Module 8: Cell Adhesion
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
What are the key processes required for multicellular development from a single fertilized egg?
Repeated mitotic divisions to produce many cells
Cell differentiation to create tissue-specific gene expression
Cell signaling between cells
Cells must associate and maintain connections during embryogenesis
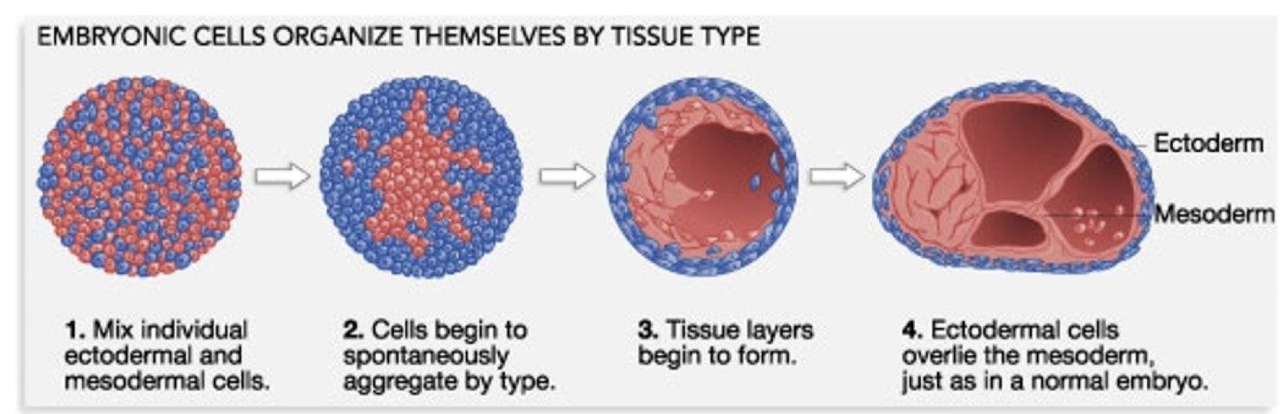
Formation of the inner cell mass forms the early embryo
Embryo cells separate into three germ layers:
Endoderm
Ectoderm
Mesoderm
These germ layers give rise to all cells and tissues in the body
Cell connections are essential to prevent a “soup of cells” and enable organ/tissue functions
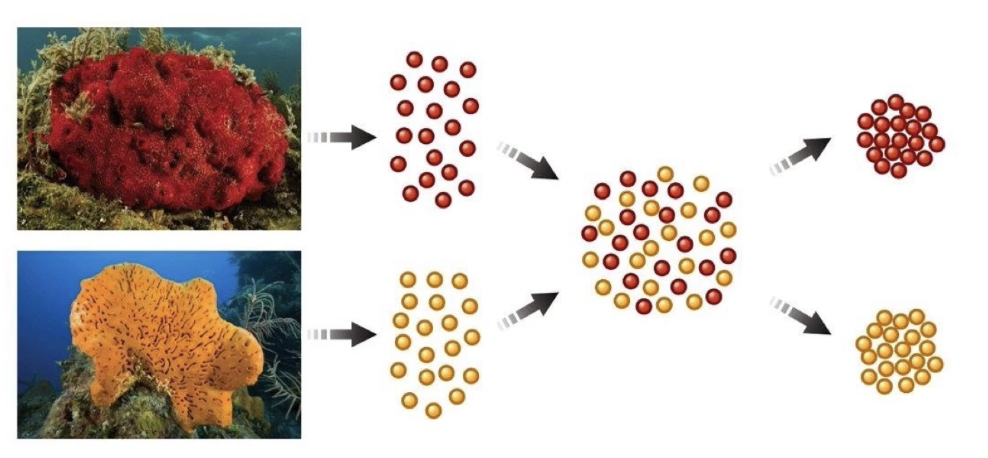
How was cell recognition and adhesion demonstrated experimentally?
In 1907, H.V. Wilson separated sponge cells of two species using a fine mesh
Mixed cells back together
Cells from the same species recognized and re-associated
Cells from different species did not associate
Demonstrated species-specific cell adhesion and recognition
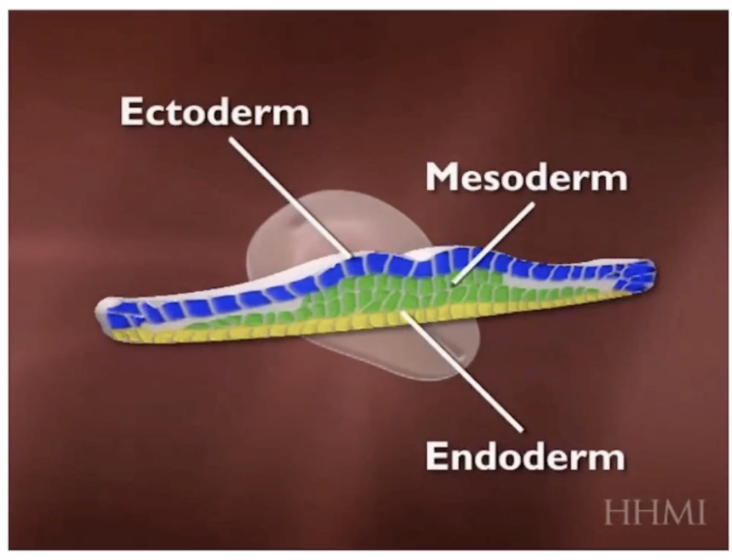
What did Johannes Holtfreter’s frog embryo experiment show about cell recognition and organization?
Separated cells from two different germ layers in frog embryos
When mixed, cells from similar tissues recognized and associated with each other
Cells organized into tissue-specific lineages mimicking original embryo organization
This demonstrates like cells recognize and adhere during embryogenesis
This adhesion requires cell adhesion molecules (CAMs), which are transmembrane proteins
After aggregation, cells form specialized cell junctions that:
Stabilize cell-to-cell interactions
Facilitate communication between neighboring cells
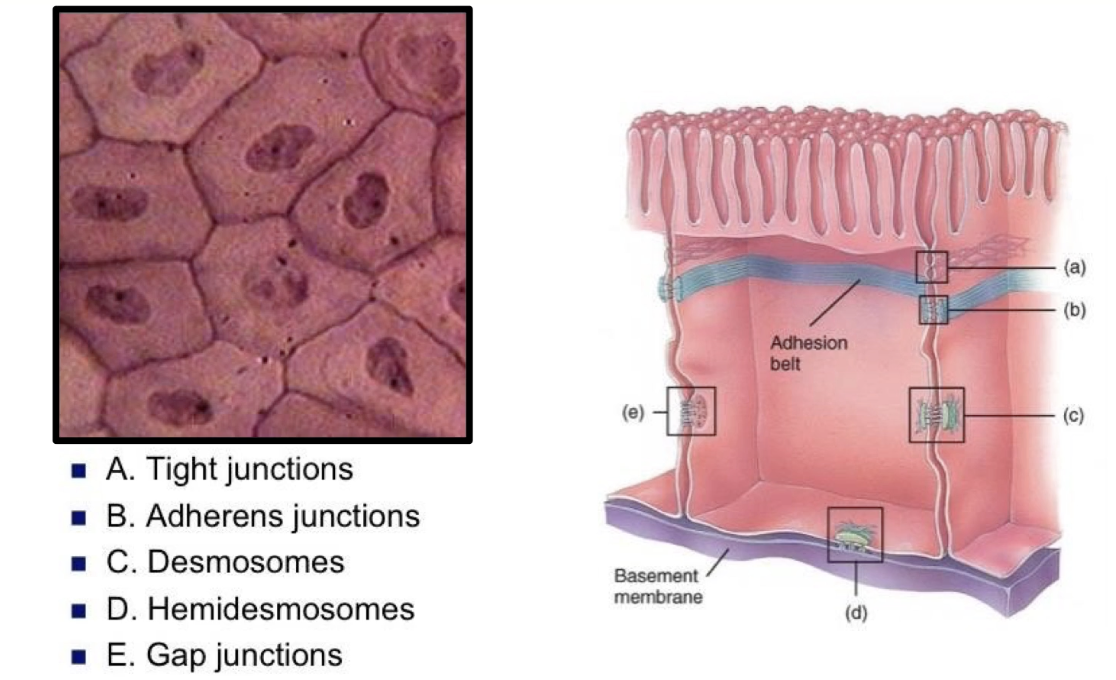
What are the key features of cell junctions in epithelial sheets, and what are the four main types of adhesion complexes on lateral surfaces?
Epithelial cells connect along lateral surfaces to form sheets lining body cavities
Epithelial sheets form:
Inner lining of digestive system
Outer layers of skin
Cells have distinct apical (top) and basal (bottom) surfaces with different functions
Basal surface anchors cells to extracellular matrix via hemidesmosomes
Apical surface often has microvilli (e.g., intestinal lining)
Four adhesion complexes connect lateral surfaces:
Tight junctions
Gap junctions
Desmosomes
Adherens junctions
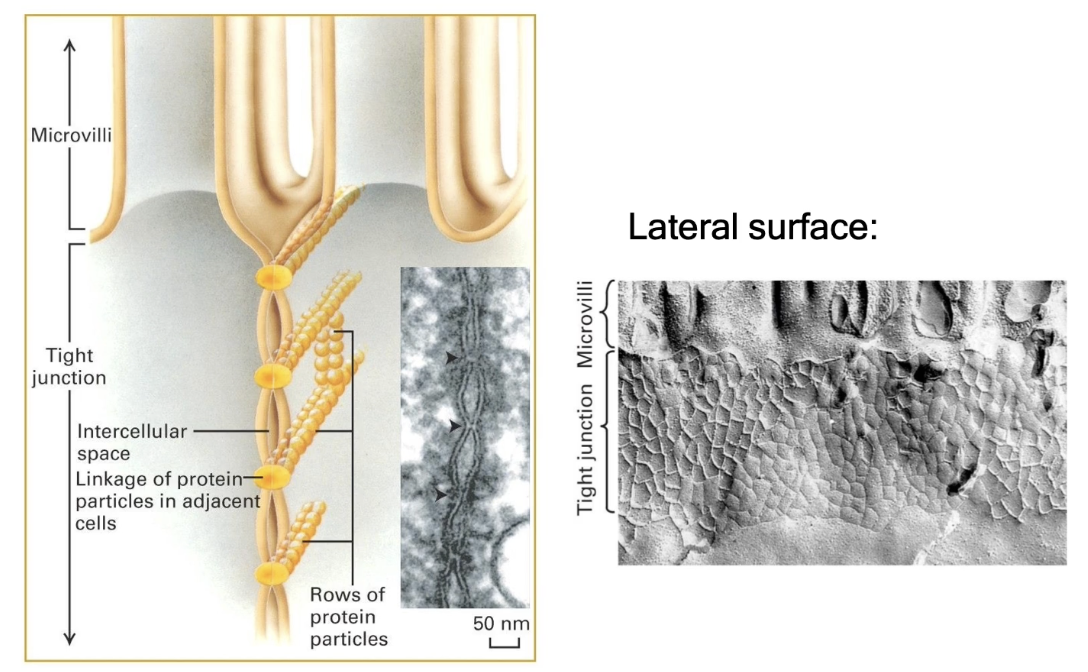
What are tight junctions and what is their function?
Also called zonula occludens
Located just below the apical surface of adjacent cells and seal off space between cells completely
Prevent fluid and small molecule diffusion across cell layers
Important in the gastrointestinal tract to prevent enzyme leakage
Made of linear arrays of occludin and claudin proteins
Appear as points where membranes are pinched together under electron microscopy
Form a complete junctional band (not just a single junction)
Freeze fracturing (cells frozen with liquid nitrogen and then broken at weak points) reveals a web-like network of tight junction proteins
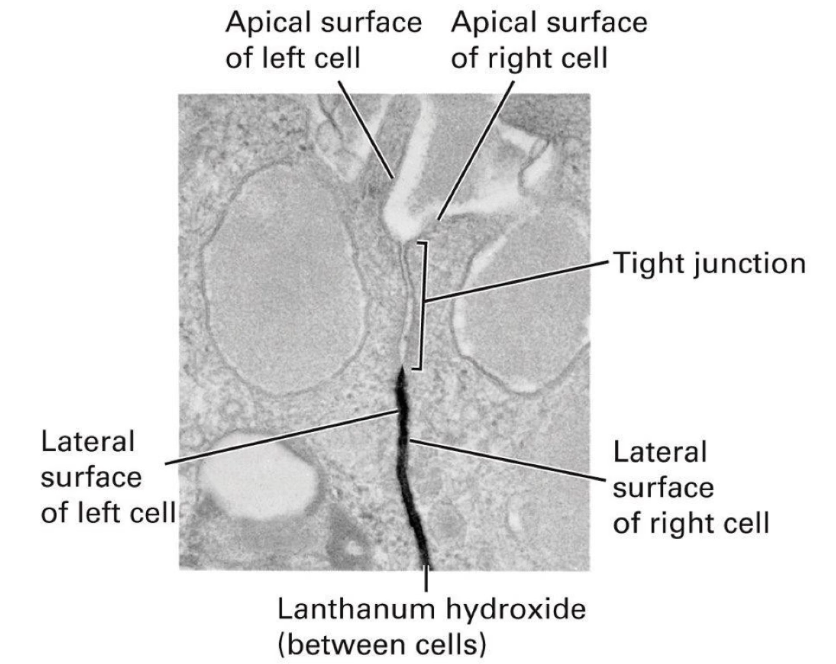
How do tight junctions affect membrane protein and molecule diffusion?
Prevent diffusion of membrane proteins between apical and basolateral regions
Block diffusion of molecules in extracellular space between cells
Example: lanthanum hydroxide (electron-dense) cannot diffuse past tight junctions
Tight junctions create a barrier limiting molecule movement from basal to apical surface
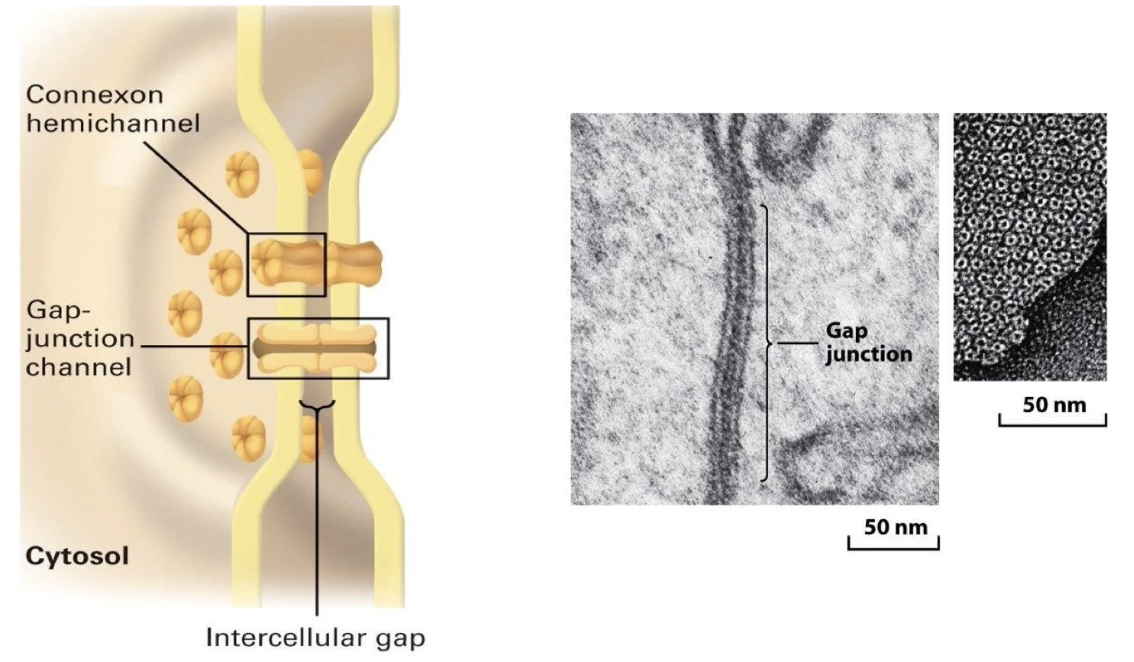
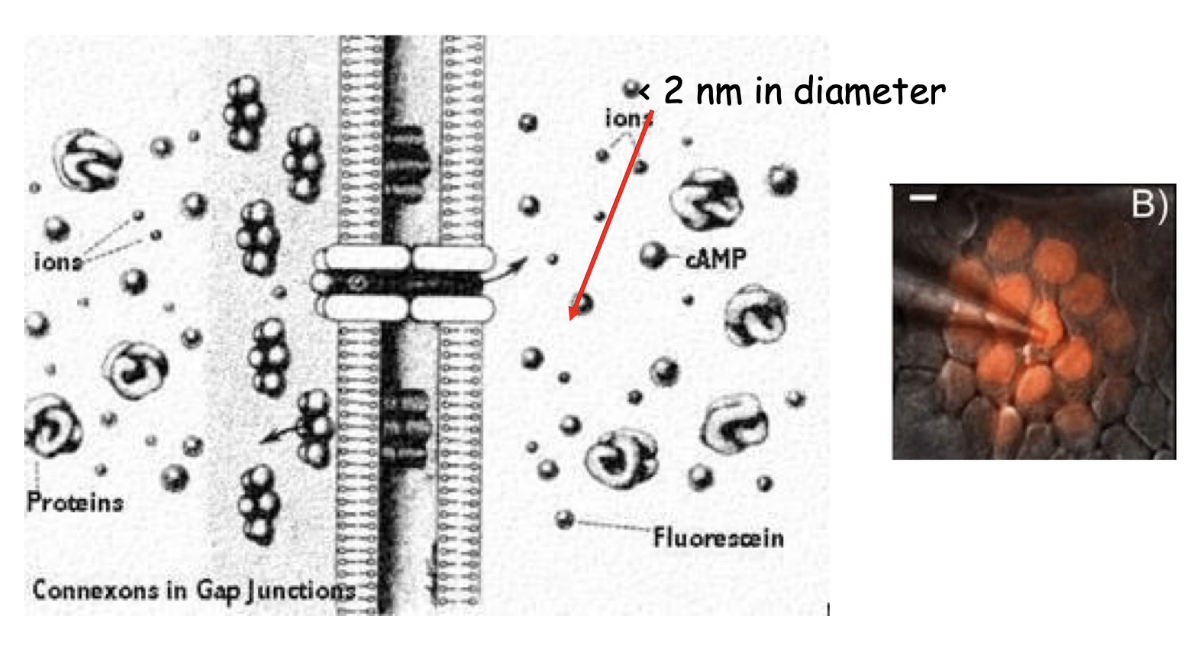
What are gap junctions and their role in cell communication?
Link cytosol of adjacent cells directly
Important for metabolic integration of tissue cells
1.5 - 2.0 nm in diameter to allow exchange of ions and small molecules (up to ~1 kDa)
Includes secondary messengers like cAMP and Ca²⁺
Structure: 6 connexin protein subunits make up 1 hexagonal connexin hemichannel
Two connexon hemichannels from adjacent cells line up to form a gap junction channel
Found in clusters forming gap junction-rich regions
Hold cells together by pinching membranes together but still allow extracellular diffusion
Electron microscopy shows donut-shaped gap junction arrays on lateral cell surfaces
What size molecules can pass through gap junctions and why is this important?
Gap junction channels are about 2 nm in diameter
Allow diffusion of ions and secondary messengers like cAMP and calcium
Enables rapid coordination of activities (e.g., cardiac and uterine muscle contractions)
Stimulation of one cell can spread to others via cytosolic flow through gap junctions
Experiment shows fluorescent molecule injected into one cell diffuses to neighboring cells connected by gap junctions
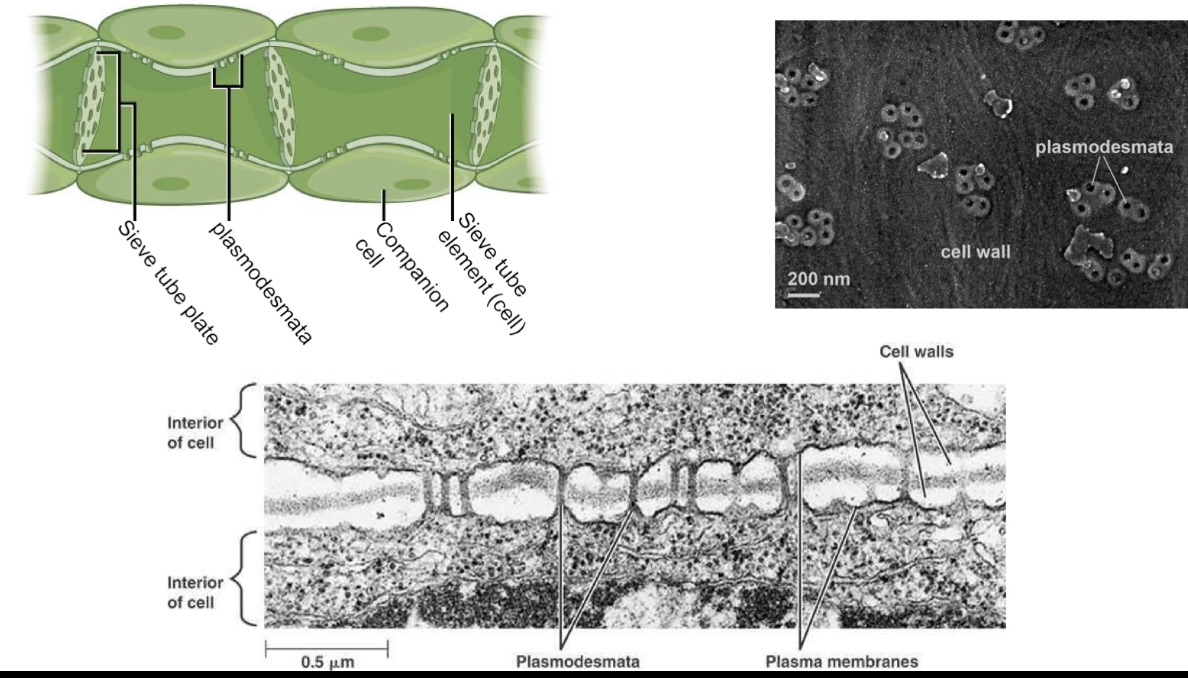
What are plasmodesmata and how do they function in plant cells?
Plasmodesmata are plant cell structures similar to animal gap junctions
Important in phloem function in flowering plants
Phloem = system of elongated tubes transporting nutrients (e.g., sucrose) from leaves to rest of plant
Phloem cells (sieve-tube elements) connected by modified/enlarged plasmodesmata forming sieve tube plates
Sieve-tube elements are metabolically inactive
Companion cells support sieve-tube elements by providing ATP, proteins, and substances
Companion cells also connected by plasmodesmata to phloem cells
TEM images show plasmodesmata channels span two cell membranes and the cell wall
Plasmodesmata appear as donut-shaped structures in electron microscopy
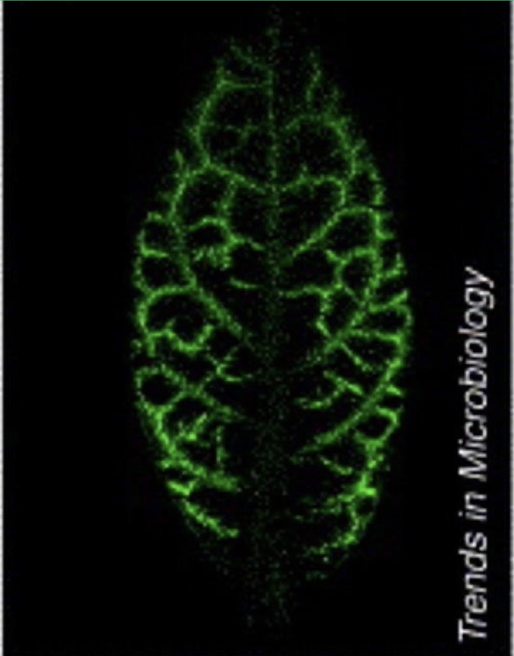
What roles do gap junctions (plasmodesmata) play in plants?
Phloem acts like a circulatory system, carrying sucrose from source cells (photosynthetic leaf cells) to the rest of the plant
Green fluorescent protein synthesized in companion cells can move within the phloem via plasmodesmata
Plasmodesmata also traffic informational macromolecules like transcription factors, gene transcripts, and small RNAs
Viral pathogens exploit plasmodesmata to facilitate intercellular viral spread
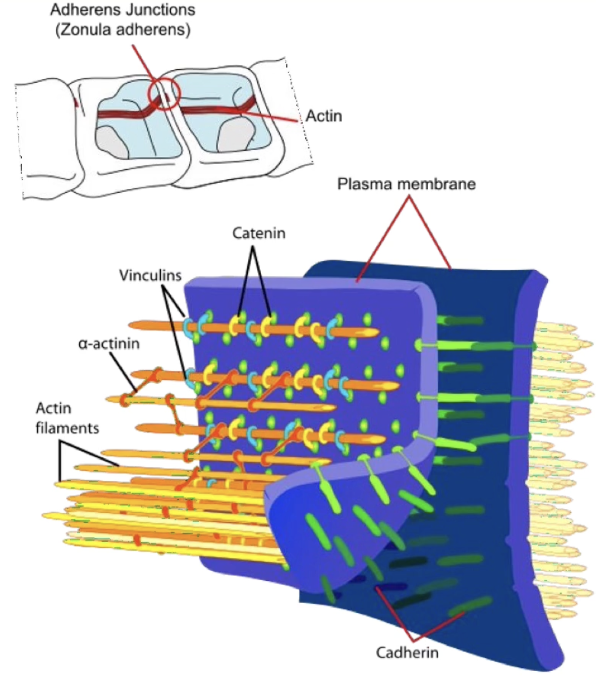
What are anchoring junctions and how do they relate to the cytoskeleton?
Anchoring junctions include: adherens junctions, desmosomes, and hemidesmosomes
Distinguished by association with the cytoskeleton, especially actin filaments
Desmosomes connect two cells
Hemidesmosomes connect cells to the extracellular matrix
Adherens junctions indirectly link the actin cytoskeleton between neighboring cells
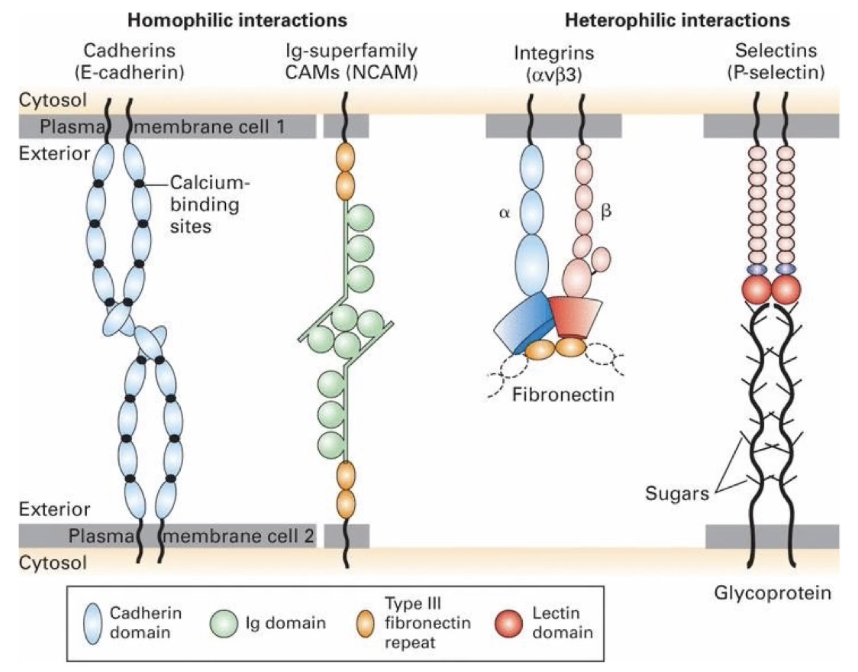
What are the major families of cell adhesion molecules (CAMs) in adherens junctions and how do their interactions differ?
Four major CAM families: cadherins, Ig-superfamily, integrins, and selectins
Homophilic interactions (same molecule on both cells): cadherins and Ig-superfamily CAMS
Heterophilic interactions (different molecules on cells): integrins and selectins
What are cadherins and what is their role in adherens junctions?
Calcium-dependent cell adhesion molecules (CAMs)
Mediate homophilic interactions (same cadherin on both cells)
Three major classes:
E-cadherin (epithelial)
N-cadherin (neural)
P-cadherin (placental)
Located near the apical surface, just below tight junctions in epithelial cells
Adhesion involves transmembrane cadherins + cytosolic cofactors called catenins that link cadherins to the actin cytoskeleton
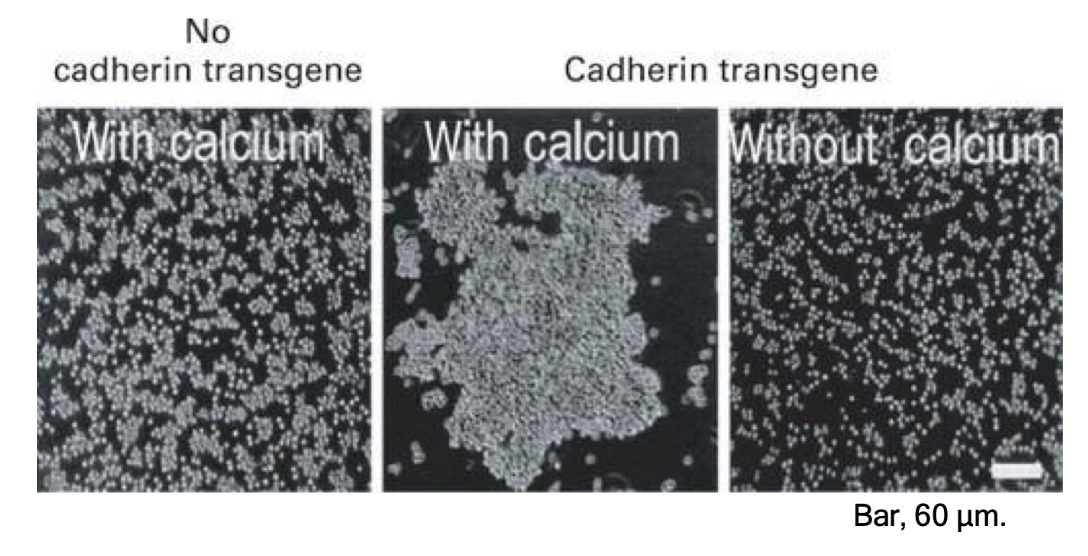
Epithelial cells without E-cadherin (mediates Ca2+ dependent adhesion) gene do not aggregate in culture
Introducing E-cadherin gene induces cell aggregation
Adhesion is calcium-dependent; without calcium, aggregation does not occur
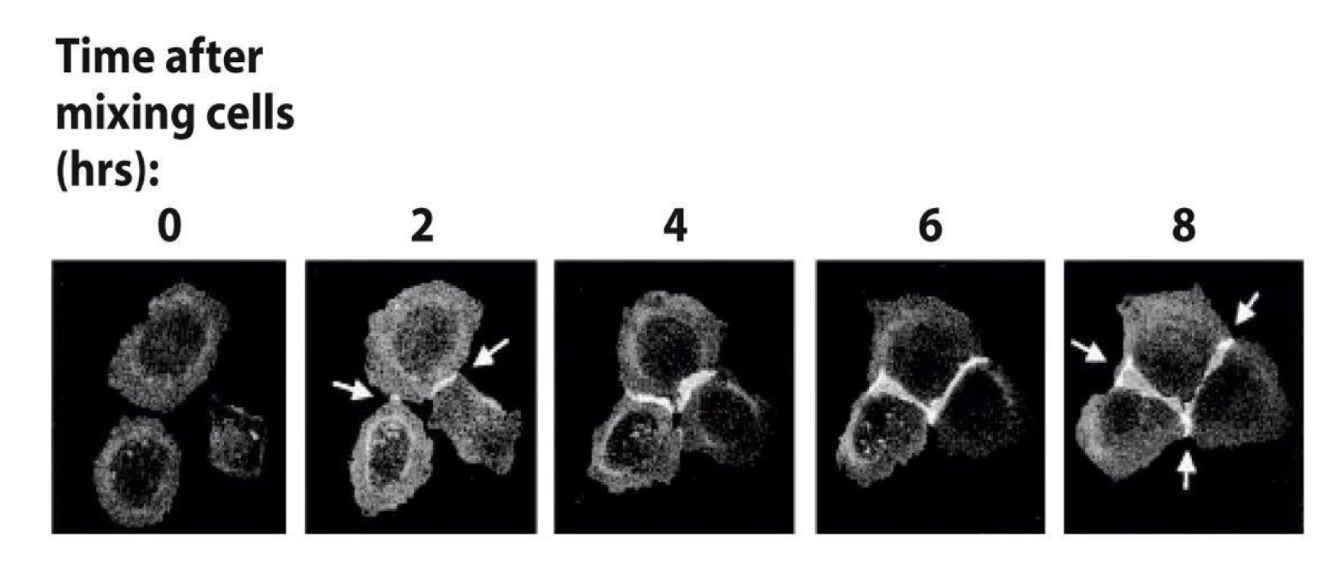
How was E-cadherin’s role in tissue-specific adhesion demonstrated using GFP?
Researchers created an E-cadherin-GFP fusion gene and introduced it into cultured cells
Cells expressing GFP-tagged E-cadherin were mixed in calcium-containing medium
Over time, cells expressing E-cadherin aggregated and adhered together
GFP fluorescence accumulated at contact surfaces, showing formation of adherens junctions
Cells only adhered to others expressing the same E-cadherin, demonstrating homophilic interaction
What is the role of neutrophils?
A type of white blood cell
Key players in the immune response
Why is transient cell adhesion important in neutrophil extravasation?
Not all cell adhesion is permanent — some are transient (temporary)
Examples of transient adhesion:
Cell migration during embryogenesis
Leukocyte migration in response to infection/injury
Leukocytes (white blood cells) exit bloodstream via extravasation
A precise sequence of adhesive interactions needed
Endothelial cells (line blood vessels) usually prevent leakage
Tight adhesion between these cells keeps blood cells inside
In immune response:
Leukocytes slow down, adhere to endothelium, and exit bloodstream
Must form and break temporary connections with endothelial cells to reach infected/injured tissue
What are the three families of white blood cells (leukocytes), and which undergo extravasation?
1. Granulocytes (target pathogens)
Include neutrophils, eosinophils, basophils
Neutrophils:
Most abundant granulocyte
First responders to bacterial infections & trauma
Undergo extravasation
Eosinophils & basophils do not extravasate
2. Monocytes
Differentiate into macrophages
Perform phagocytosis (engulf bacteria, debris)
Can undergo extravasation
3. Lymphocytes
Include NK (natural killer) cells, T cells, B cells
NK cells destroy virally infected & tumor cells
T/B cells produce antibodies
Can undergo extravasation
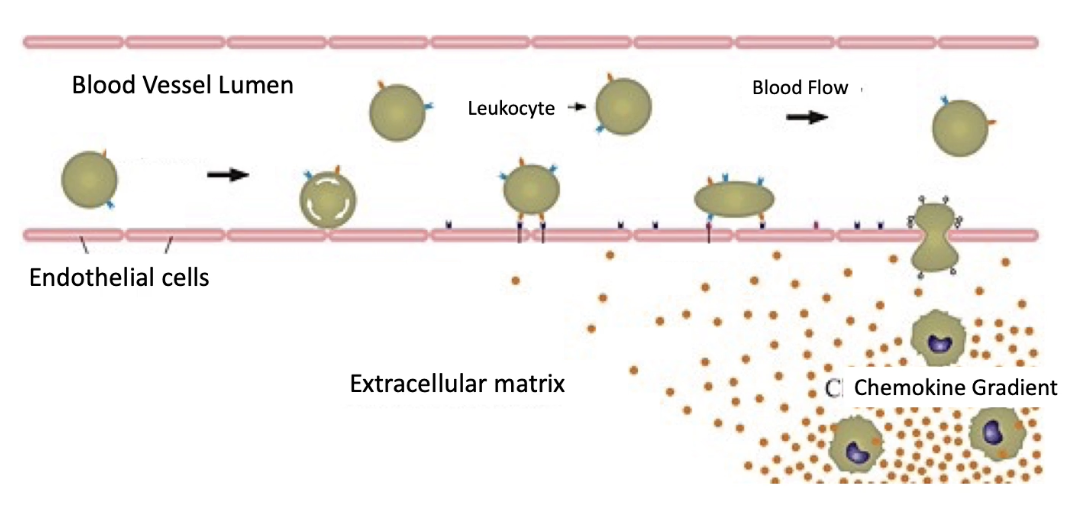
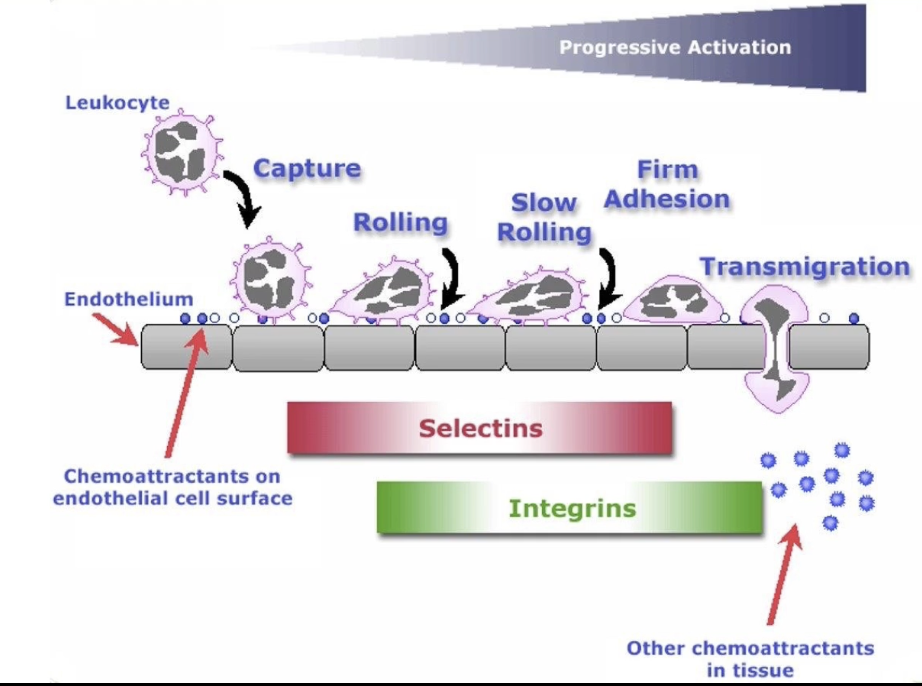
What are the 5 steps of neutrophil extravasation and what happens in each step?
Triggered by infection signals
Steps:
Capture
Temporary binding of neutrophil to endothelial cells
Neutrophil slows slightly, remains in bloodstream
Rolling
Neutrophil begins to roll along vessel wall
Mediated by weak interactions
Slow-Rolling
More adhesive interactions → neutrophil movement slows further
Firm Adhesion
Strong attachment to endothelium
Cell shape and function change
Transmigration
Neutrophil moves through endothelial barrier to tissue
Inflammation/swelling occurs due to:
Plasma leakage
Leukocyte accumulation
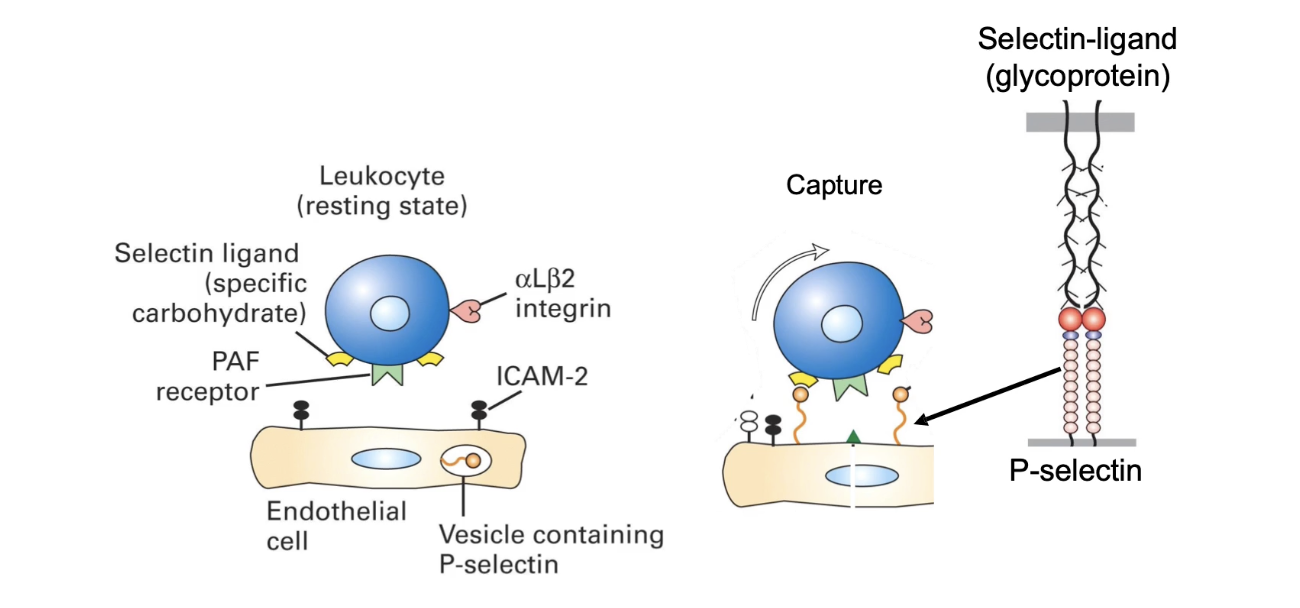
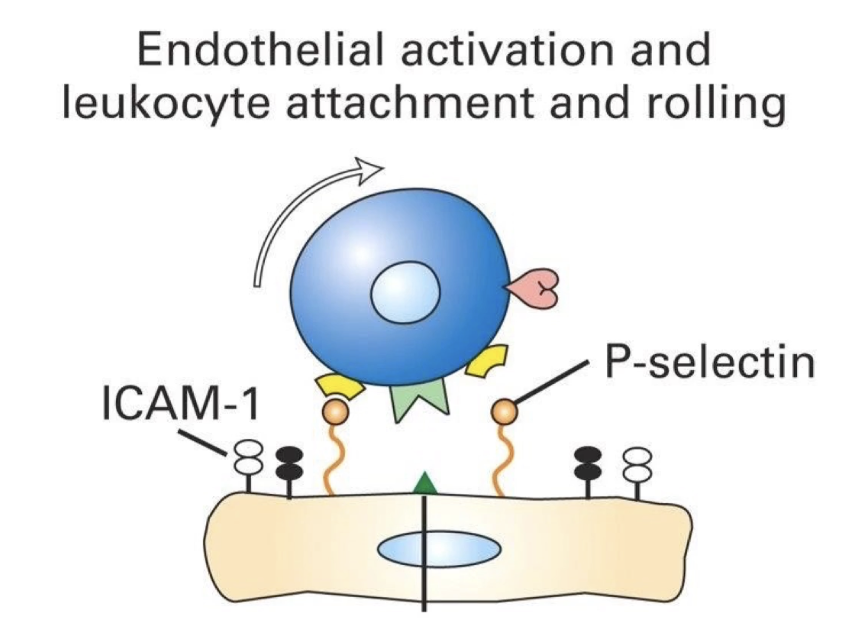
What triggers the initial capture of neutrophils during extravasation?
Infection site releases cytokines (e.g., TNF-alpha)
Cytokines affect endothelial cells lining blood vessels
TNF-alpha binds receptors on basal surface of endothelial cells
Triggers release of P-selectins from secretory vesicles
P-selectins move to apical surface of endothelial cells
P-selectins bind to ligands (selectin-specific glycoproteins) on neutrophils
Enables transient attachment of neutrophil to vessel wall
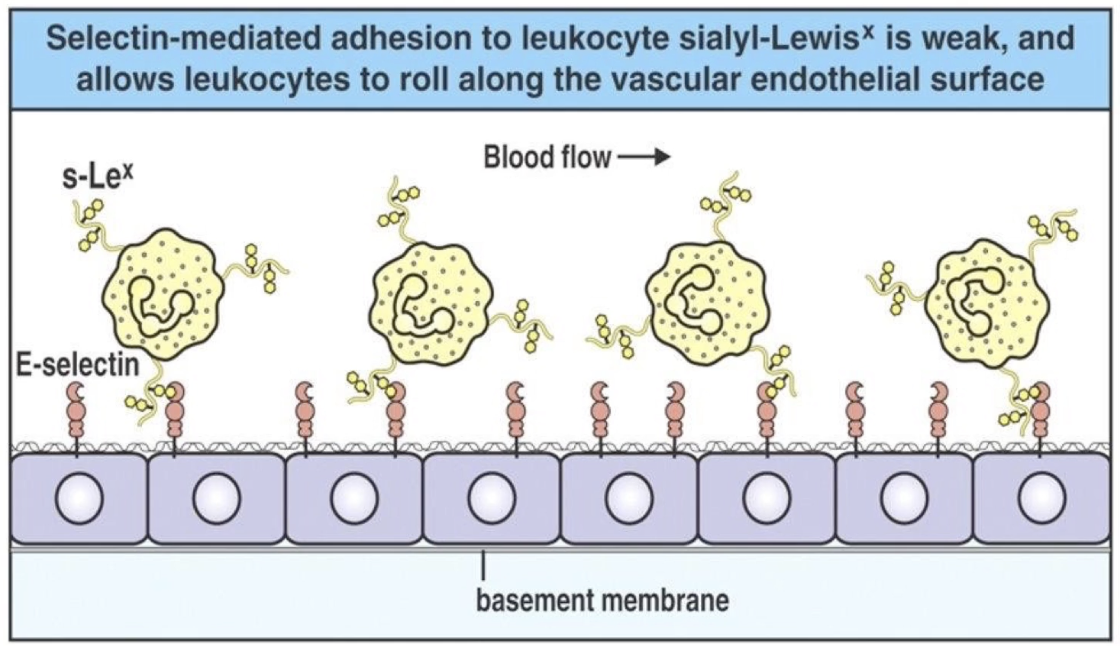
What happens during the rolling stage of neutrophil extravasation?
Neutrophil slows down due to weak selectin-ligand binding
Begins rolling along the endothelial surface
Rolling = transient attachments and detachments
Movement driven by blood flow, but slowed by interactions with selectins
What causes neutrophils to slow-roll during extravasation?
Closer to infection site → more selectins (P-selectin + E-selectin) on endothelium
Higher selectin density = more binding opportunities
Increased interactions between selectins and neutrophil ligands
Leads to further slowing of the neutrophil
Neutrophil now in slow rolling phase
How does firm adhesion occur during neutrophil extravasation?
During slow rolling, neutrophil interacts with PAF (platelet activating factor) on endothelium
PAF binds to PAF receptor (a GPCR) on neutrophil
Other neutrophil receptors: CXCR1, CXCR2 (also GPCRs)
PAF binding triggers intracellular signaling
Changes in gene expression
Activation of integrin adhesion molecules
Activated integrins bind to ICAMs (intercellular adhesion molecules) on endothelium
Result: Firm adhesion (tight binding) to endothelial surface
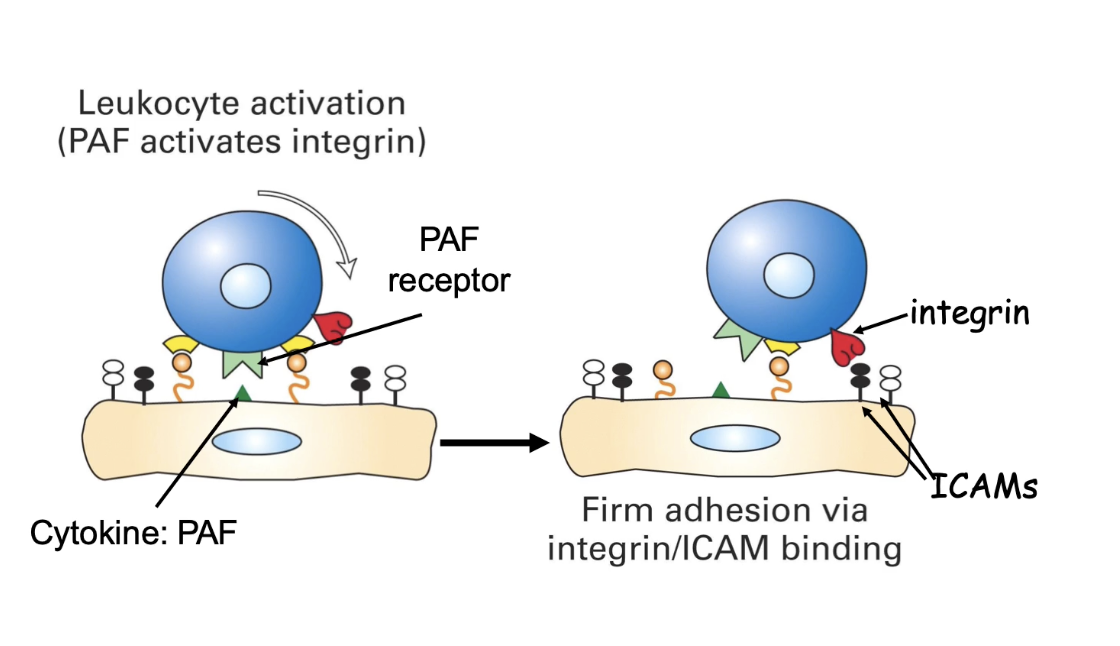
How do integrins contribute to firm adhesion during neutrophil extravasation?
Inactive integrin = folded conformation (propeller & β-A domains bent down)
Can't bind ligands (e.g. ICAMs)
PAF signaling triggers conformational change
Activates integrins (ligand-binding domain exposed)
Active integrins bind ICAMs on endothelial cells
Much stronger adhesion than selectins
Slows neutrophils further, establishing firm adhesion
Triggers actin cytoskeleton reorganization
Prepares neutrophil for migration

What occurs during the final stage of neutrophil extravasation: transmigration?
Neutrophil crawls between endothelial cells
Produces enzymes to break endothelial cell junctions
Involves shape changes to squeeze through gaps
Allows movement from blood vessel to infection site
Confocal image shows:
Neutrophil (red) actively migrating
Endothelial cells (green)

What is the sequence of neutrophil activation and cell adhesion molecule involvement in extravasation?
Five stages: Capture → Rolling → Slow rolling → Firm adhesion → Transmigration
Selectins:
Mediate early stages (capture, rolling, slow rolling)
Integrins:
Activated by PAF/GPCR signals during slow rolling
Mediate firm adhesion
Enable transmigration
Activation of cell adhesion molecules is sequential and regulated
How does the animation summarize neutrophil extravasation and activation?
Red blood cells flow freely; neutrophils roll on endothelium
Rolling due to PSGL-1 on neutrophil binding P-selectin
P-selectin expression increases at infection site → slows neutrophil
PAF binds GPCR on neutrophil → triggers integrin activation
Activated integrins bind ICAMs on endothelial cells → firm adhesion
Neutrophil:
Secretes proteases to break endothelial junctions
Reorganizes cytoskeleton for migration
Applied Lecture
Anti-adhesion in infectious disease treatment
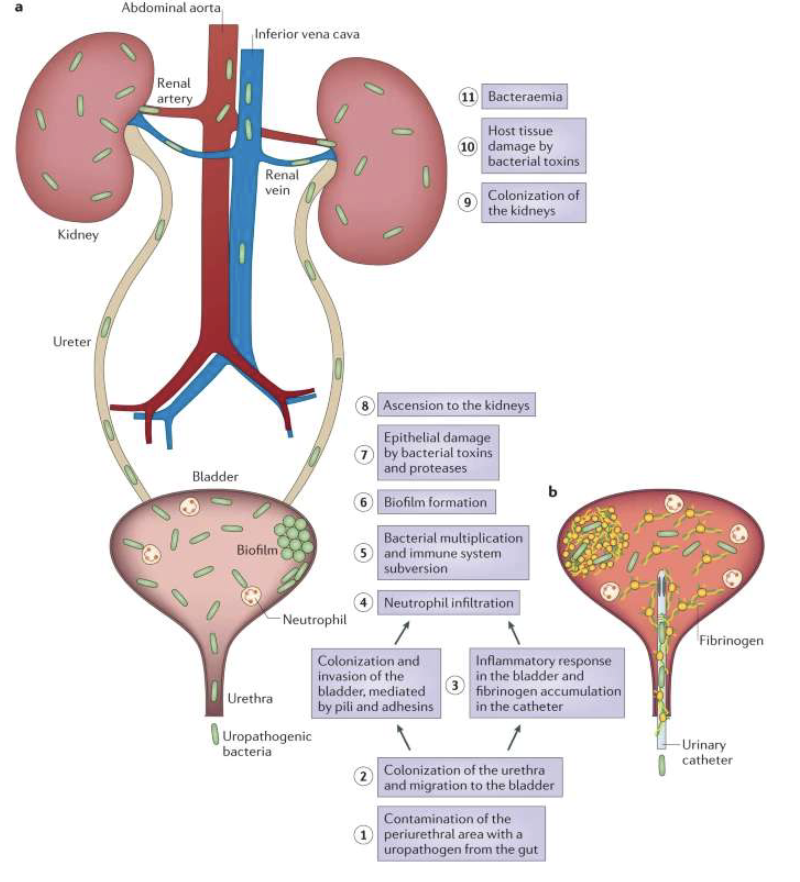
What is the main cause of UTIs and how common are they?
Uropathogenic E. coli (UPEC) is the main cause
~150 million cases of UTIs annually
Can cause inflammation and long-term damage despite treatments
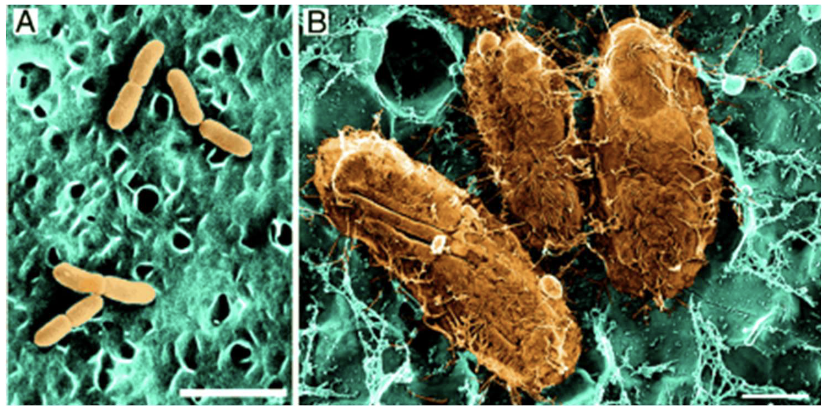
How do UPEC bacteria cause UTIs?
Use pili and adhesins to adhere to epithelium
More common in females
Steps:
Invade urethra and bladder
Replicate
Immune system suppression
Bacteria form biofilms
Damage epithelium
Can ascend up ureters to kidneys and renal arteries → bacteremia or septicemia
What is required for UPEC to initiate infection and what follows?
Adhesion via pili prevents removal by urine flow/immune defenses
Steps: attachment → invasion → replication → host cell damage
Secrete toxins → cell death and exfoliation
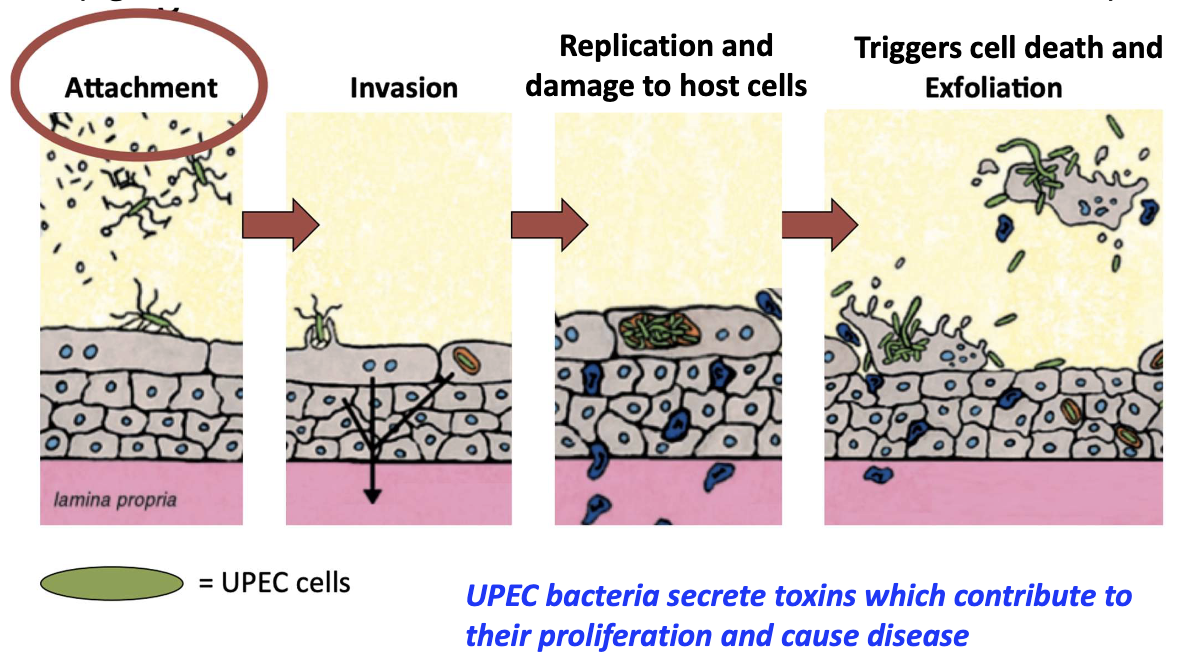
Why is bacterial adhesion critical in UTIs?
Allows bacteria to stay, multiply, and cause damage
Initial non-covalent binding weak → becomes stronger over time as more interactions occur
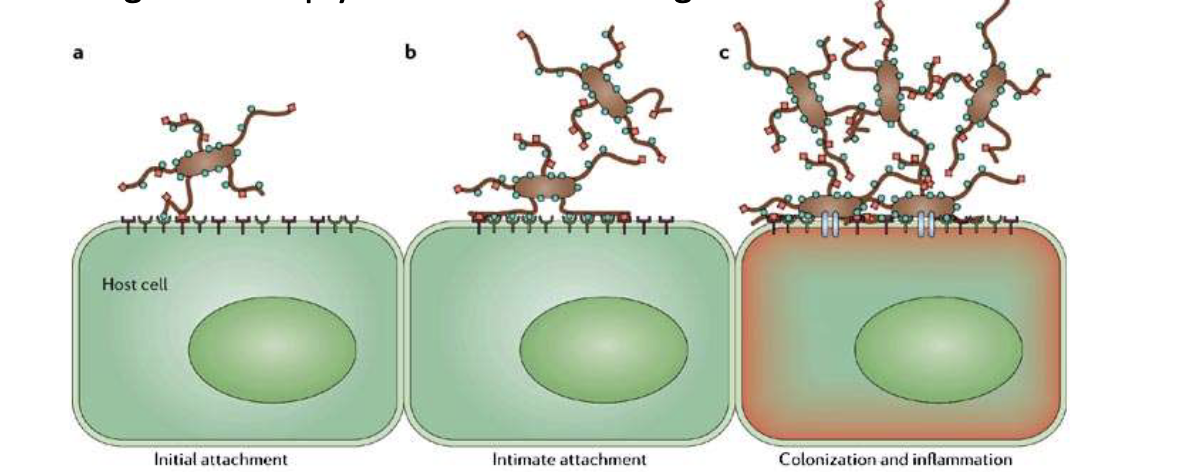
What role do lectins play in adhesion?
Lectins = proteins with sugar-binding specificity
Example: P-selectin binds sugar residues on glycoproteins
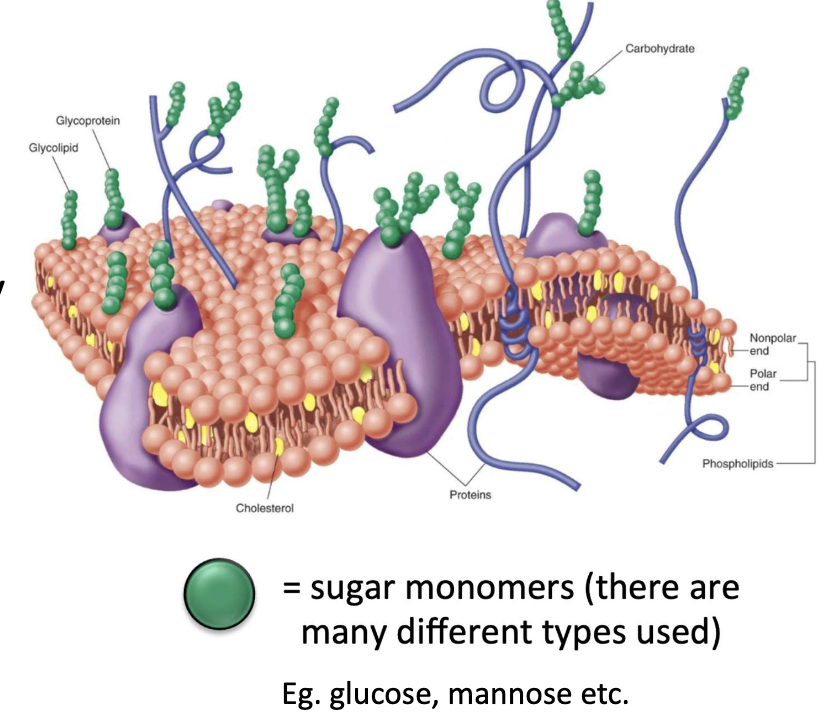
Why is glycosylation important in host cells?
Occurs in Golgi
Essential for:
Protein folding
Stability
Function
Cell-cell interactions
How do UPEC pili interact with host cells?
Pili/fimbriae (hair-like projections) have lectin proteins at their tips
Bind to mannose-containing glycoproteins on urinary tract epithelium
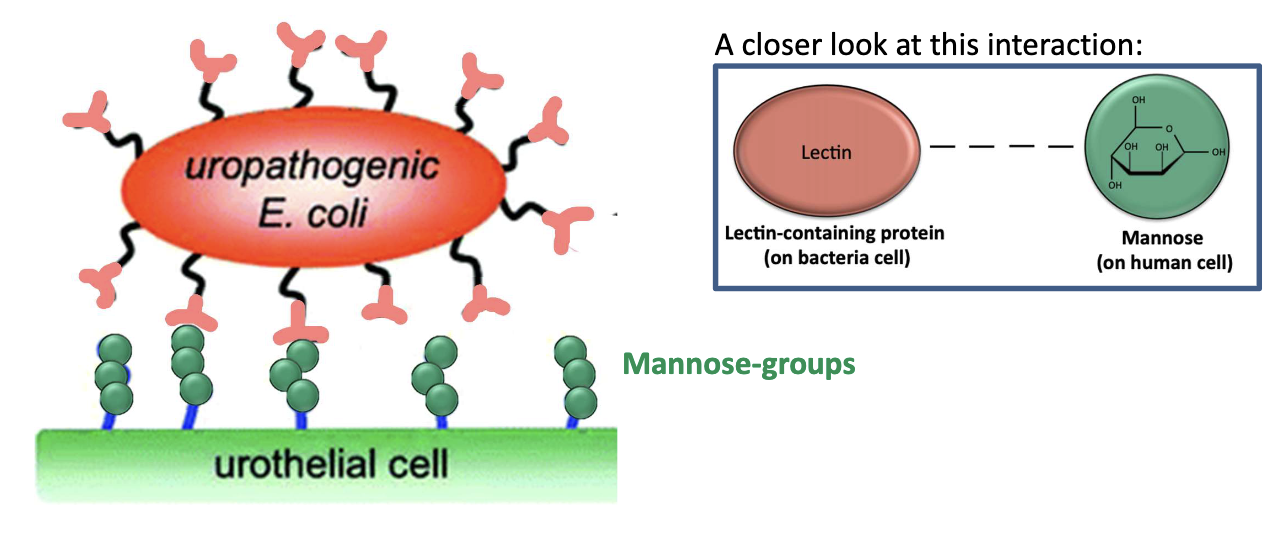
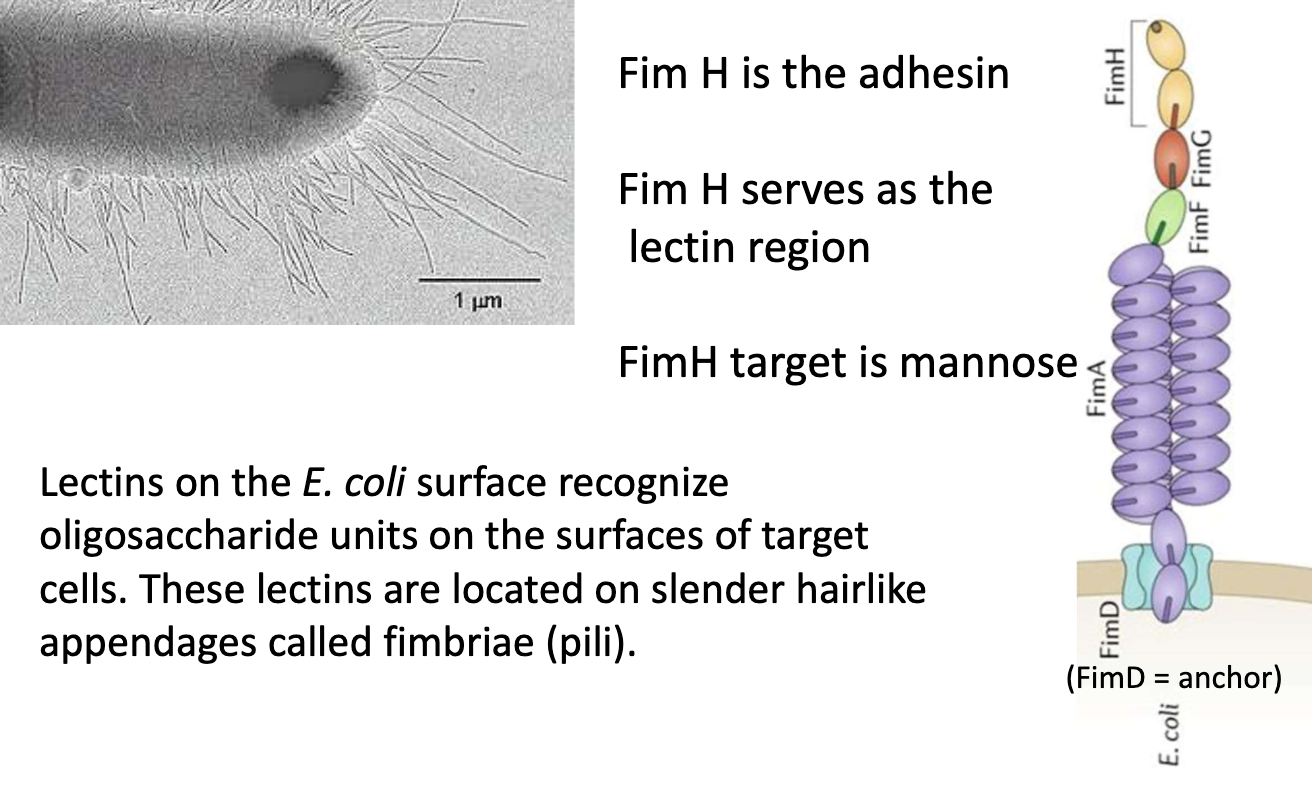
What is FimH and what does it bind?
FimD = anchor
FimA = amino acids that create the length
FimF + FimG = connector domains
FimH = adhesin + lectin domain
Located at tip of Type 1 pili (fimbriae)
Binds mannose (oligosaccharide) on host cell surface
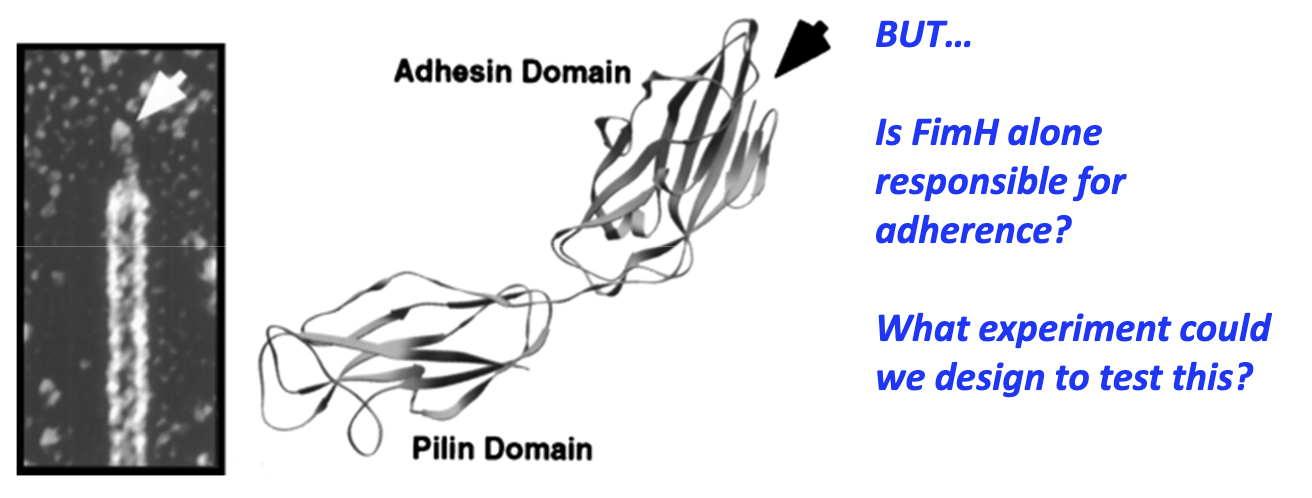
What are the domains of FimH and their functions?
C-terminal (pilin domain): connected to pili
N-terminal (adhesin domain): contains a carbohydrate binding pocket and binds D-mannose
Experiment needed to test if FimH alone enables adherence
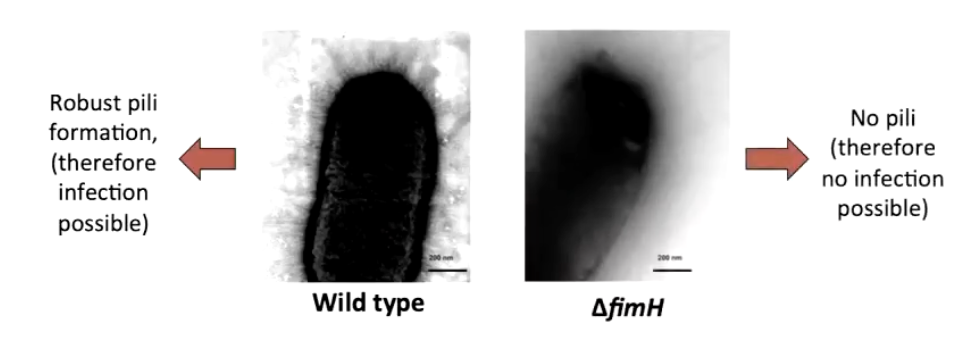
What happens in a ΔfimH mutant strain?
Cannot form pili → no infection
Shows FimH is necessary for pili assembly
Is FimH alone sufficient for adhesion/internalization? → needs testing
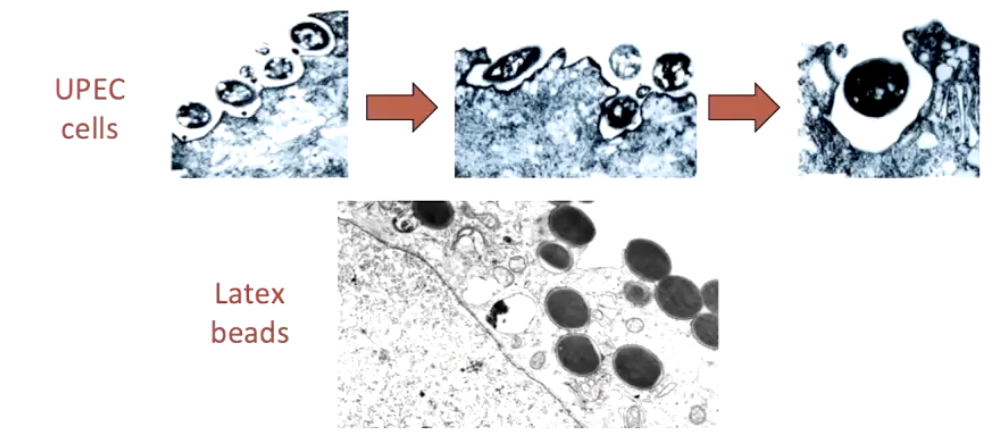
Is FimH sufficient for adherence and internalization?
Compare UPEC vs. FimH-coated latex beads
Visualized with electron microscopy
FimH enables adherence & internalization
Key in persistent & recurrent UTIs
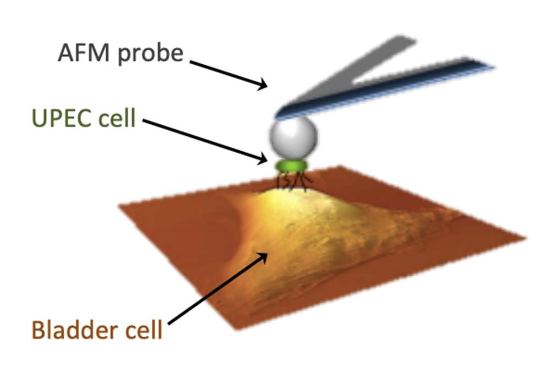
How does Single Cell Force Spectroscopy (SCFS) work?
Attach UPEC cell to AFM (atomic force microscopy) probe
Lower to contact target surface
Measure interaction forces between cell & host
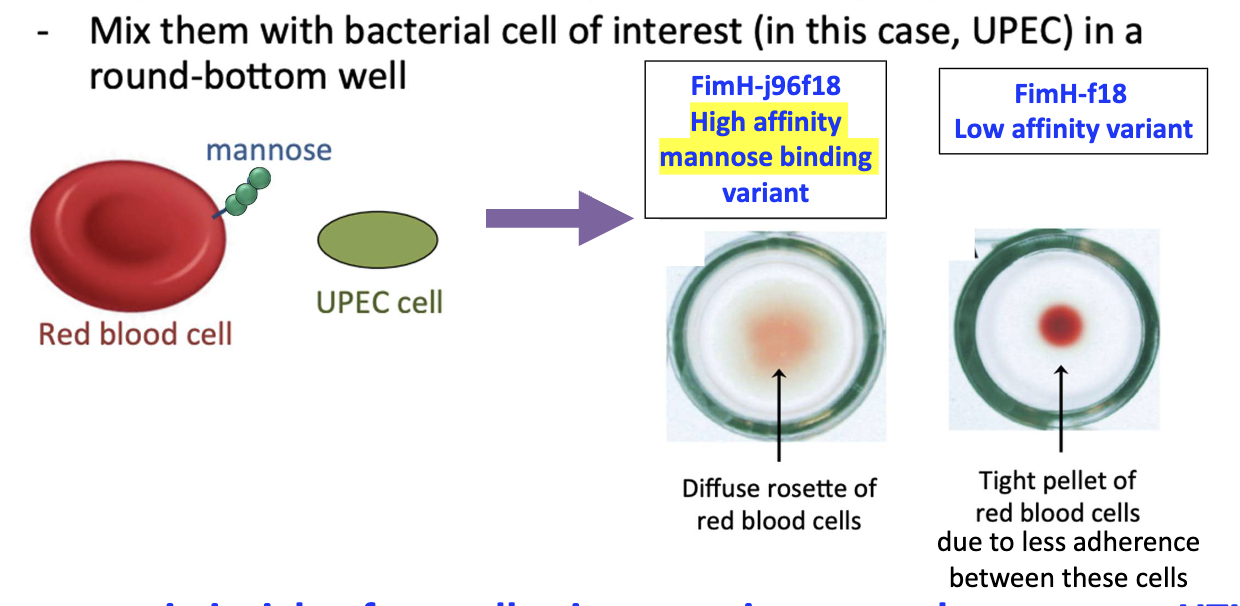
What is the Rosette Formation Assay?
RBCs engineered to express mannose
Mixed with UPEC in well
High-affinity mannose binding→ diffuse rosette of RBCs
Low-affinity mannose binding → tight pellet of RBCs due to less adherence
Used to compare adhesion strength
How are UTIs commonly treated and what does it cost?
Common treatment: antibiotics
~11 million U.S. cases/year
~$5 billion annually
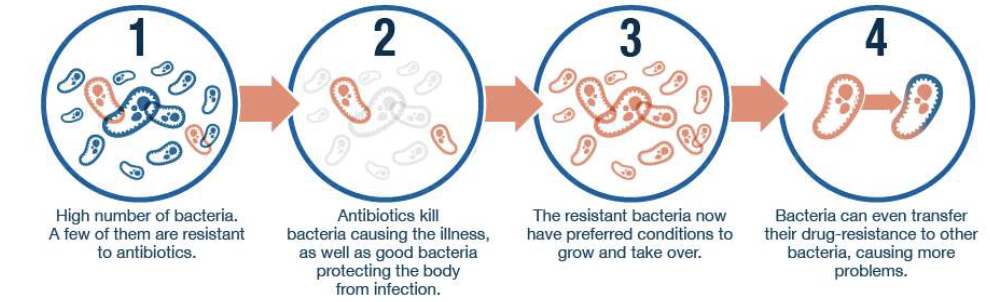
What are the main problems with antibiotic use for UTIs?
Antibiotic resistance → relapsing infections
Harms gut microbiome
Rising concern for multi-drug resistance
What happens if bacterial adhesion is blocked?
Bacteria washed out by urine flow
No attachment = no infection
Strategy: interrupt adhesion step
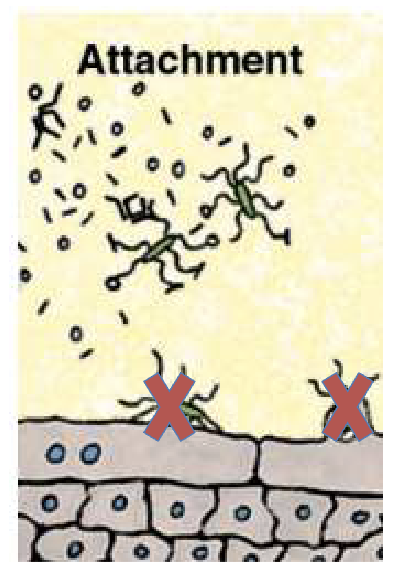
Can soap be used to disrupt adhesion in the urinary tract?
Soap disrupts adhesion externally
Not usable internally in urinary tract
Need safer, targeted alternatives

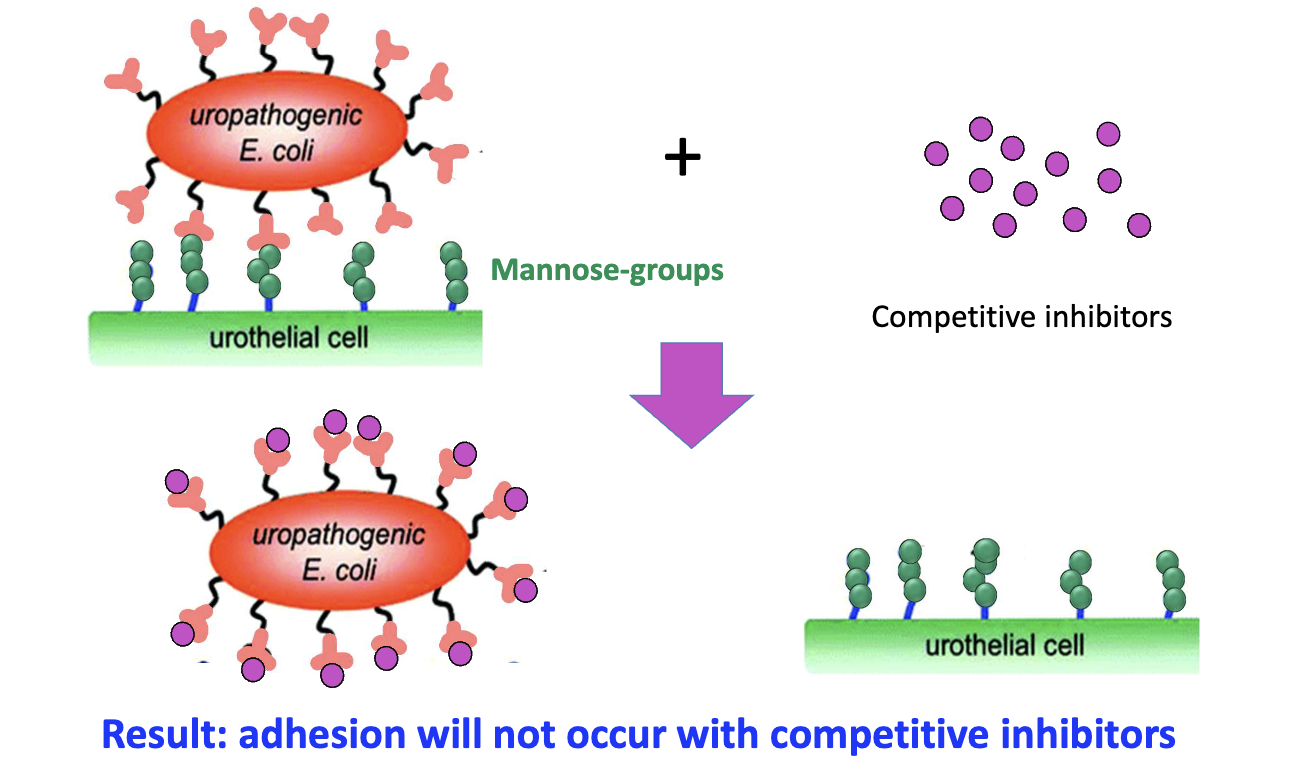
How do mannose analogs work as anti-adhesion drugs?
Mannose + competitive inhibitors bind FimH
Blocks E. coli binding
Prevents adhesion to host cells
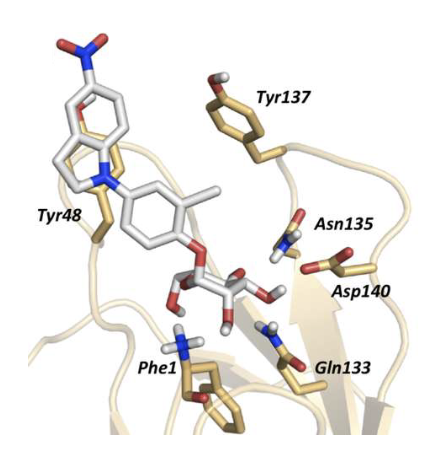
Why is FimH a strong target for drug design?
X-ray structures available
Enables rational drug design
Inhibitors = low molecular weight + high potency
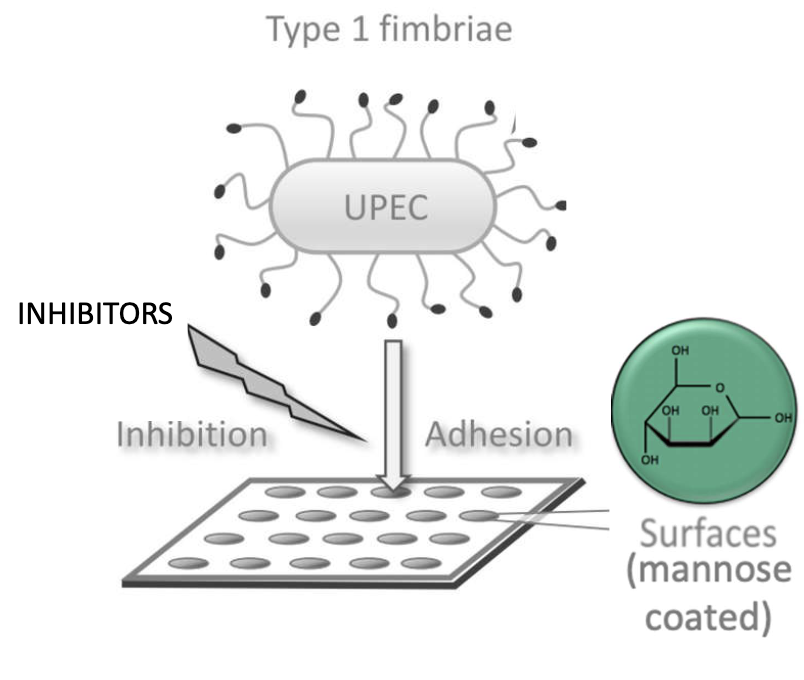
How are FimH inhibitors tested?
Coat plates with mannose
Incubate UPEC with inhibitors
Add to coated wells
Best inhibitors prevent bacterial binding
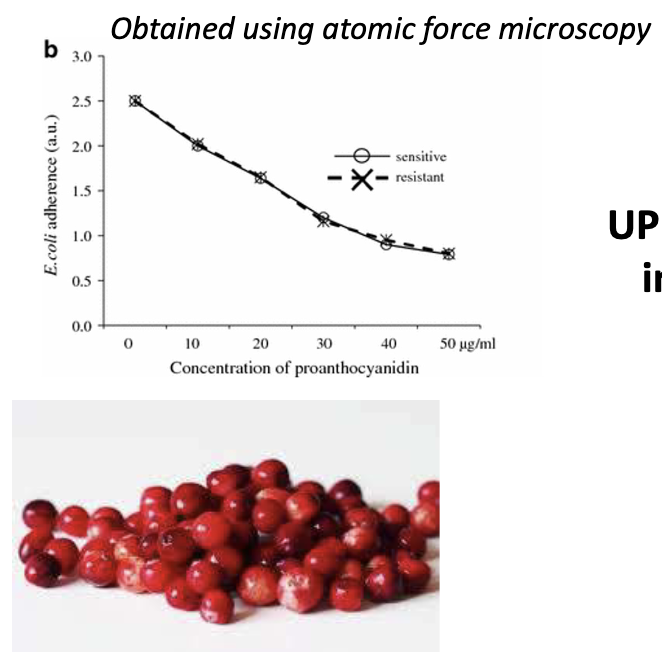
How do cranberries reduce UPEC adhesion?
Contain proanthocyanidins
Bind FimH → reduce adhesion
Graph: Dose-dependent effect shown by AFM
UPEC adherence decreases with increasing concentrations of proanthocyanidins
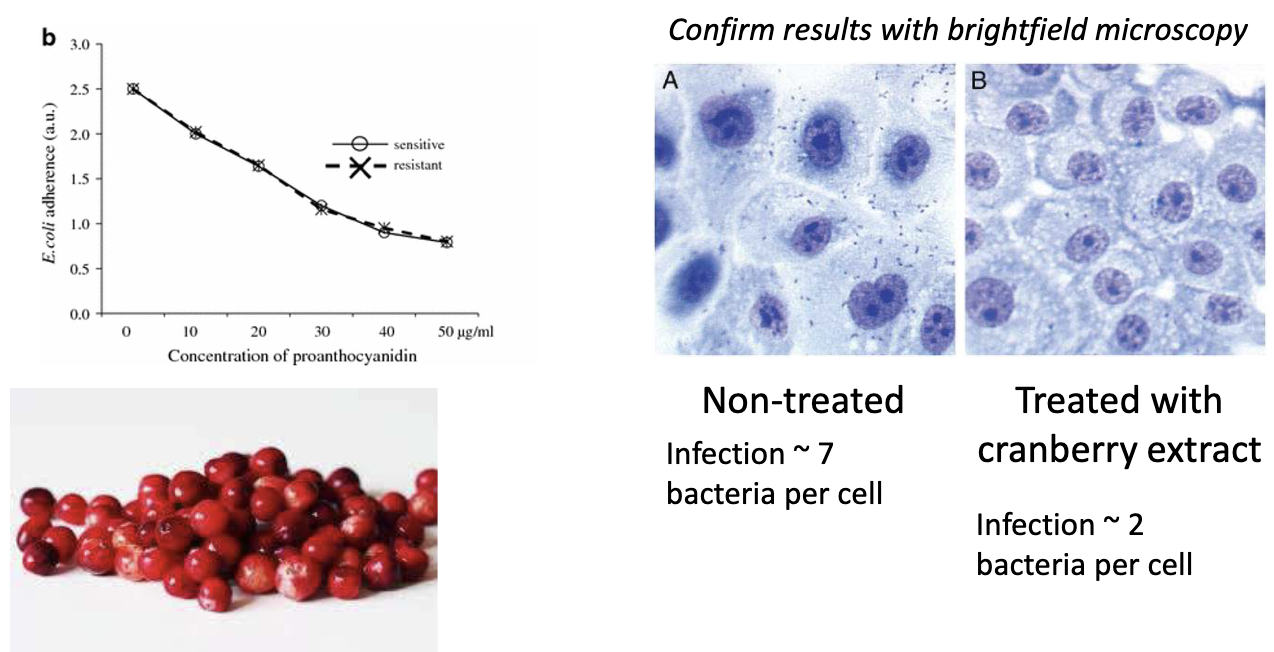
What is the evidence that cranberry extract works?
Non-treated: ~7 bacteria/cell
Treated: ~2 bacteria/cell
Confirmed with brightfield microscopy
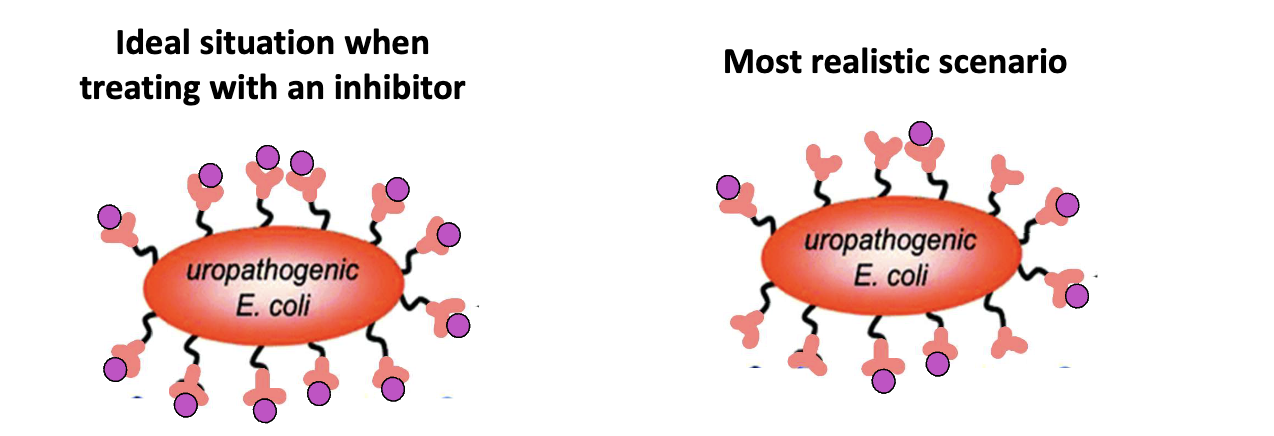
What are the challenges in blocking bacterial adhesion?
Hard to block all pili on E. coli
Bacteria have multiple adhesins
FimH inhibition alone may be insufficient
Future: adhesion inhibitor cocktails + antibiotics