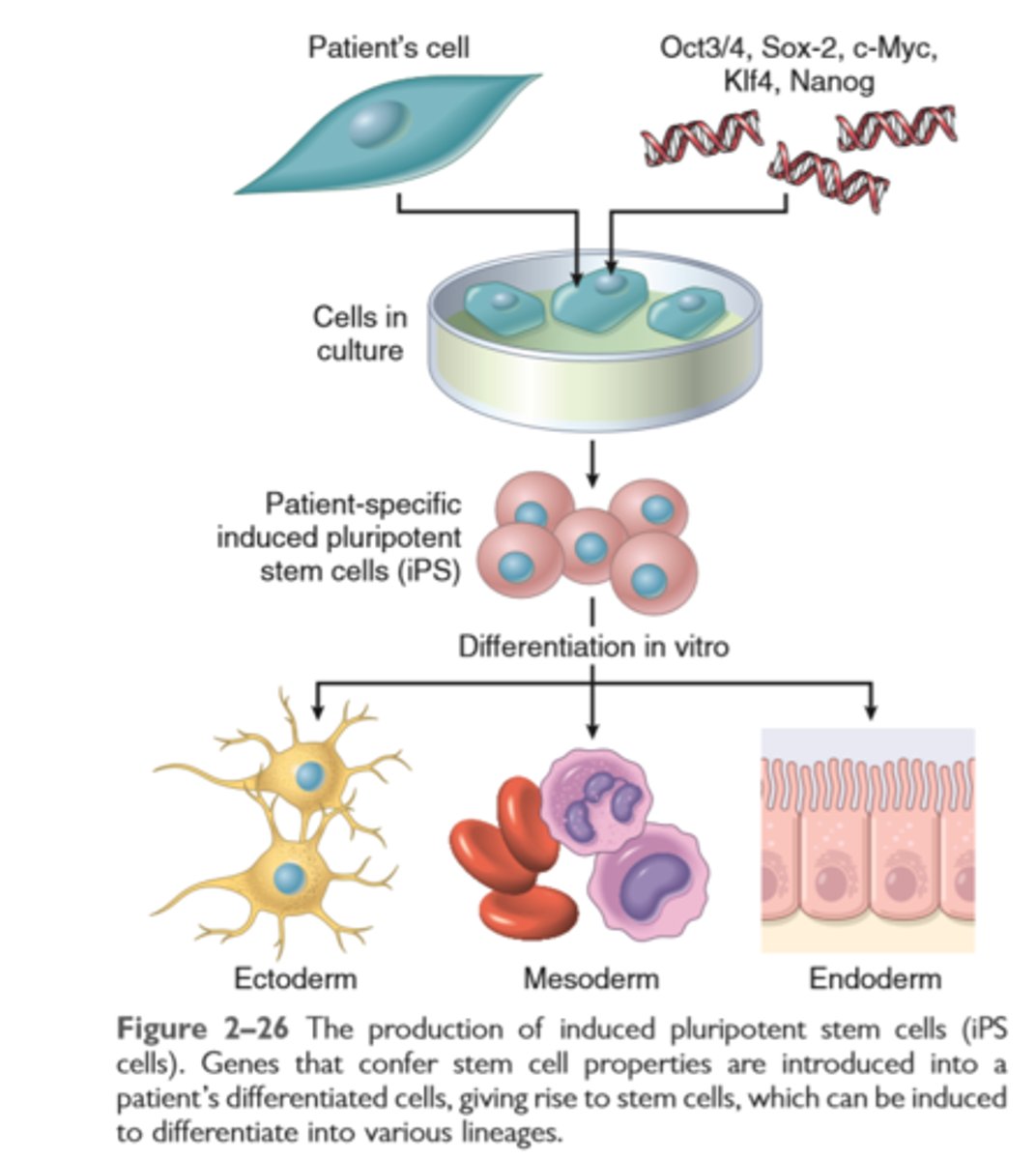
Production of induced pluripotent stem cells
Cell proliferation, the cell cycle and stem cells
Regeneration of tissues is driven by proliferation of uninjured (residual) cells and replacement from stem cells
Cell proliferation occurs when quiescent cells enter the cell cycle. The cell cycle is tightly regulated by stimulators and inhibitors and contains intrinsic checkpoint controls to prevent replication of abnormal cells
Tissues are divided into labile, stable, and permanent, according to the proliferative capacity of their cells
Continuously dividing tissues (labile tissues) contain mature cells that are capable of dividing and stem cells that differentiate to replenish lost cells
Stem cells from embryos (ES cells) are pluripotent; adult tissues, particularly the bone marrow, contain adult stem cells capable of generating multiple cell lineages
Induced pluripotent stem cells (iPS cells) are derived by introducing into mature cells genes that are characteristic of ES cells
iPS cells acquire many characteristics of stem cells.
Growth factors involved in regeneration and repair
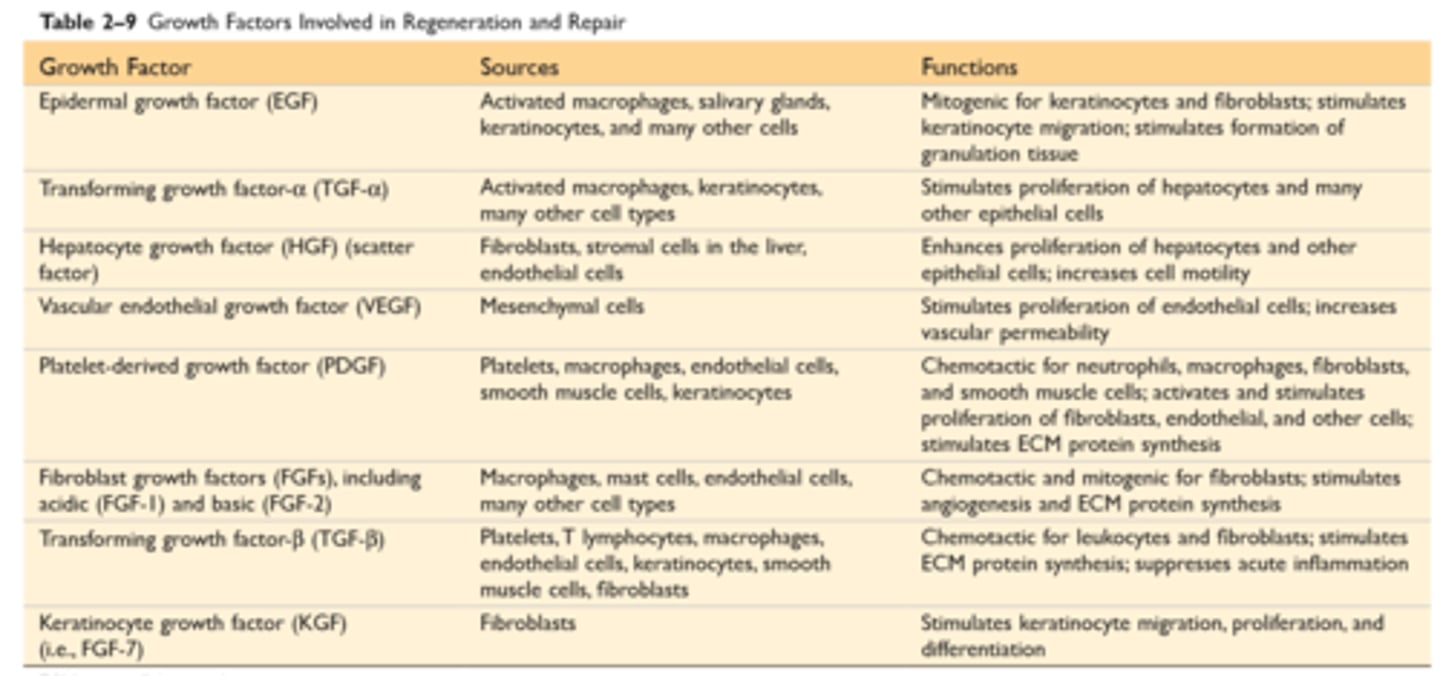
Growth factors, receptors and signal transduction
Polypeptide growth factors act in autocrine, paracrine, or endocrine manner
Growth factors are produced transiently in response to an external stimulus and act by binding to cellular receptors. Different classes of growth factor receptors include receptors with intrinsic kinase activity, G protein-coupled receptors and receptors without intrinsic kinase activity
Growth factors such as epidermal growth factor (EGF) and hepatocyte growth factor (HGF) bind to receptors with intrinsic kinase activity, triggering a cascade of phosphorylating events through MAP kinases, which culminate in transcription factor activation and DNA replication
G protein-coupled receptors produce multiple effects via the cAMP and Ca2+ pathways
Chemokines utilize such receptors
Cytokines generally bind to receptors without kinase activity; such receptors interact with cytoplasmic transcription factors that move into the nucleus
Most growth factors have multiple effects, such as cell migration, differentiation, stimulation of angiogenesis, and fibrogenesis, in addition to cell proliferation
Principal signalling pathways used by cell surface receptors
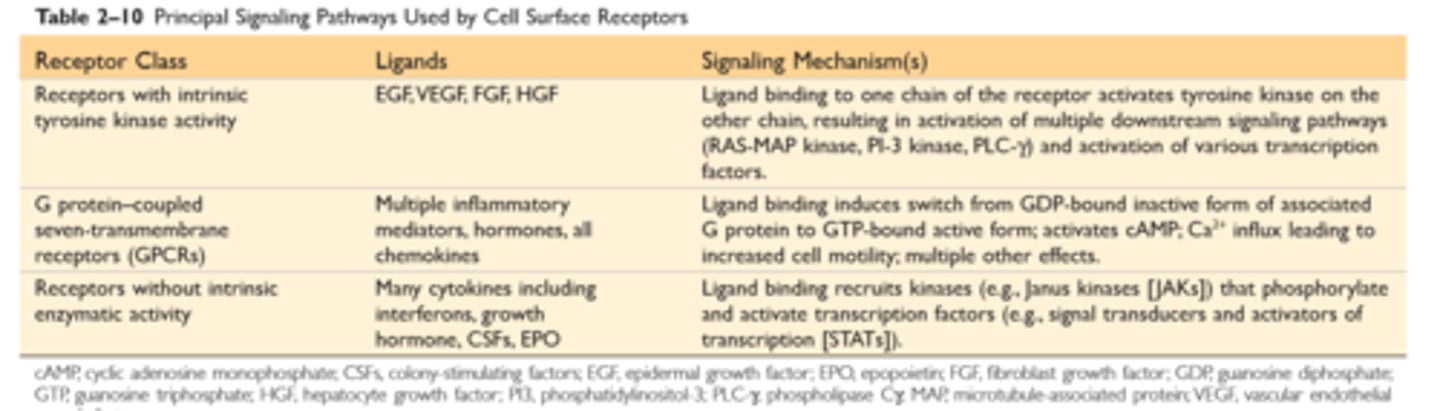
The major components of ECM
ECM and tissue repair
The ECM consists of the interstitial matrix between cells, made up of collagens and several glycoproteins, and basement membranes underlying epithelia and surrounding vessels, made up of nonfibrillar collagen and laminin
The ECM serves several important functions: It provides mechanical support to tissues; this is the role of collagens and elastin. It acts as a substrate for cell growth and the formation of tissue microenvironments. It regulates cell proliferation and differentiation; proteoglycans bind growth factors and display them at high concentration, and fibronectin and laminin stimulate cells through cellular integrin receptors
An intact ECM is required for tissue regeneration, and if the ECM is damaged, repair can be accomplished only by scar formation
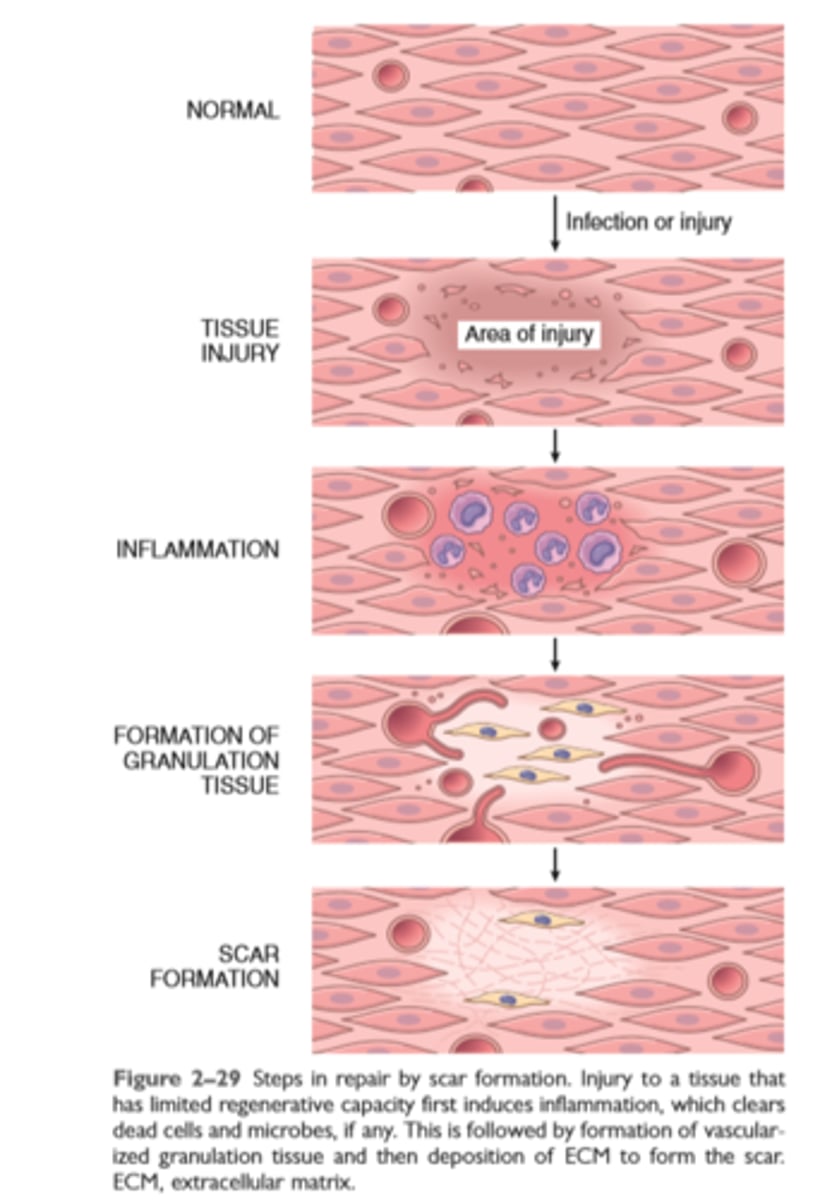
Steps in repair by scar formation
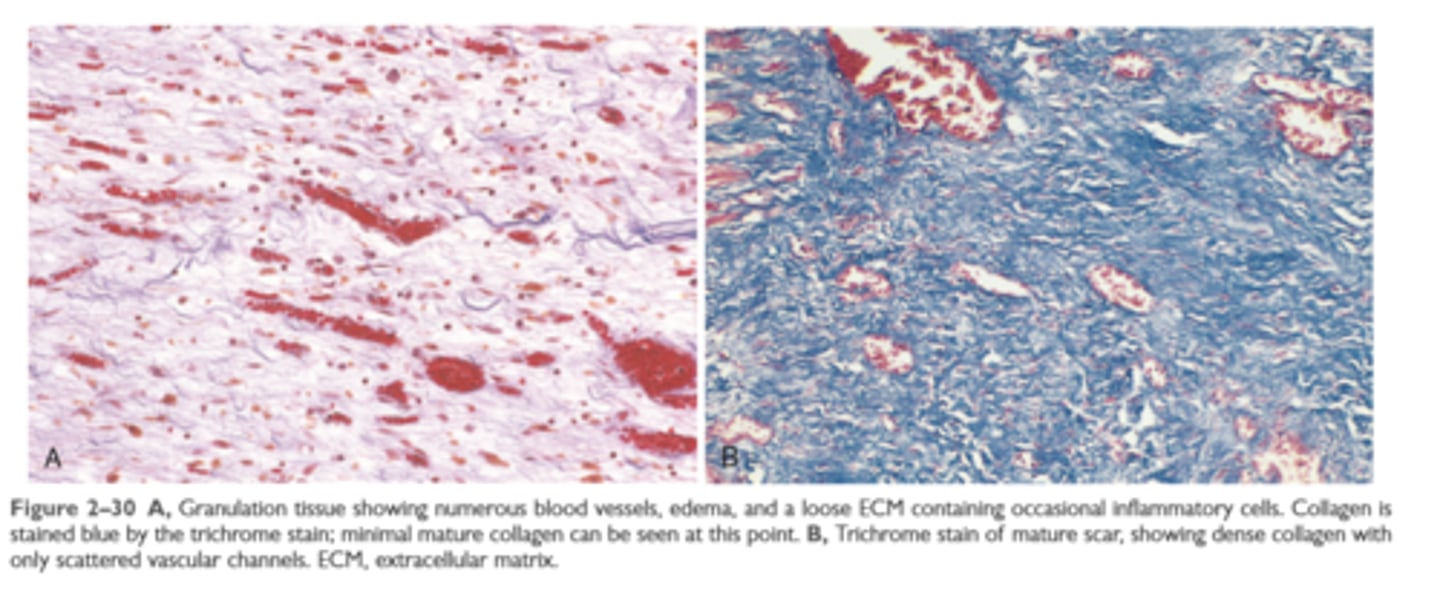
A: granulation tissue using trichrome stain
B: scar using trichrome stain
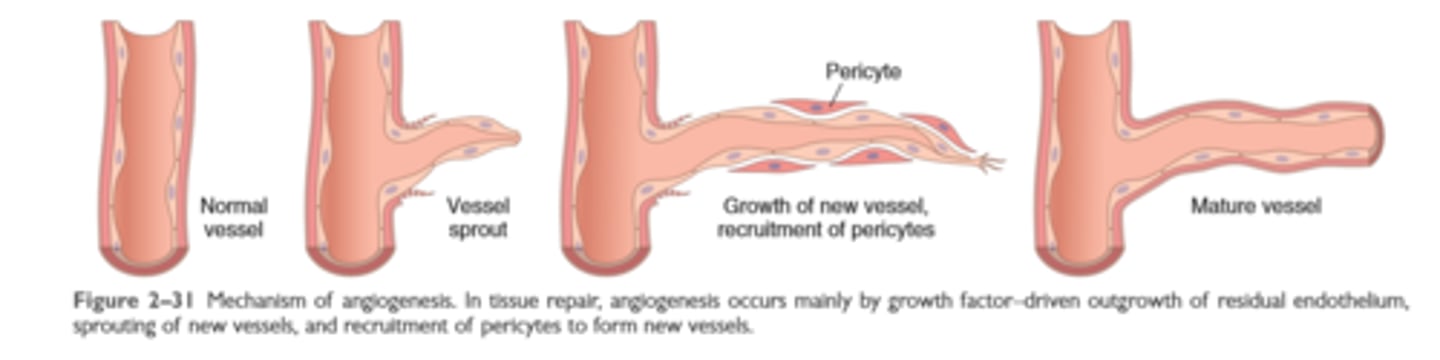
Mechanisms of angiogenesis
Repair by scar formation
Tissues can be repaired by regeneration with complete restoration of form and function, or by replacement with connective tissue and scar formation
Repair by connective tissue deposition involves angiogenesis, migration and proliferation of fibroblasts, collagen synthesis, and connective tissue remodeling
Repair by connective tissue starts with the formation of granulation tissue and culminates in the laying down of fibrous tissue.
Multiple growth factors stimulate the proliferation of the cell types involved in repair
TGF-β is a potent fibrogenic agent; ECM deposition depends on the balance among fibrogenic agents, the metalloproteinases (MMPs) that digest ECM, and the TIMPs
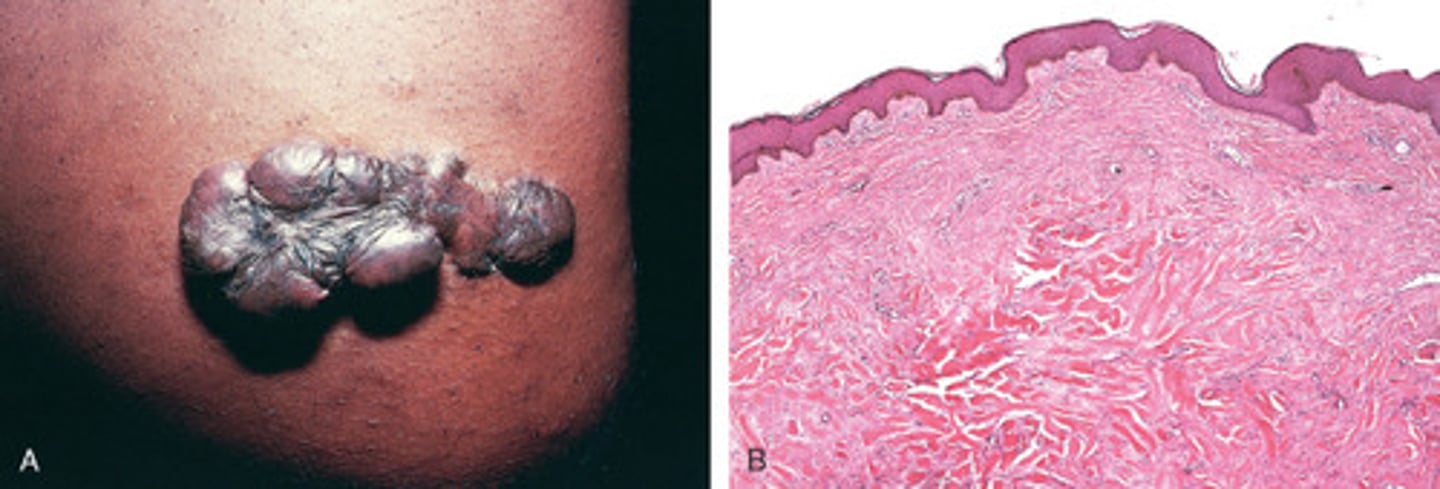
Keloid (excess collagen deposition)
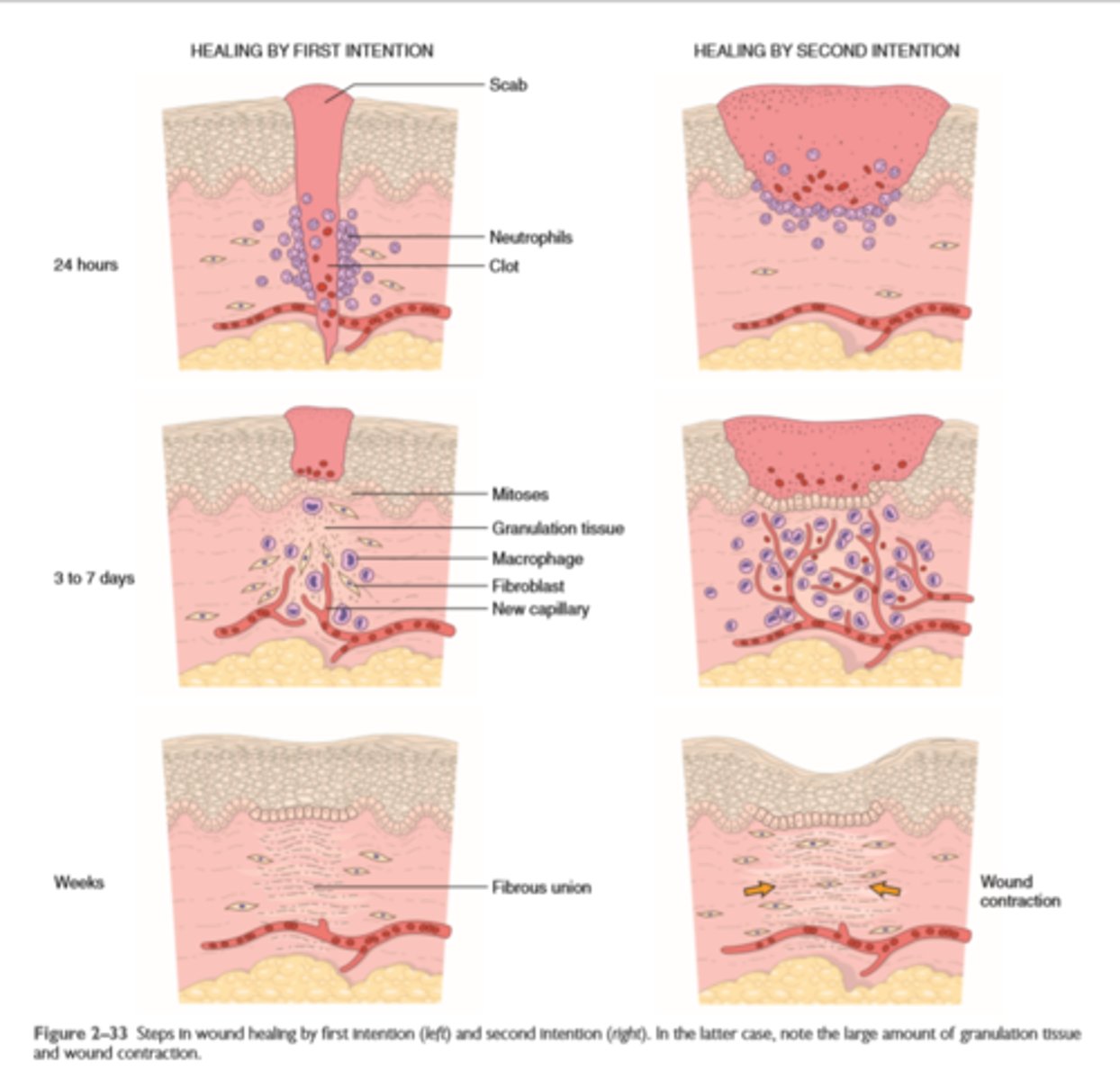
Wound healing by first and second intention
Cutaneous wound healing and pathologic aspects of repair
Cutaneous wounds can heal by primary union (first intention) or secondary union (second intention); secondary healing involves more extensive scarring and wound contraction
Wound healing can be altered by many conditions, particularly infection and diabetes; the type, volume, and location of the injury are also important factors in healing
Excessive production of ECM can cause keloids in the skin
Persistent stimulation of collagen synthesis in chronic inflammatory diseases leads to fibrosis of the tissue