ORGANIC CHEMISTRY EXAM 1 REVIEW
0.0(0)
Card Sorting
1/28
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
1
New cards
H-Cl
-7
2
New cards
CH3CO2-H
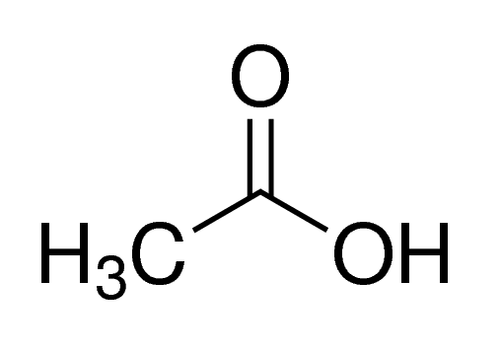
4.8 (4.76)
3
New cards
NH4+ (ammonium)
9.27
4
New cards
HO-H
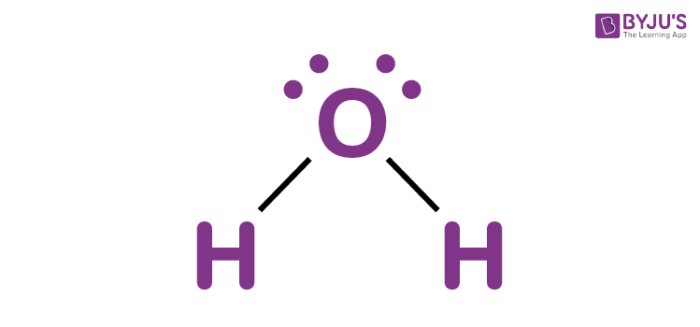
15.7 (14)
5
New cards
CH3CH2O-H
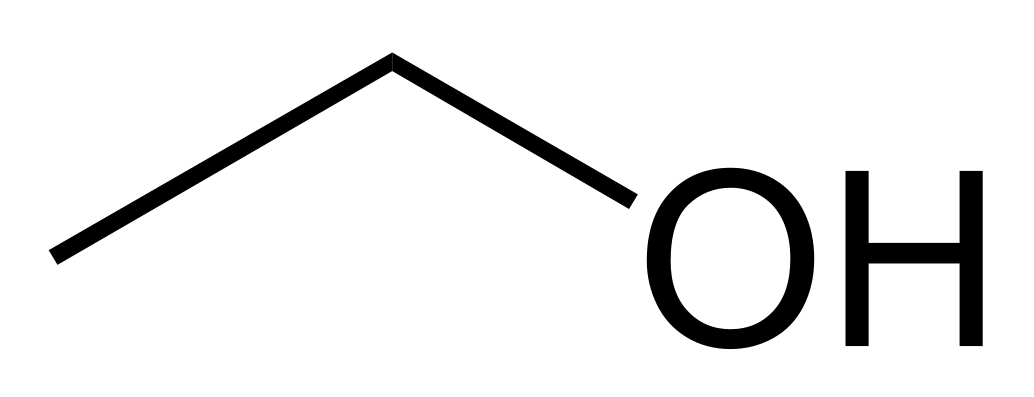
16
6
New cards
HC≡CH (≡—H)
25
7
New cards
H-H
35
8
New cards
H2N-H
38
9
New cards
CH2=CH2
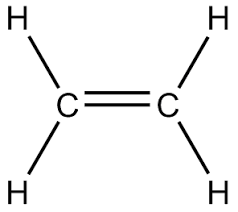
44
10
New cards
H3C-H
50
11
New cards
number of pi electrons formula
2 x (# of pi bonds + # of delocalized lone pairs)
12
New cards
Degree of Unsaturation formula (DoU)
2C + 2 - H + N - X / 2
13
New cards
What are the 3 rules to find significant resonance?
Greatest filled octet
Least amount of formal charges
A structure w a negative charge on the more EN element is more important
14
New cards
Alkene

15
New cards
Alkyne
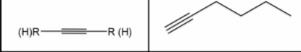
16
New cards
Aromatic compound/ring
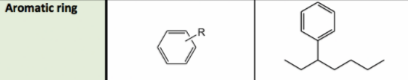
17
New cards
Alkyl Halide

18
New cards
Alcohol
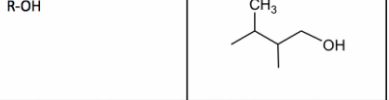
19
New cards
Ether
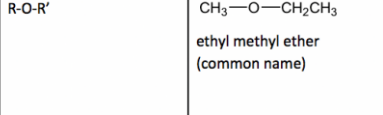
20
New cards
Amine

21
New cards
thiol
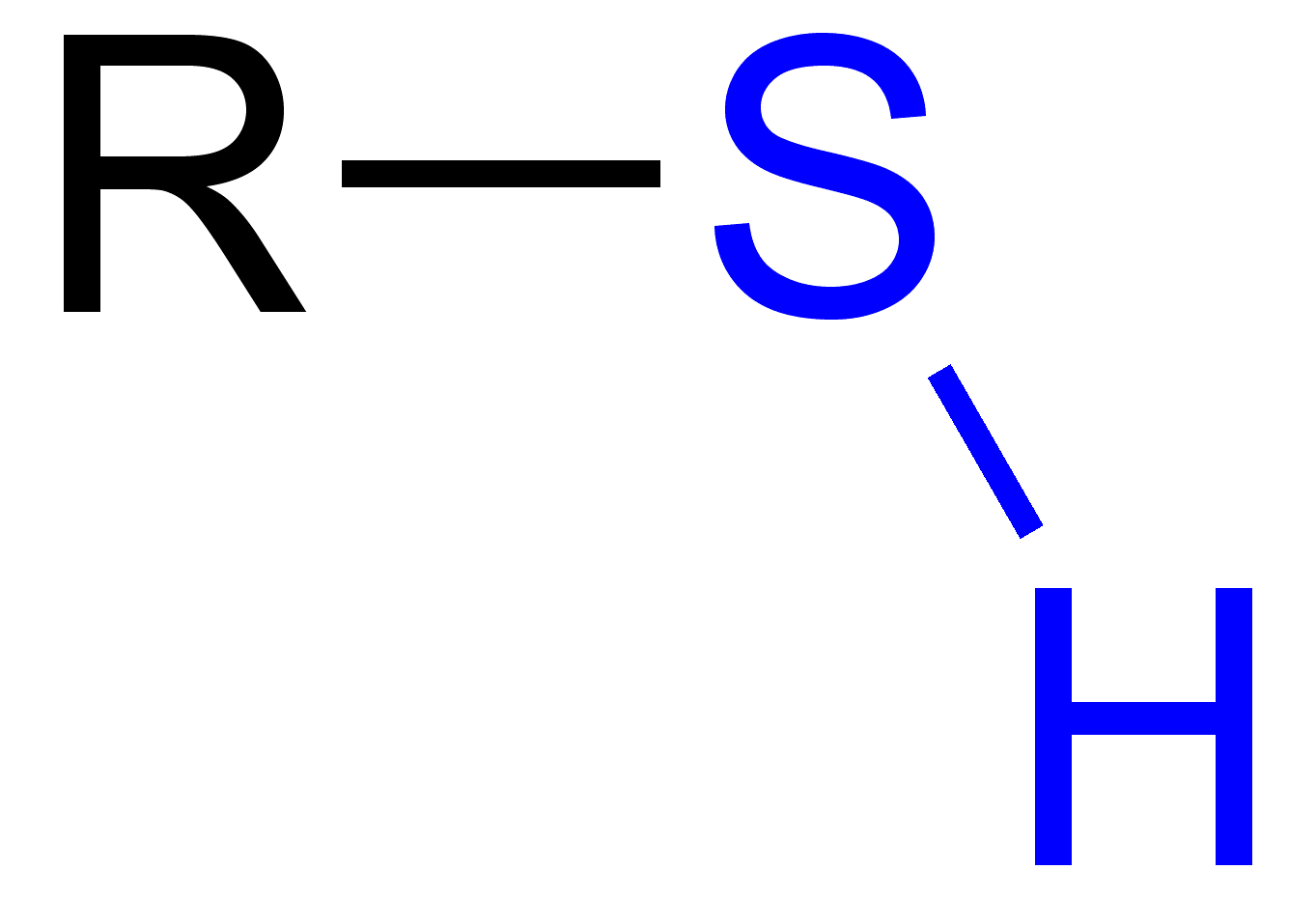
22
New cards
Sulfide
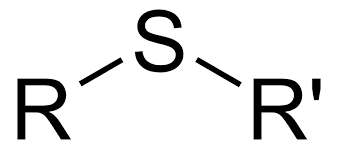
23
New cards
Aldehyde
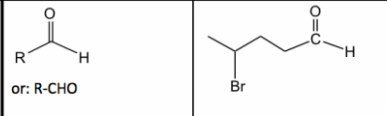
24
New cards
Ketone

25
New cards
Carboxylic Acid
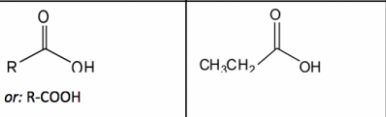
26
New cards
Ester
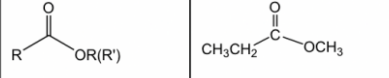
27
New cards
amide
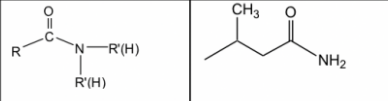
28
New cards
Acid Chloride
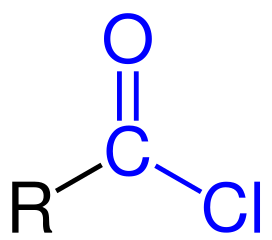
29
New cards
2 rules to determine which compound is more acidic?
E.N for same row
SIZE for same column