Acids and Bases
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Acid
Produces H+ in solution;
Accepts an electron pair in a reaction
Donates H+ (hydrogen proton) - Gives away H+
Base
Creates OH- in solution
Donates an electron pair in a reaction
H+ acceptor (hydrogen proton)
Is Water a Acid or a Base?
It can act as both an acid and a base! It depends on what else is present!
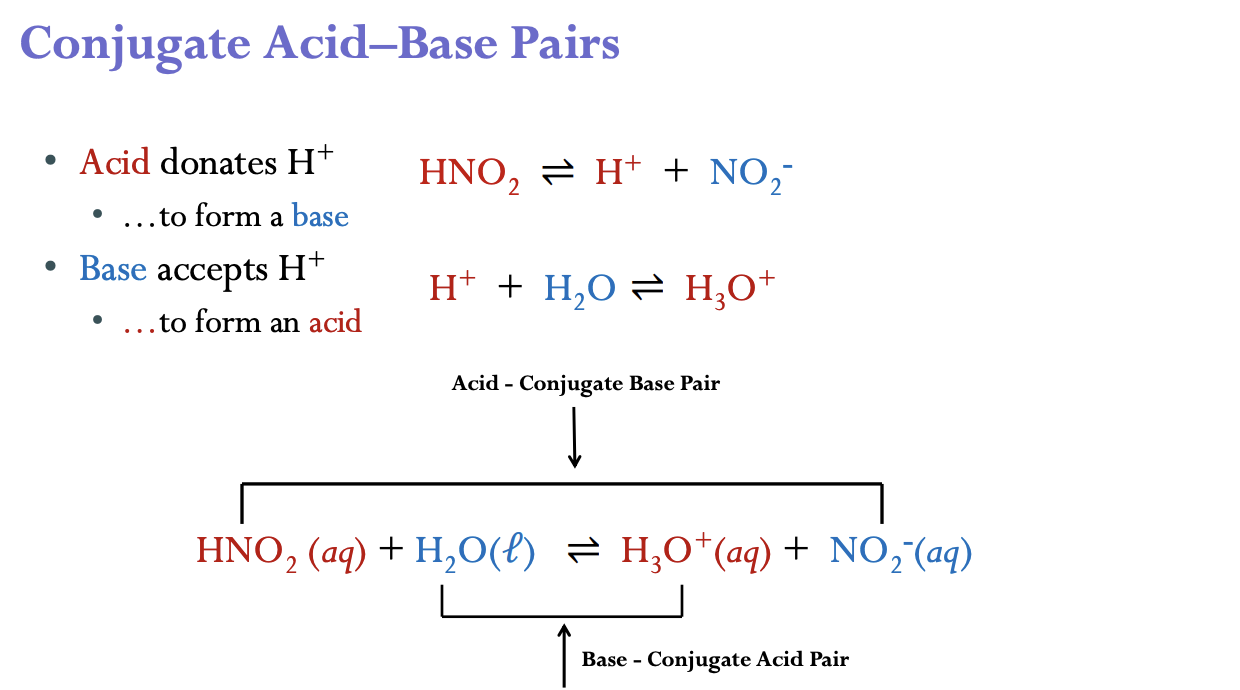
Conjugate Acid–Base Pairs in reactions
HCl - The Big 6
Hydrochloric acid
HBr - The Big 6
Hydrogen Bromide
HI - The Big 6
Hydrogen Iodine
HClO4 - The Big 6
Perchloric Acid
HNO3 - The Big 6
Nitric Acid
H2SO4 - The Big 6
Sulfuric Acid
Factors Affecting Acid Strength in Binary Acids
The more polar the H-X bond (the bond between hydrogen (H) and a halogen or another nonmetal (X) in a binary acid), the weaker the binary acid.
- (This means that if the difference in electronegativity between H and X is large (i.e., the bond is highly polar), the acid is weaker because the bond holds onto the hydrogen more tightly, making it harder to ionize (release H⁺ in solution).
The weaker the H-X bond, the stronger the binary acid
Factors Affecting Acid Strength in Oxoacids
H—O—Y
The more electronegative the element adjacent to the oxygen (Y), the stronger the acid
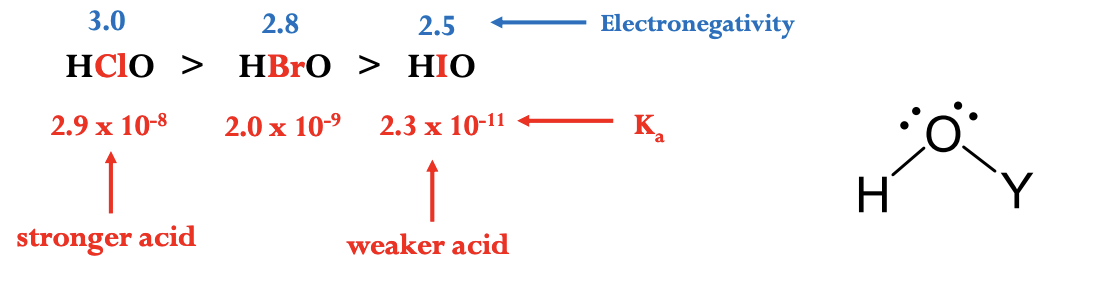
Factors Affecting Acid Strength
The more oxygen atoms in an anion, the greater stability, and the weaker the H-O-Y bond
Delocalization of the negative charge over more electronegative atoms - Stronger the acid
More oxygen atoms affect the oxidation number of adjacent elements in the polyatomic anion
The higher the oxidation number of element adjacent to oxygen, the stronger the acid. (slide 15)
pH = -log[H3O+]
Used to calculate the pH of a solution based on the concentration of hydronium ions (H₃O⁺) in moles per liter (M). It expresses the acidity of a solution on a logarithmic scale.
Kw = 1 × 10^-14 = [H3O+]*[OH-]

Degree of Ionization
Indication of Acid Strength expressed as a decimal
Ionized Acid (H+)/ Initial Acid(HA)
Percent Ionization
Degree of ionization expressed as a percentage
[Ionized Acid (H+)/ Initial Acid(HA)] * 100%
pH of Mixtures of Weak Acids (or Bases)
The pH of the strong acid alone can estimate the pH of a mixture of strong and weak acids. So if you have to estimate the PH of a mix of strong and weak acids then just use the strong acid to make that estimate
Checking the ‘x is small’ assumption
Multiply the Ka value by the initial concentration, then take the square root of the result.
Next, divide that value by the initial concentration and multiply by 100 to get a percentage.
If the percentage is less than 5%, the assumption is valid, so you can ignore x in the denominator and skip the quadratic equation.
If it's more than 5%, the assumption is invalid, and you need to solve using the quadratic formula.
Strong Bases
Most oxides (O2-) and hydroxides (OH-) of Groups 1 and 2 (Alkaline Metals and Alkaline Earth Metals)
Completely dissociate in solution
Weak Bases
Nitrogen-containing compounds
Do not completely dissociate from the solution
Difference between pH and pOH
Both pH and pOH measure the concentration of ions in a solution, but they focus on different aspects of acidity and basicity
If pH = 3, then pOH = 14 - 3 = 11 (acidic solution).
If pOH = 2, then pH = 14 - 2 = 12 (basic solution).
Relationship between Ka and Kb
Kw = Ka*Kb
If we know one, we can figure out the other one, Kw is given
Polyprotic Acids
They can donate more than one proton (H⁺) in solution
• Harder to dissociate the second proton
Diprotic Acids
Can donate two protons (H⁺)
Ka1 > Ka2
• More difficult to remove H+ ion from negatively charged anion
• Typically, only first H+ dissociation affects pH (Ka1)
Triprotic Acids
Can donate three protons (H⁺)
Ka1 >> Ka2 & Ka3
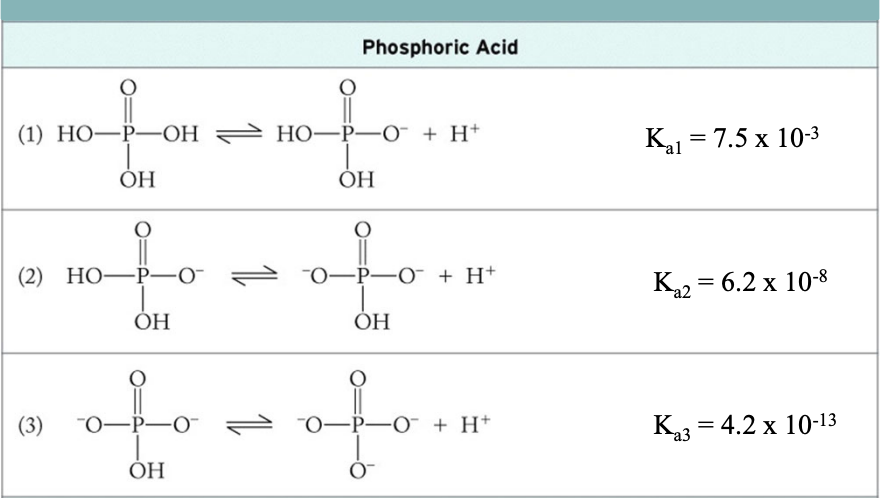
Triprotic Acids: Phosphoric Acid
Formula: H3PO4
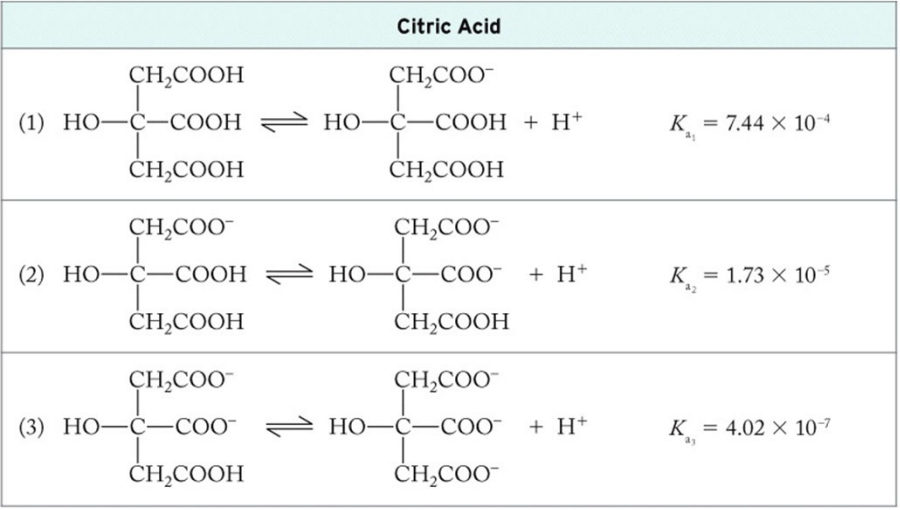
Triprotic Acids: Citric Acid
Formula: C6H8O7
What ions are always soluble in compounds with no exceptions?
Li⁺, Na⁺, K⁺, NH₄⁺
NO₃⁻, C₂H₃O₂⁻
Are Cl⁻, Br⁻, and I⁻ soluble?
Yes, generally soluble.
Exception: Insoluble when paired with Ag⁺, Hg₂²⁺, or Pb²⁺.
Is SO₄²⁻ (sulfate) soluble?
Yes, generally soluble.
Exceptions: Insoluble when paired with Sr²⁺, Ba²⁺, Pb²⁺, Ag⁺, or Ca²⁺.
Are OH⁻ and S²⁻ soluble?
Generally insoluble.
Exceptions:
Soluble with Li⁺, Na⁺, K⁺, NH₄⁺.
S²⁻ is soluble with Ca²⁺, Sr²⁺, Ba²⁺.
OH⁻ is slightly soluble with Ca²⁺, Sr²⁺, Ba²⁺.
Are CO₃²⁻ (carbonate) and PO₄³⁻ (phosphate) soluble?
Generally insoluble.
Exception: Soluble with Li⁺, Na⁺, K⁺, NH₄⁺.