Reactions in chemistry
1/176
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
177 Terms
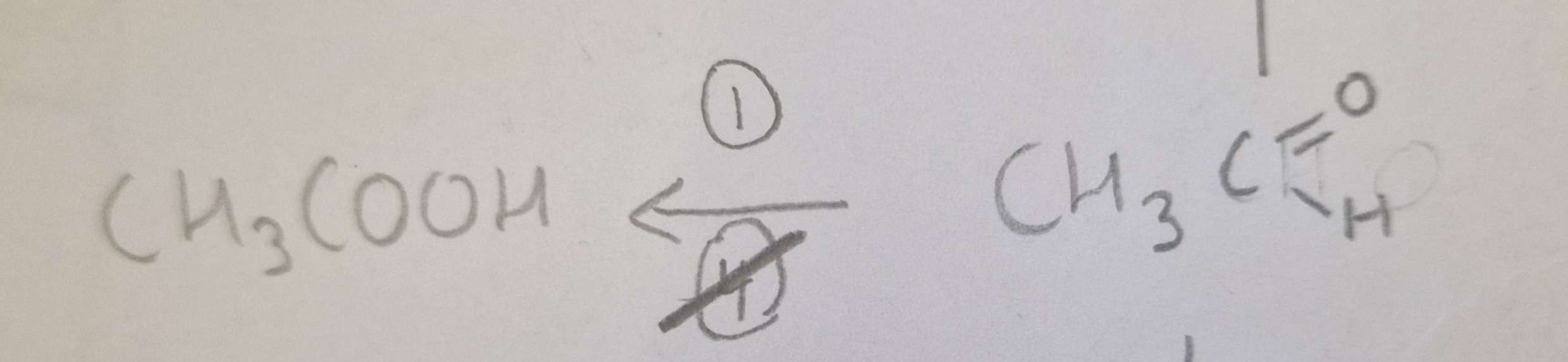
Aldehyde to Carboxylic Acid Type of Reaction:
Oxidation
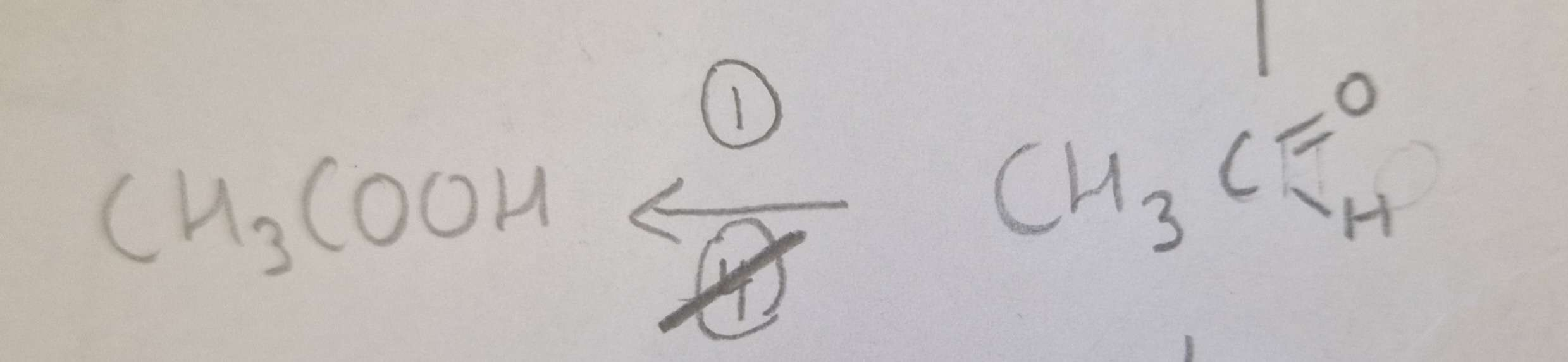
Aldehyde to Carboxylic Acid Mechanism:
None
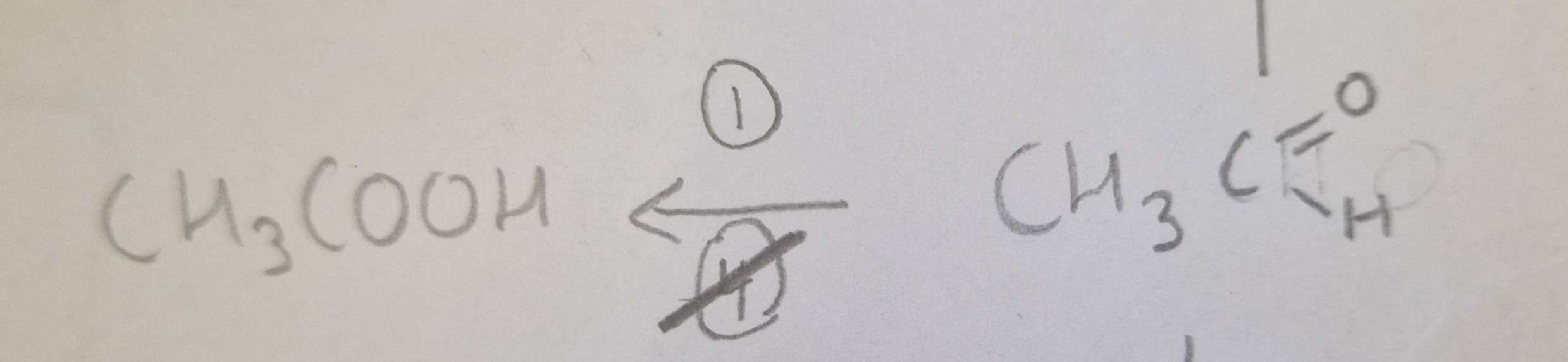
Aldehyde to Carboxylic Acid Reagents:
Kr2Cr2O7 + Dilute H2SO4
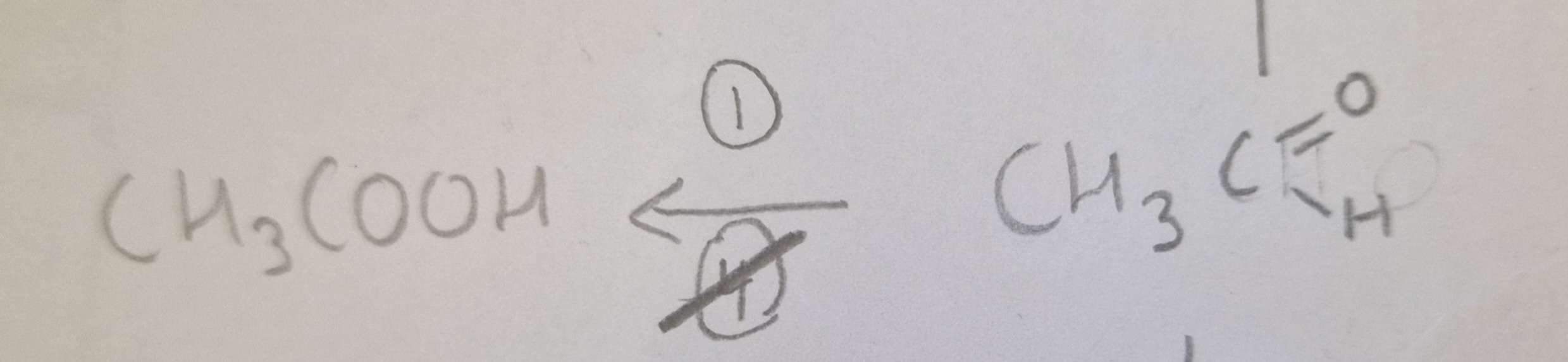
Aldehyde to Carboxylic Acid Conditions:
H.U.R or Tollens
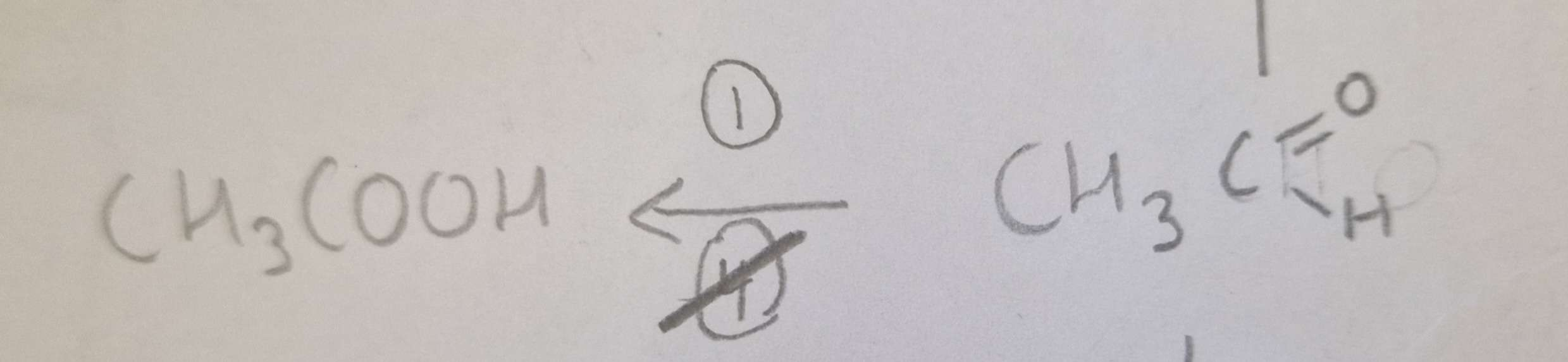
Aldehyde to Carboxylic Acid Name of Product:
Propanoic Acid
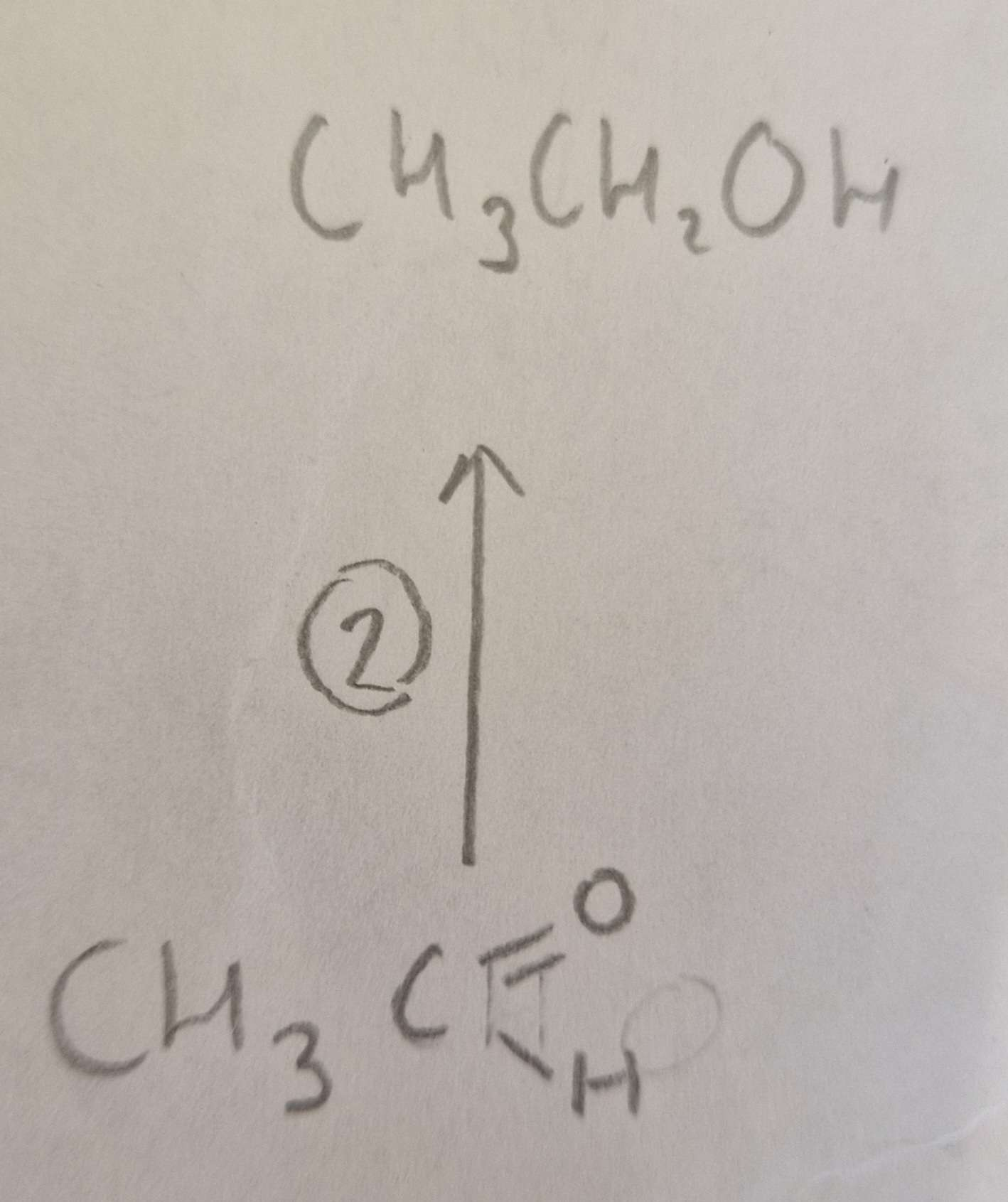
Aldehyde to Alcohol Type of Reaction:
Reduction
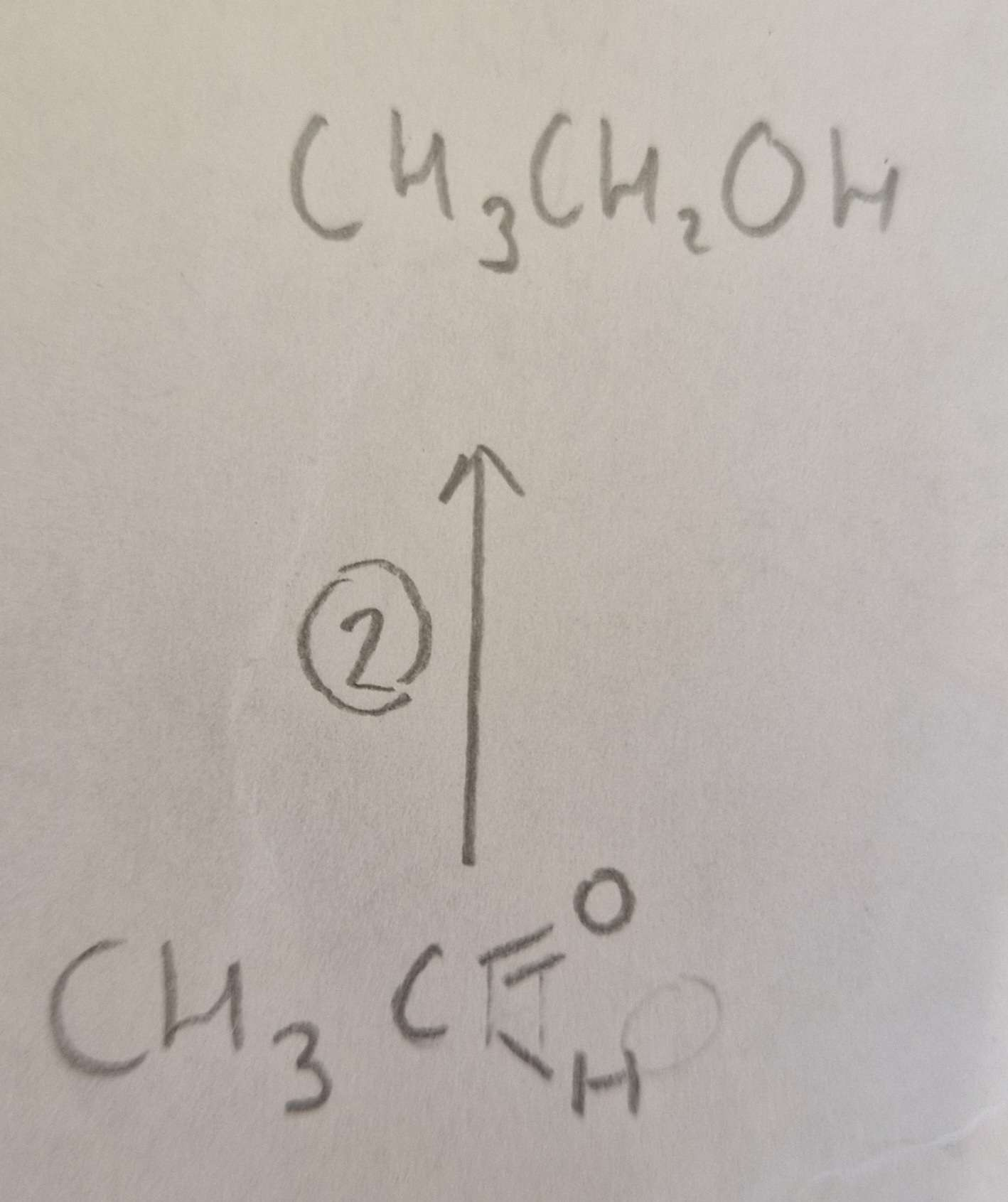
Aldehyde to Alcohol Mechanism:
None
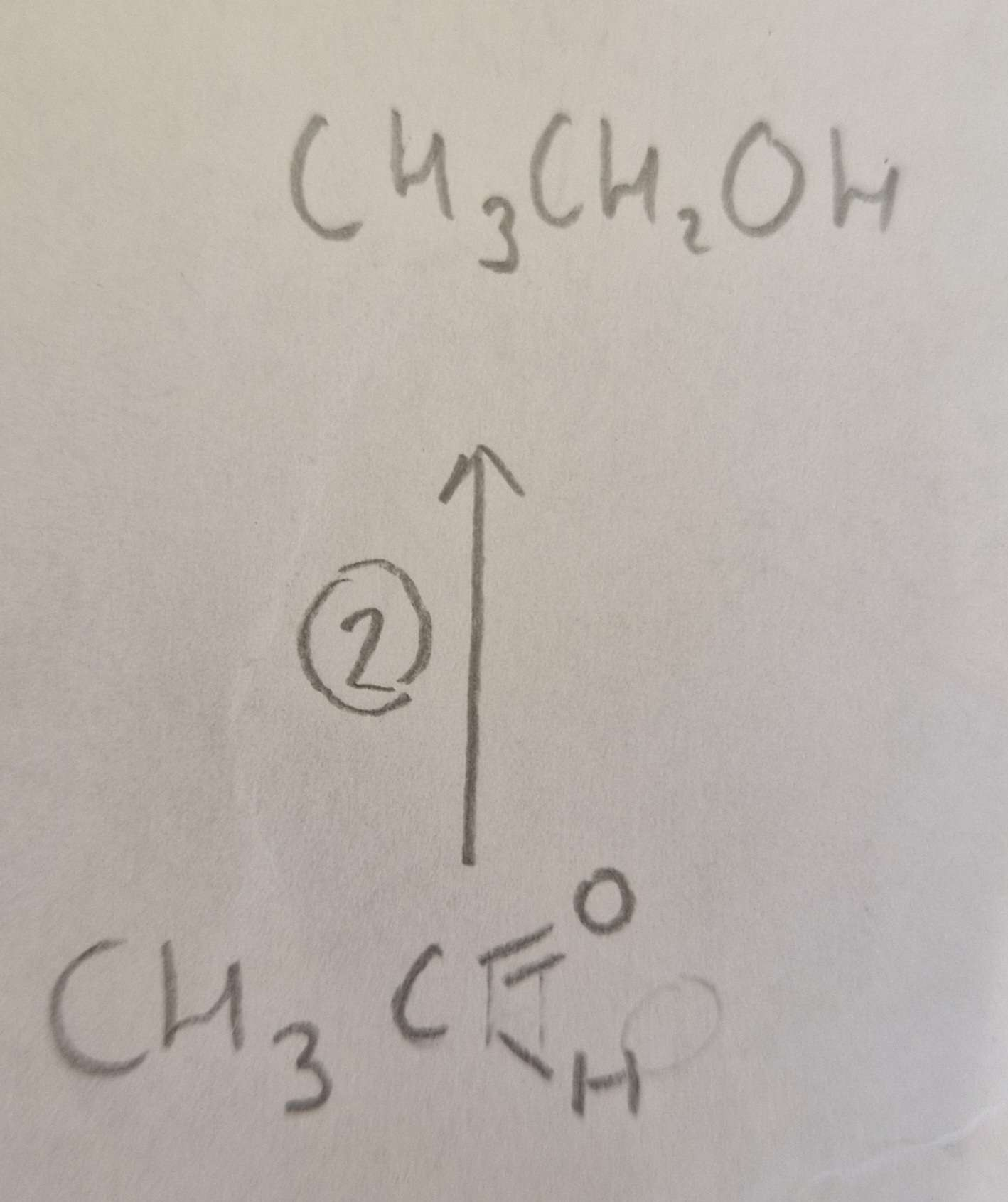
Aldehyde to Alcohol Reagents:
NaBH4 (aq)

Aldehyde to Alcohol Conditions:
H.U.R
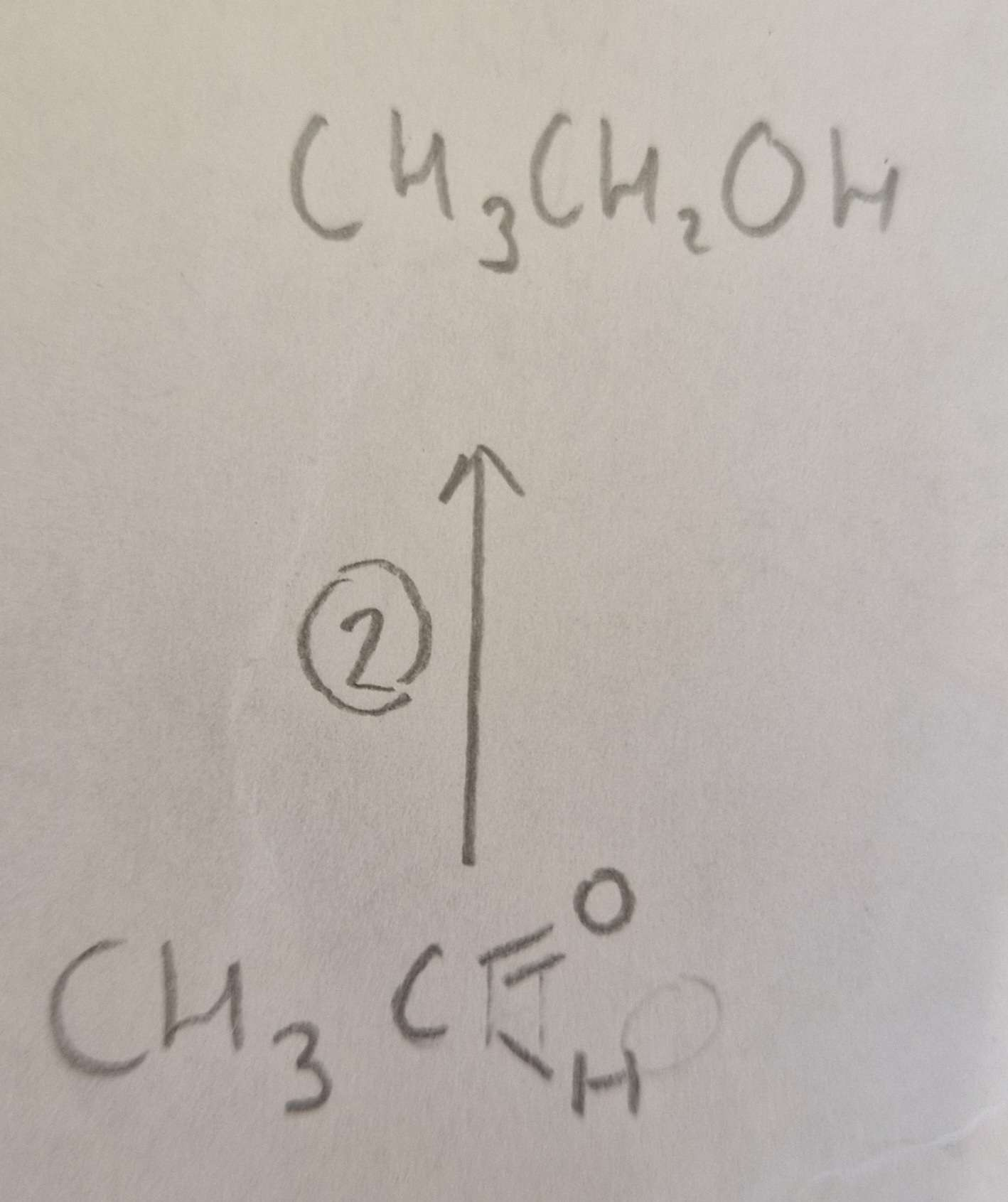
Aldehyde to Alcohol Name of Product:
Ethanol ( propan-2-ol otherwise)
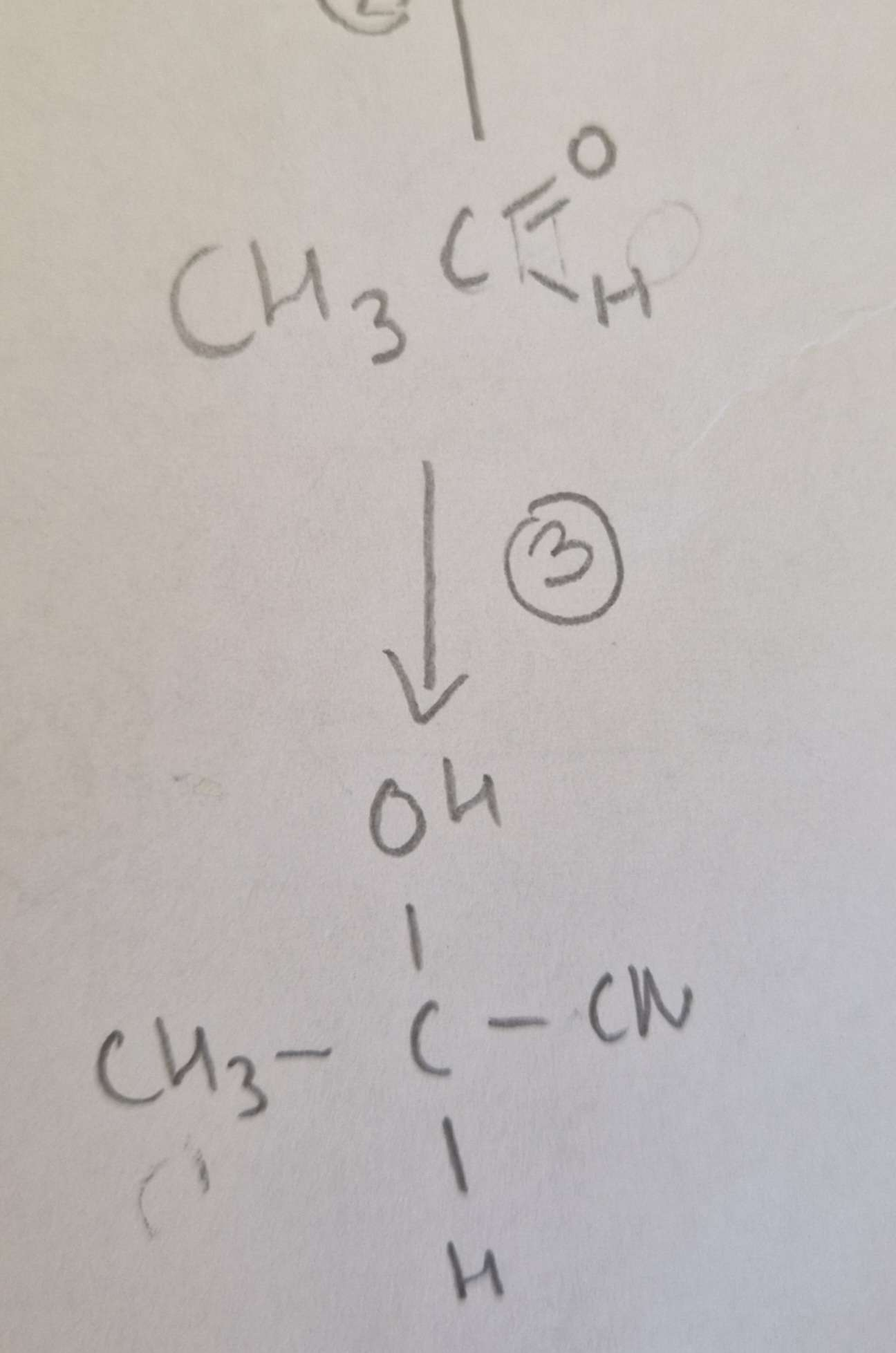
Aldehyde to Hydroxynitrile Type of Reaction:
Addition
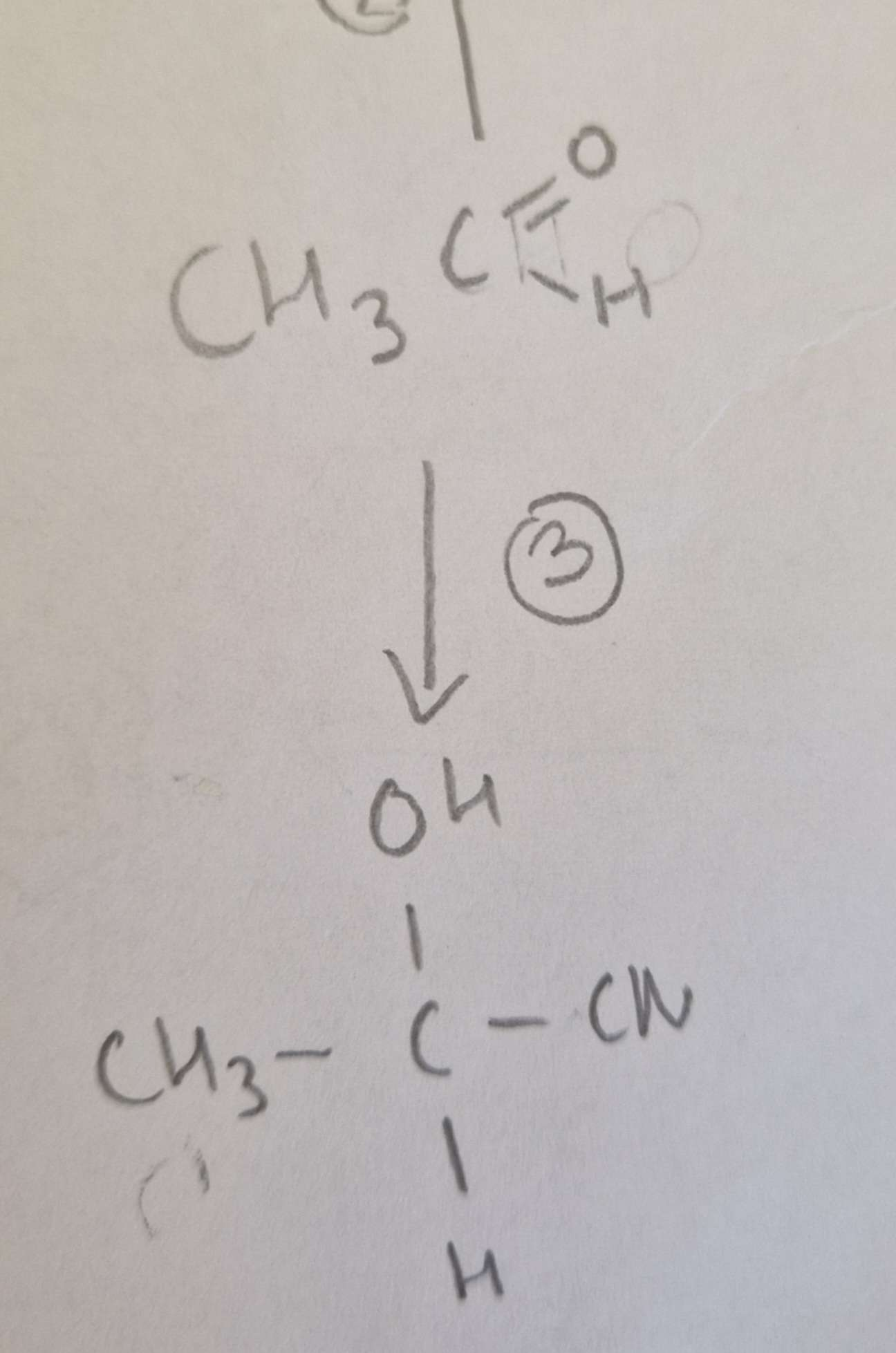
Aldehyde to Hydroxynitrile Mechanism:
Nucleophilic
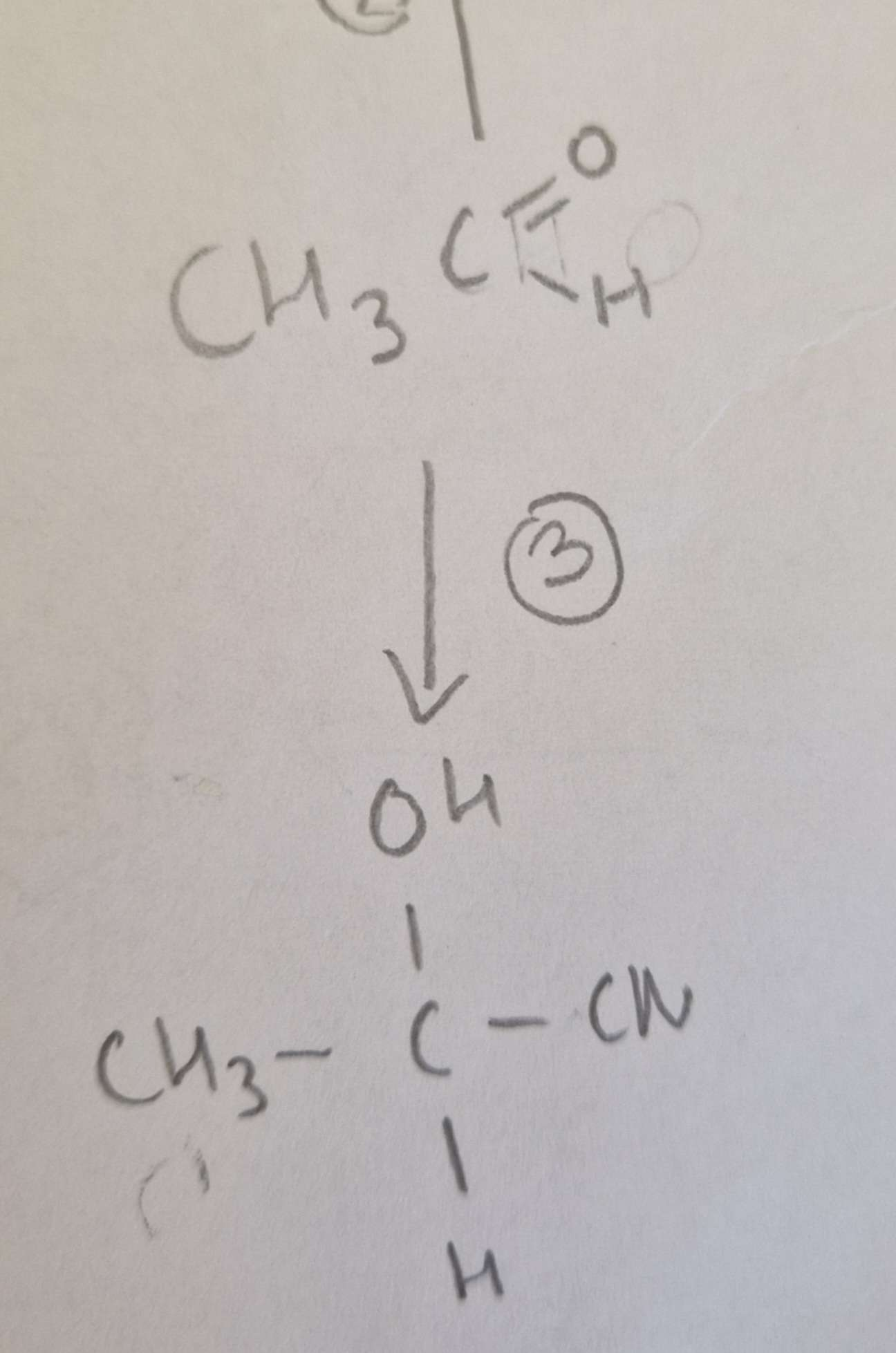
Aldehyde to Hydroxynitrile Reagents:
KCN + Dilute H2SO4
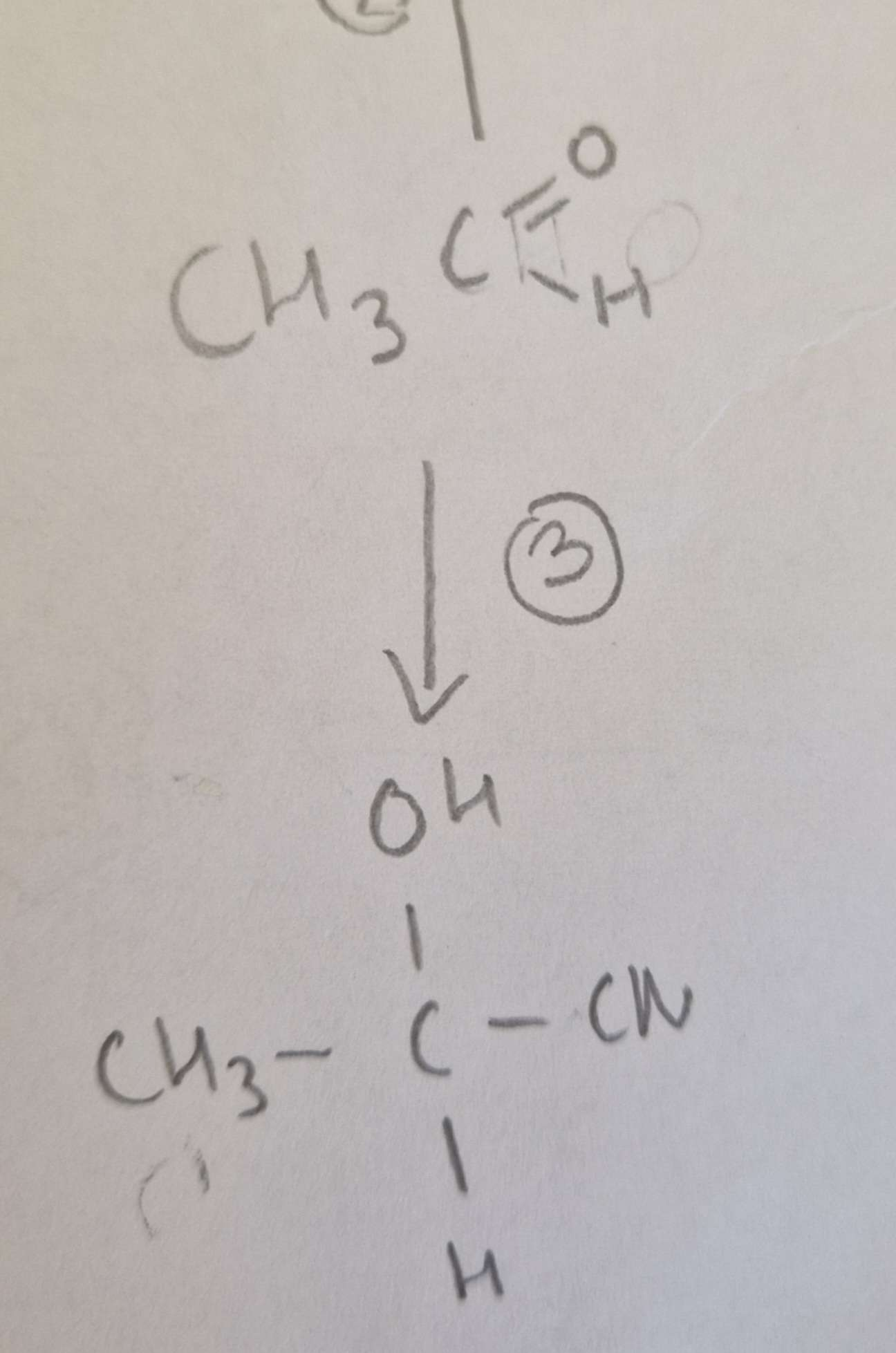
Aldehyde to Hydroxynitrile Conditions:
H.U.R
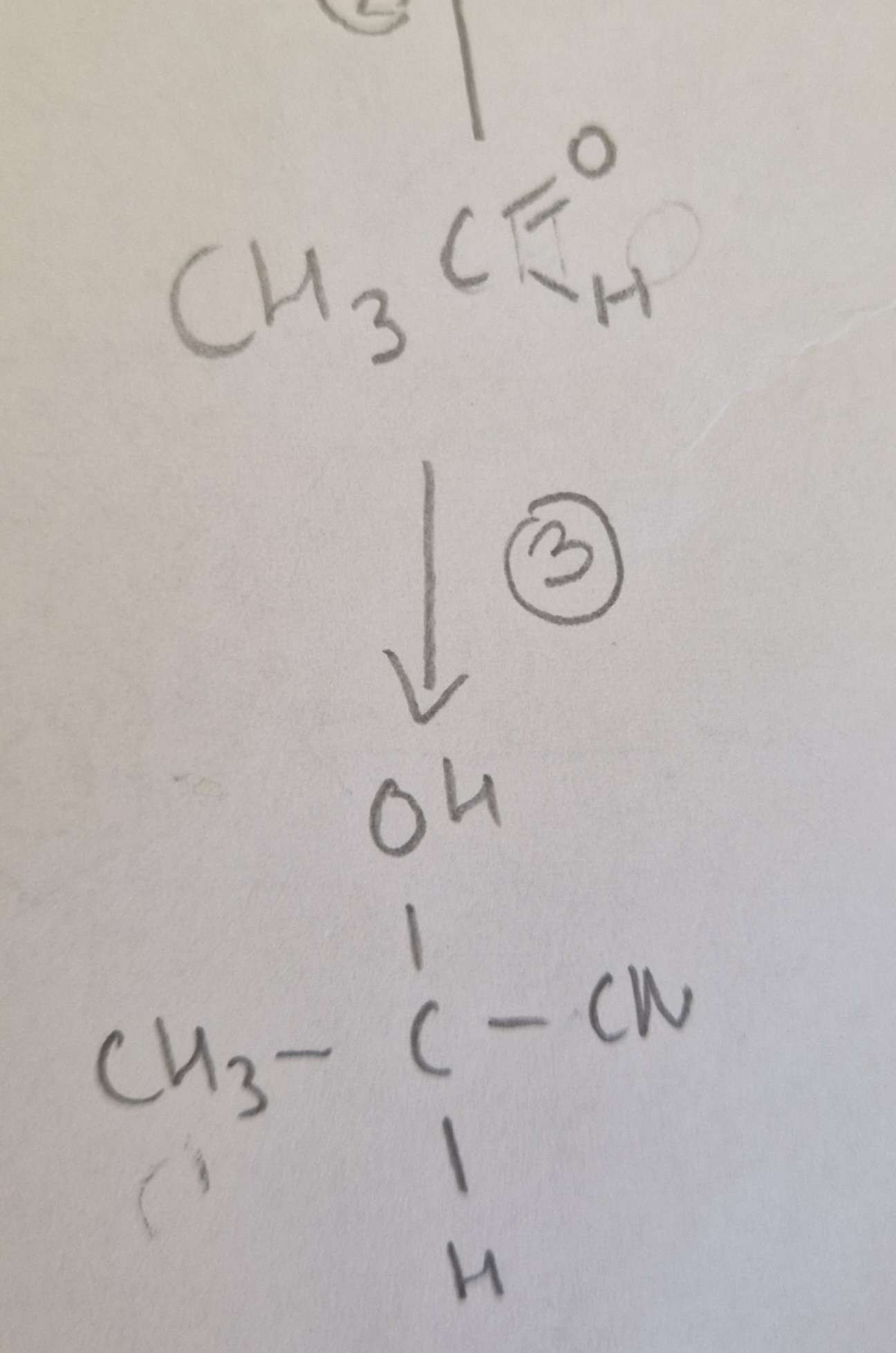
Aldehyde to Hydroxynitrile Name of Product:
2-Hydroxypropanenitrile
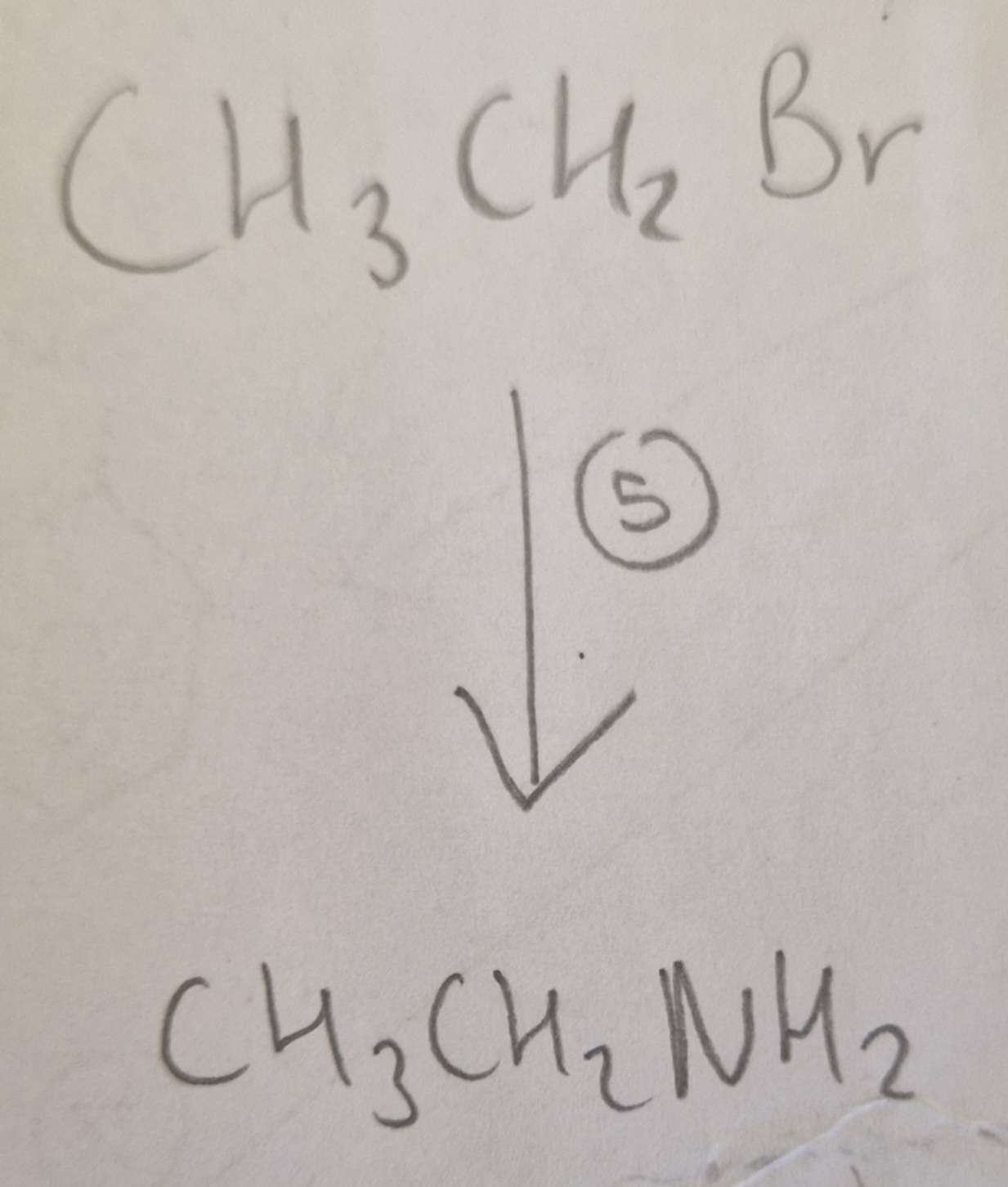
Haloalkane to Amine Type of Reaction:
Subsitution
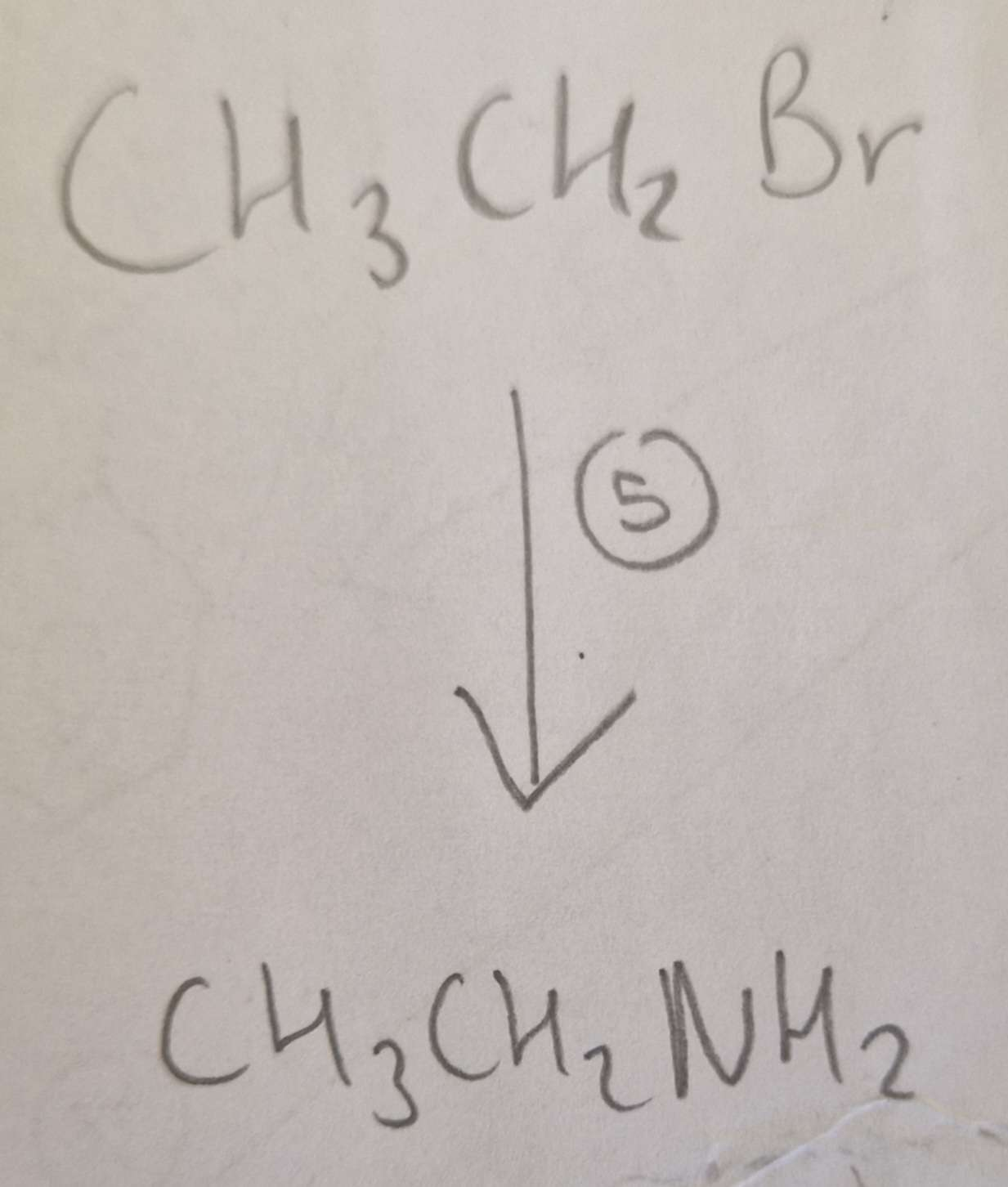
Haloalkane to Amine Mechanism:
Nucleophilic
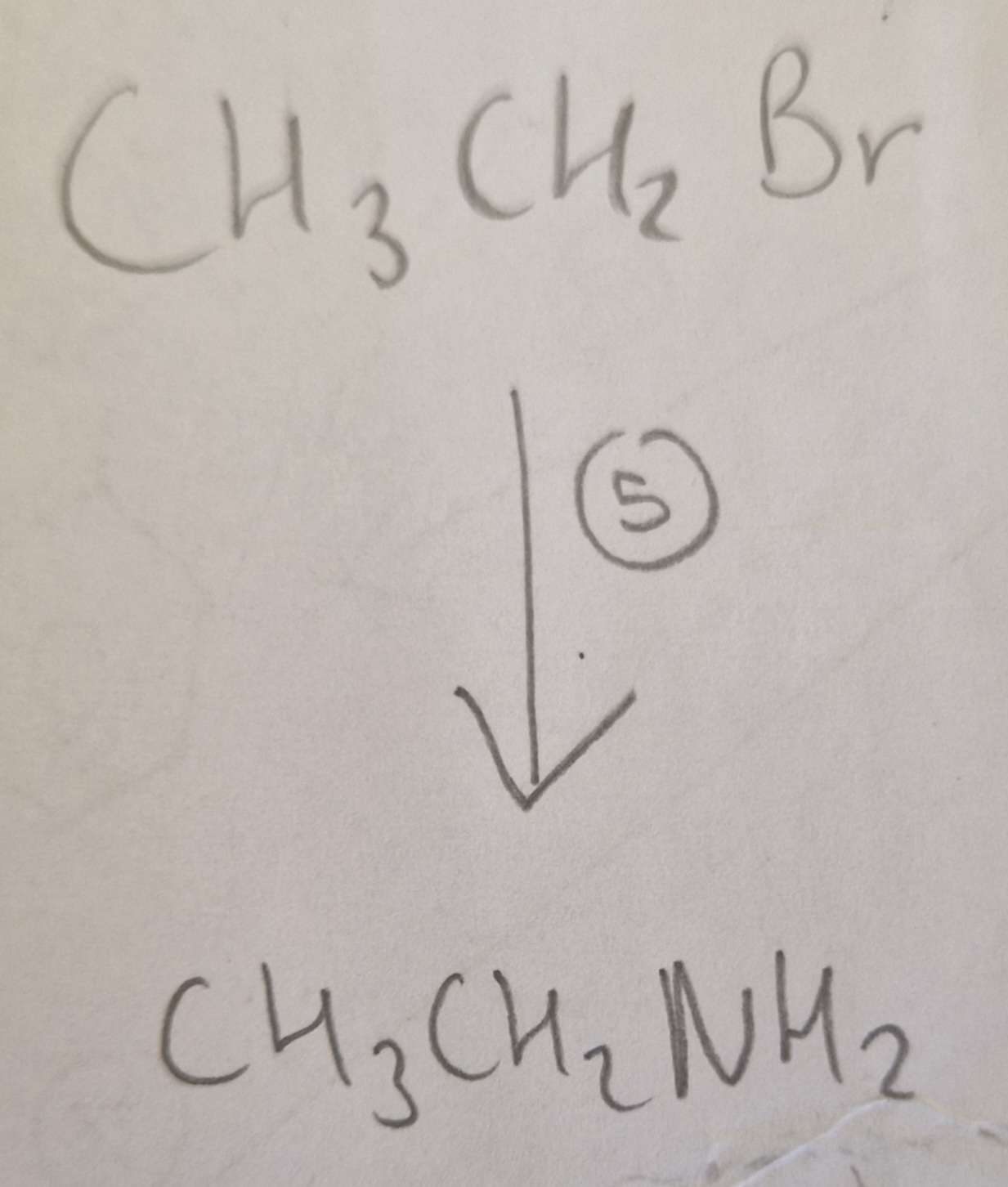
Haloalkane to Amine Reagents:
NH3 (CH3CH2Br afterwards aswell)
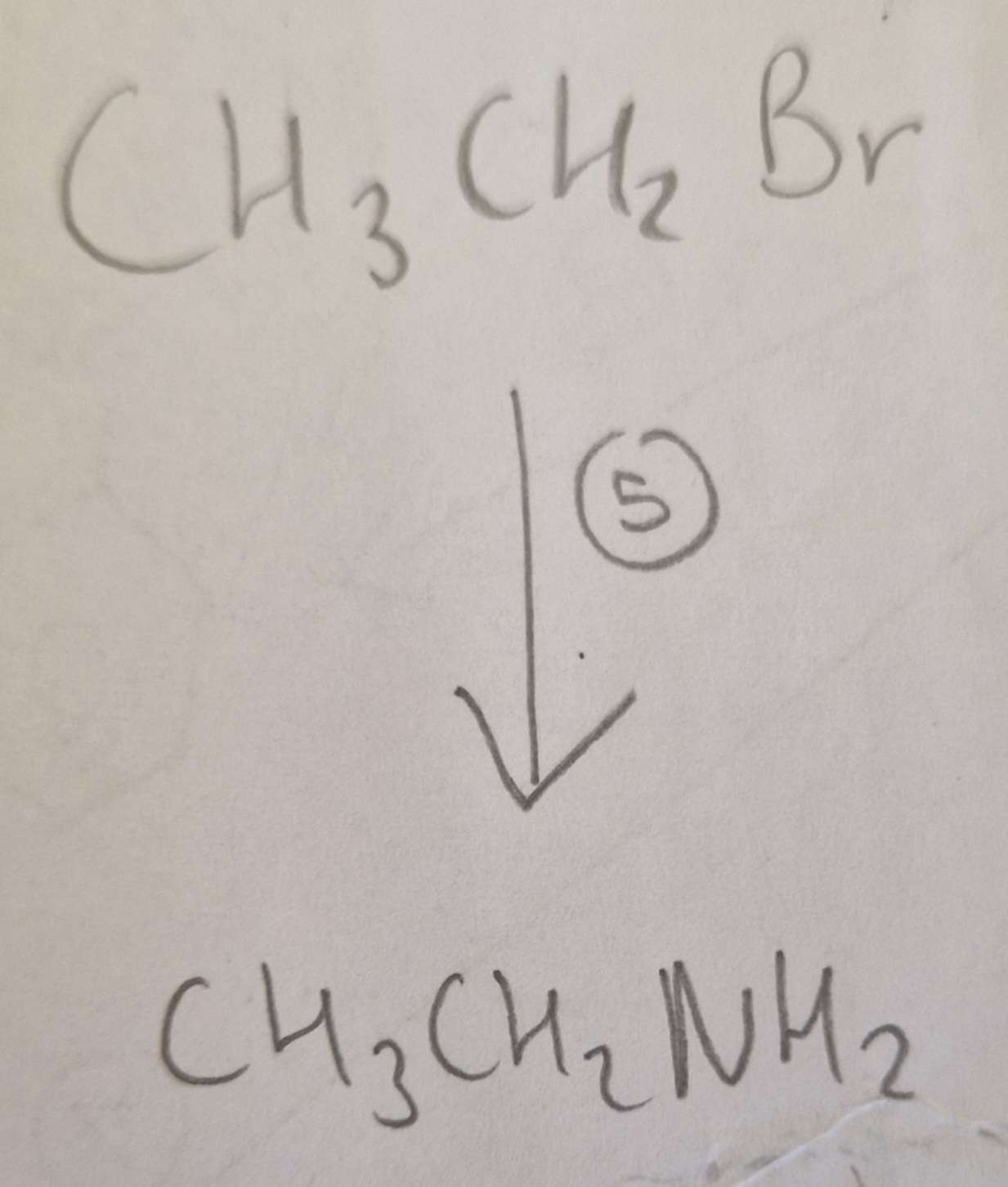
Haloalkane to Amine Conditions:
Ethanol, H.U.R
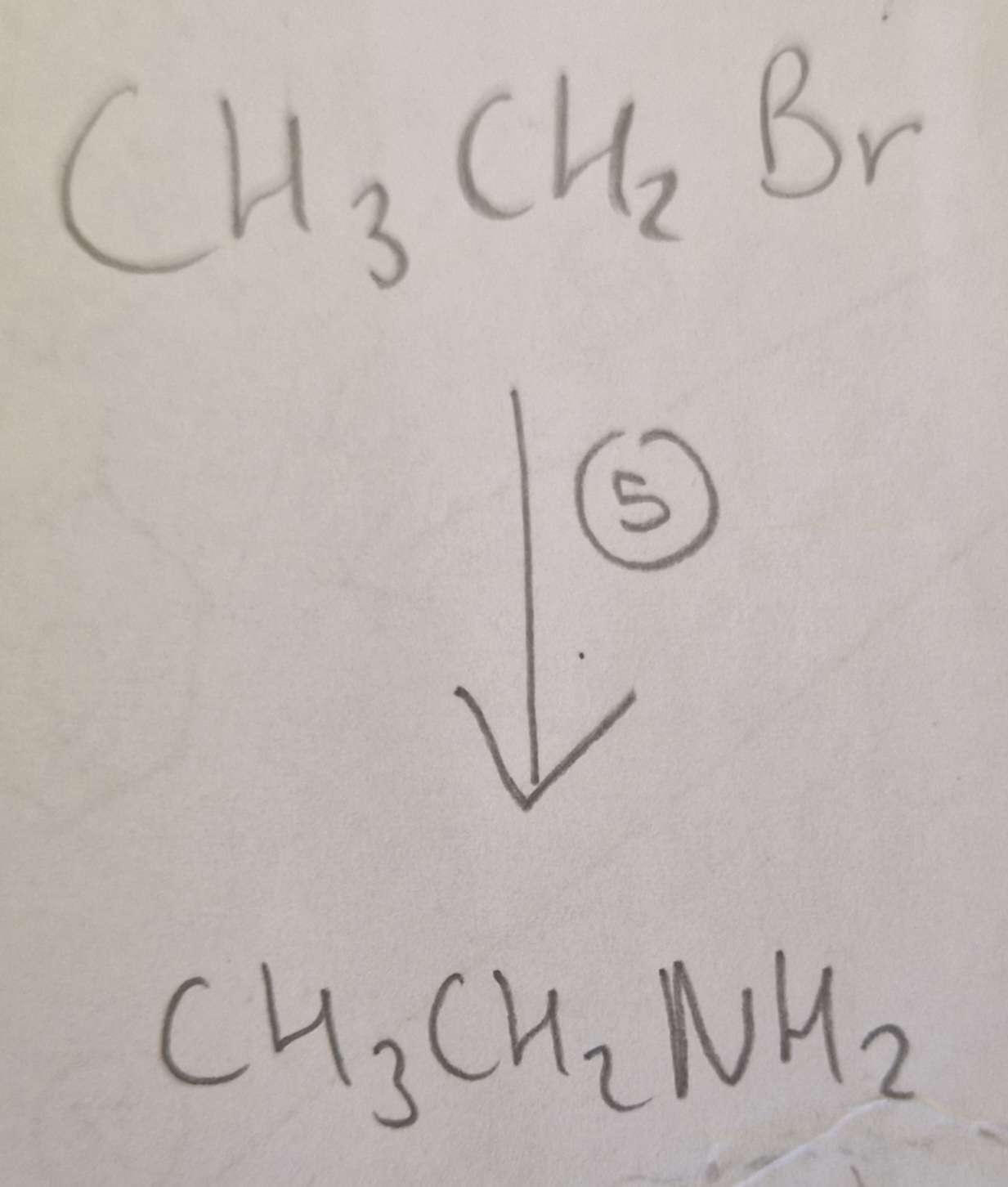
Haloalkane to Amine Name of Product:
Ethylamine
Haloalkane to Nitrile Type of Reaction:
Subsitution
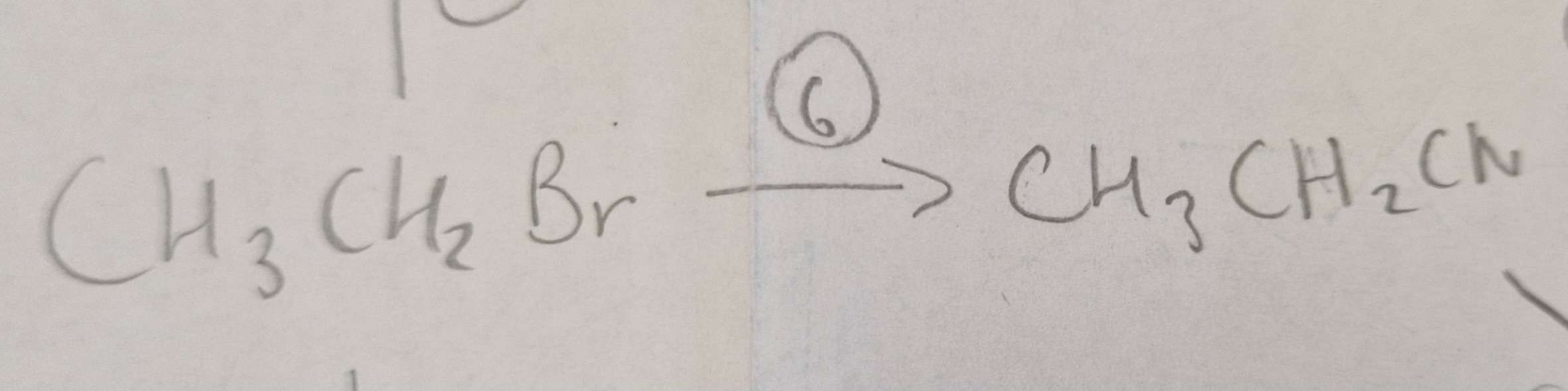
Haloalkane to Nitrile Mechanism:
Nucleophilic
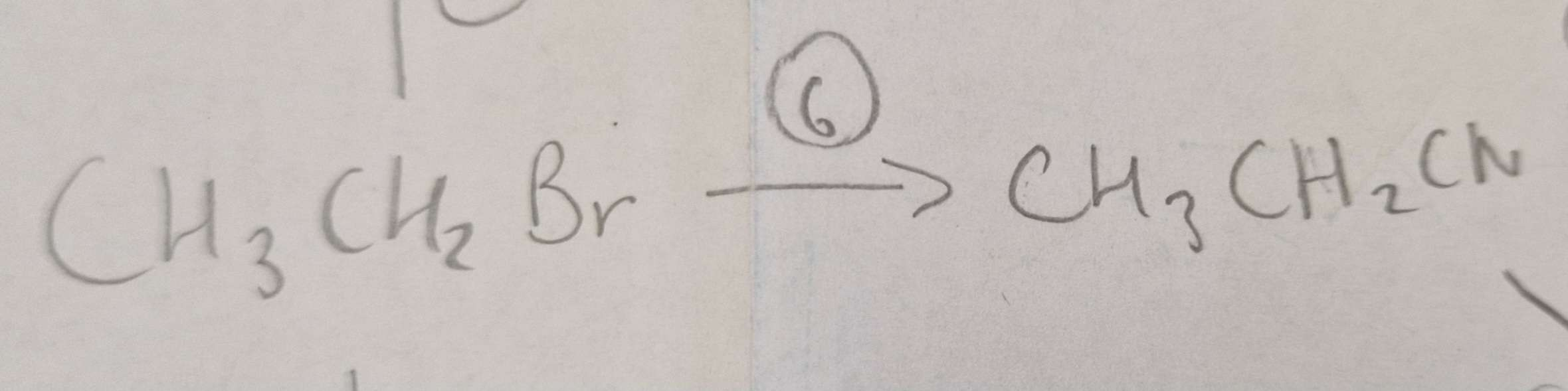
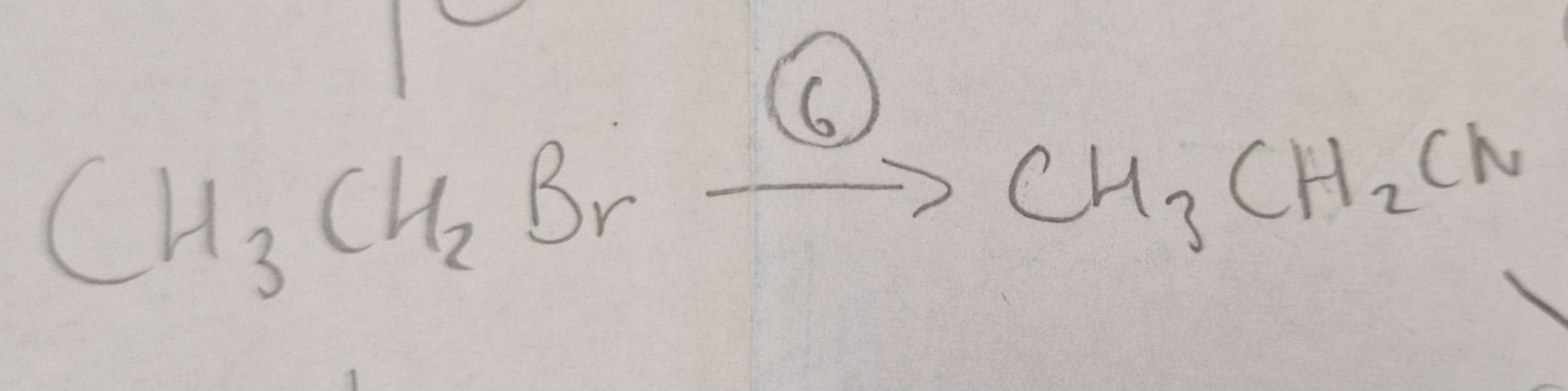
Haloalkane to Nitrile Reagents:
KCN (conditions in ethanol as a solvent, bc haloaklanes dont mix well with water)
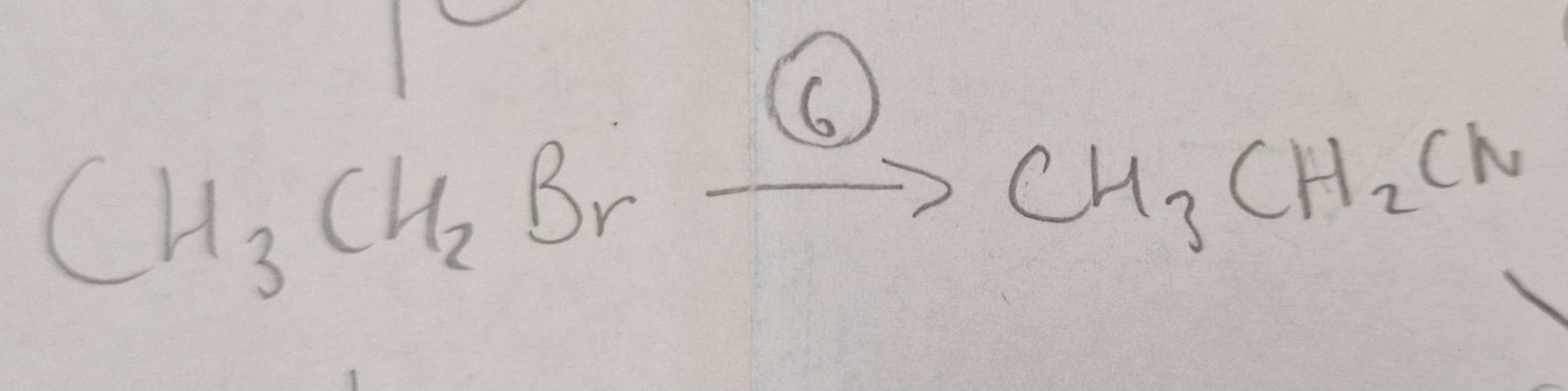
Haloalkane to Nitrile Conditions:
Propanone Solvent, H.U.R
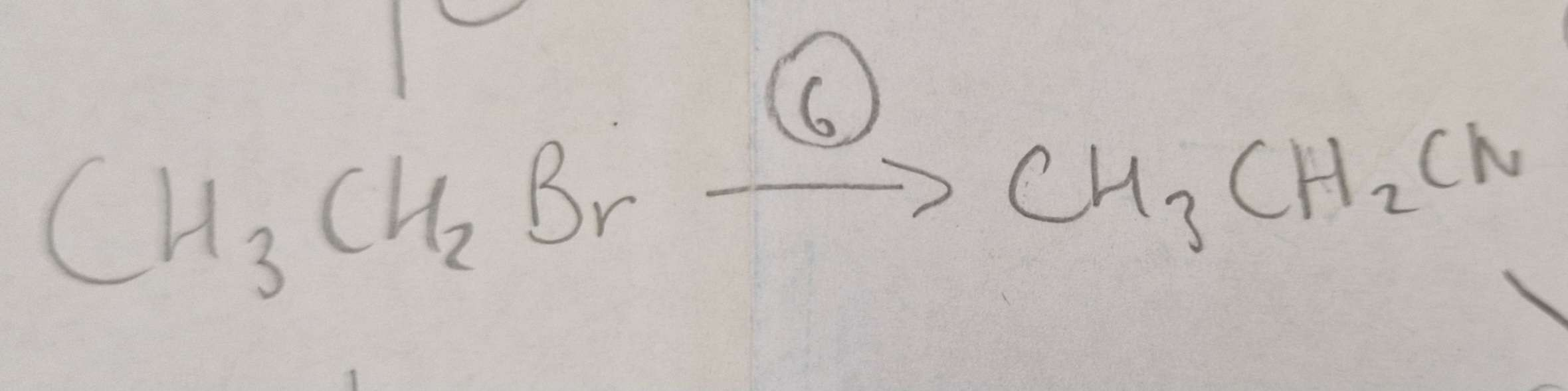
Haloalkane to Nitrile Name of Product:
Propanenitrile
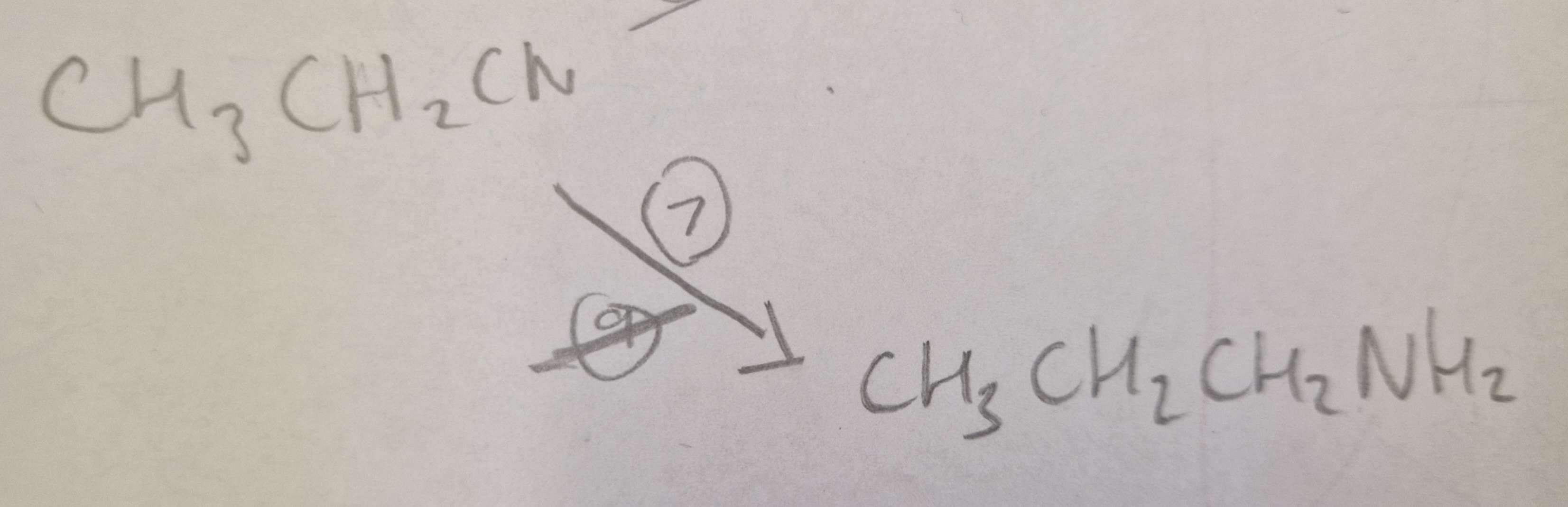
Nitrile to Amine Type of Reaction:
Reduction
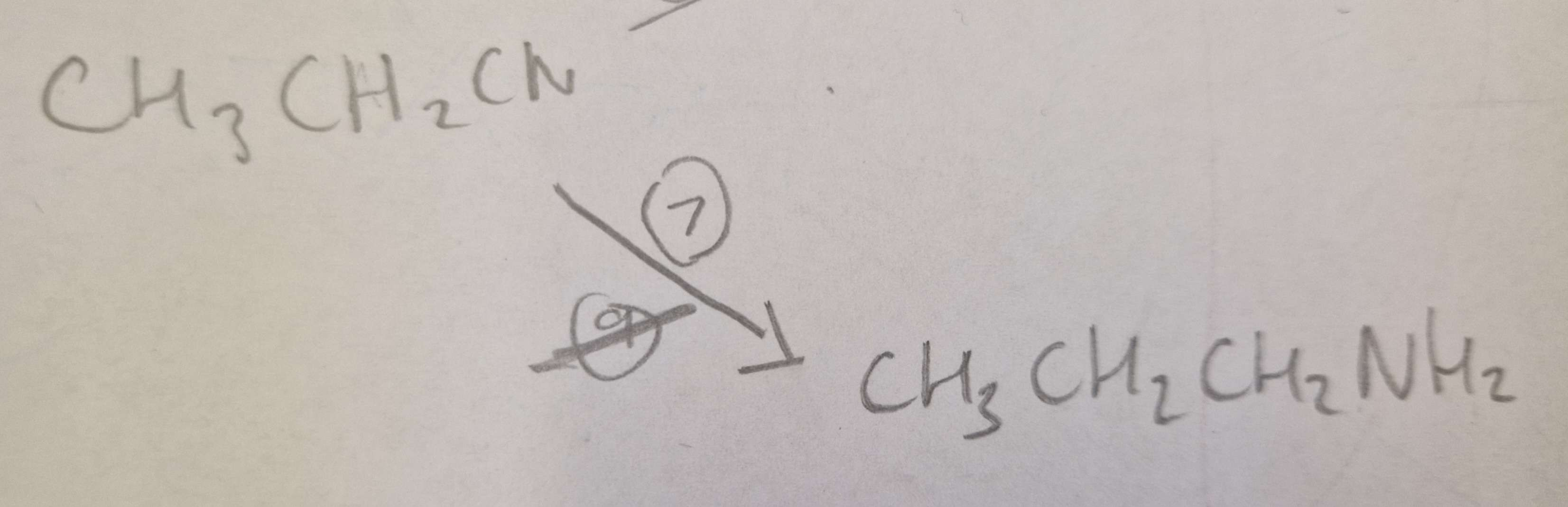
Nitrile to Amine Mechanism:
None
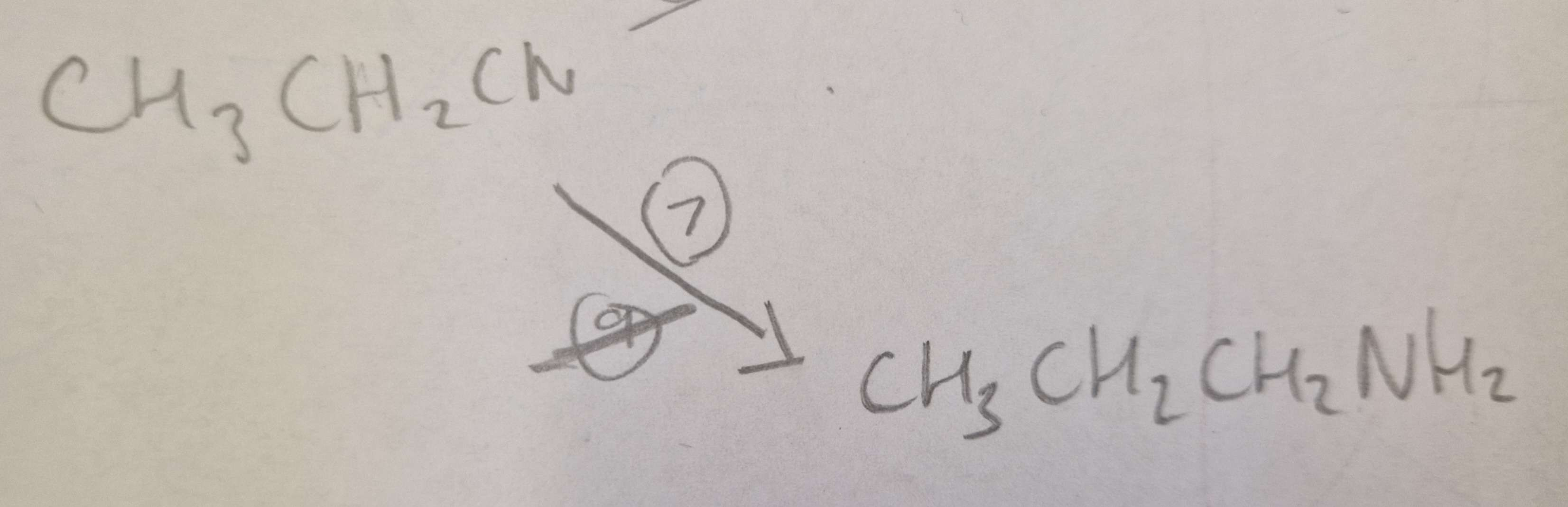
Nitrile to Amine Reagents:
LiAlH4 (Lithium Aluminium Hydride)
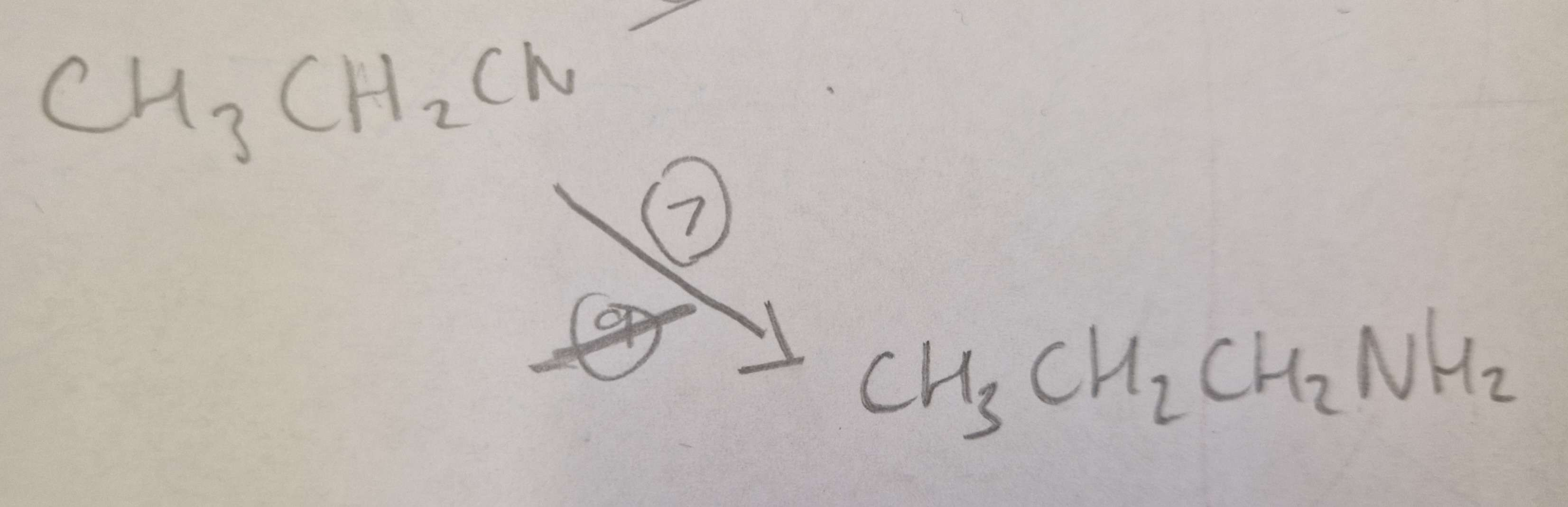
Nitrile to Amine Conditions:
in Ether, H.U.R
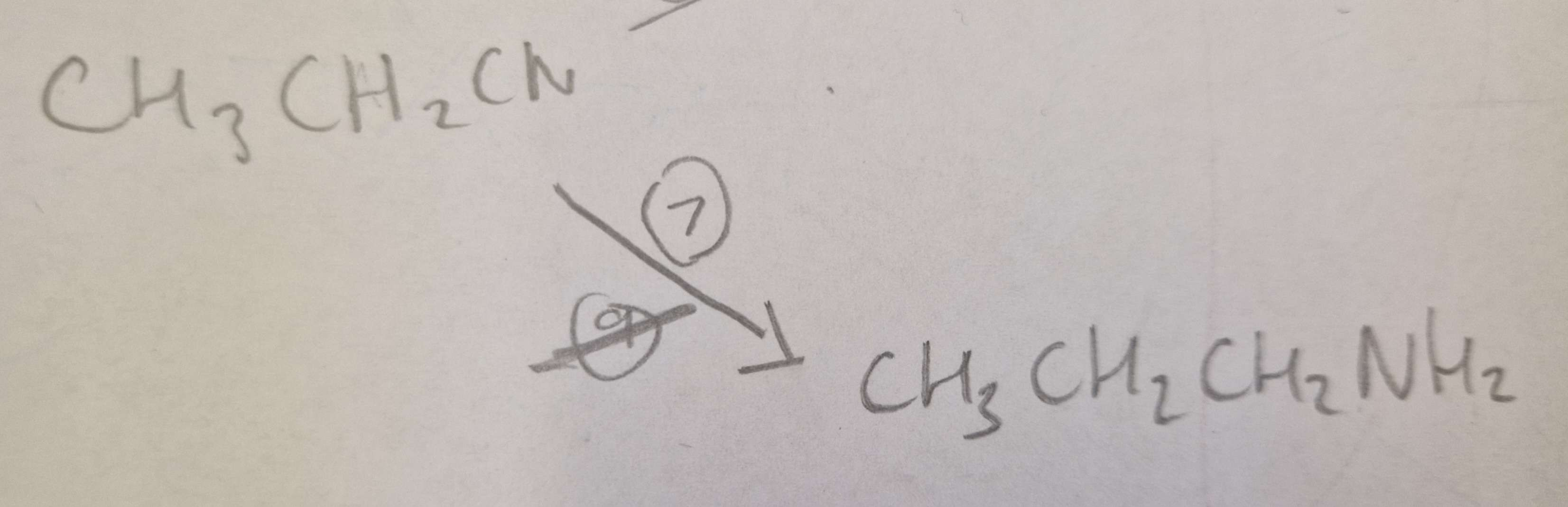
Nitrile to Amine Name of Product:
Propylamine
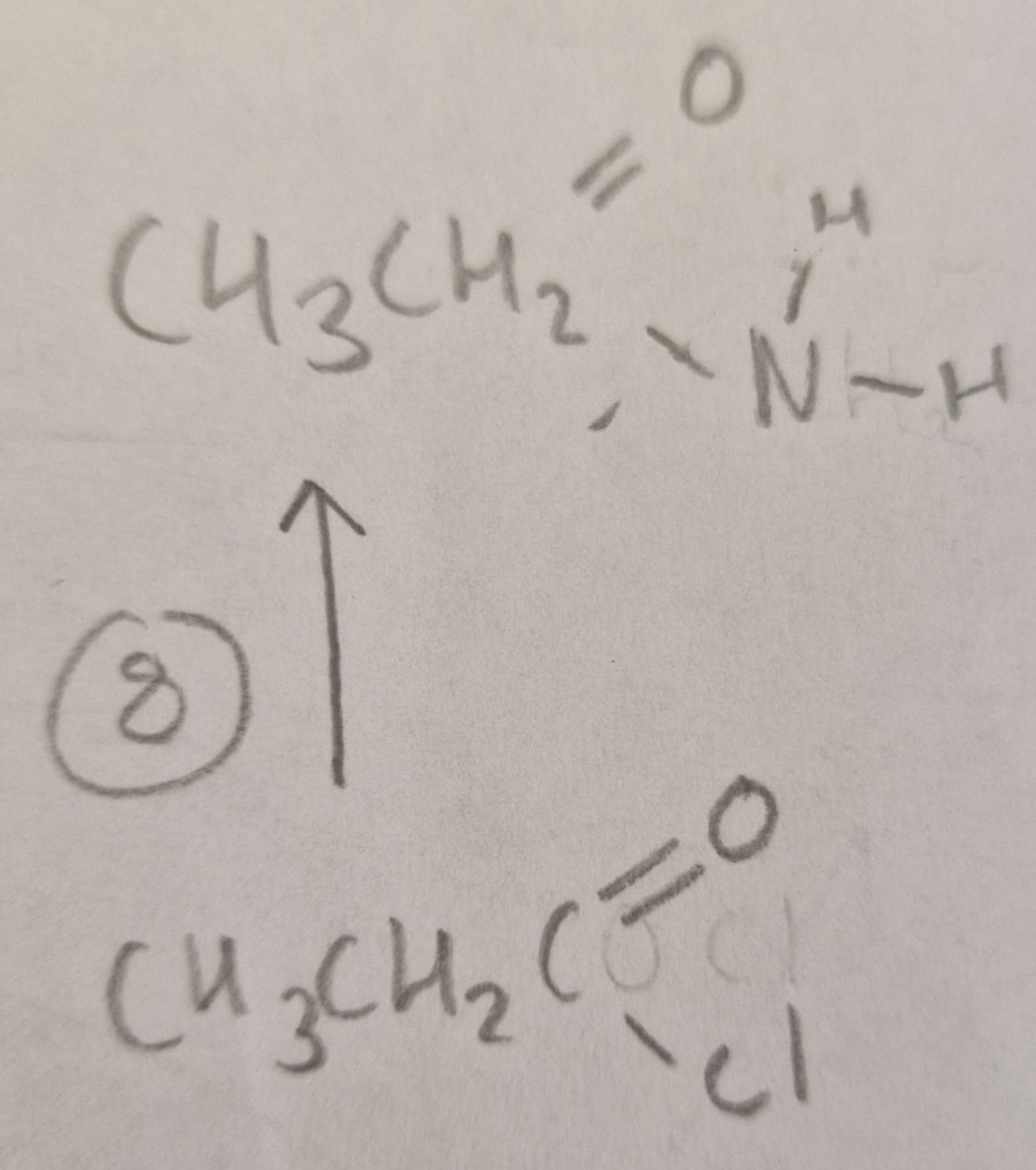
Acyl Chloride to Acid Amide Type of Reaction:
Subsitution
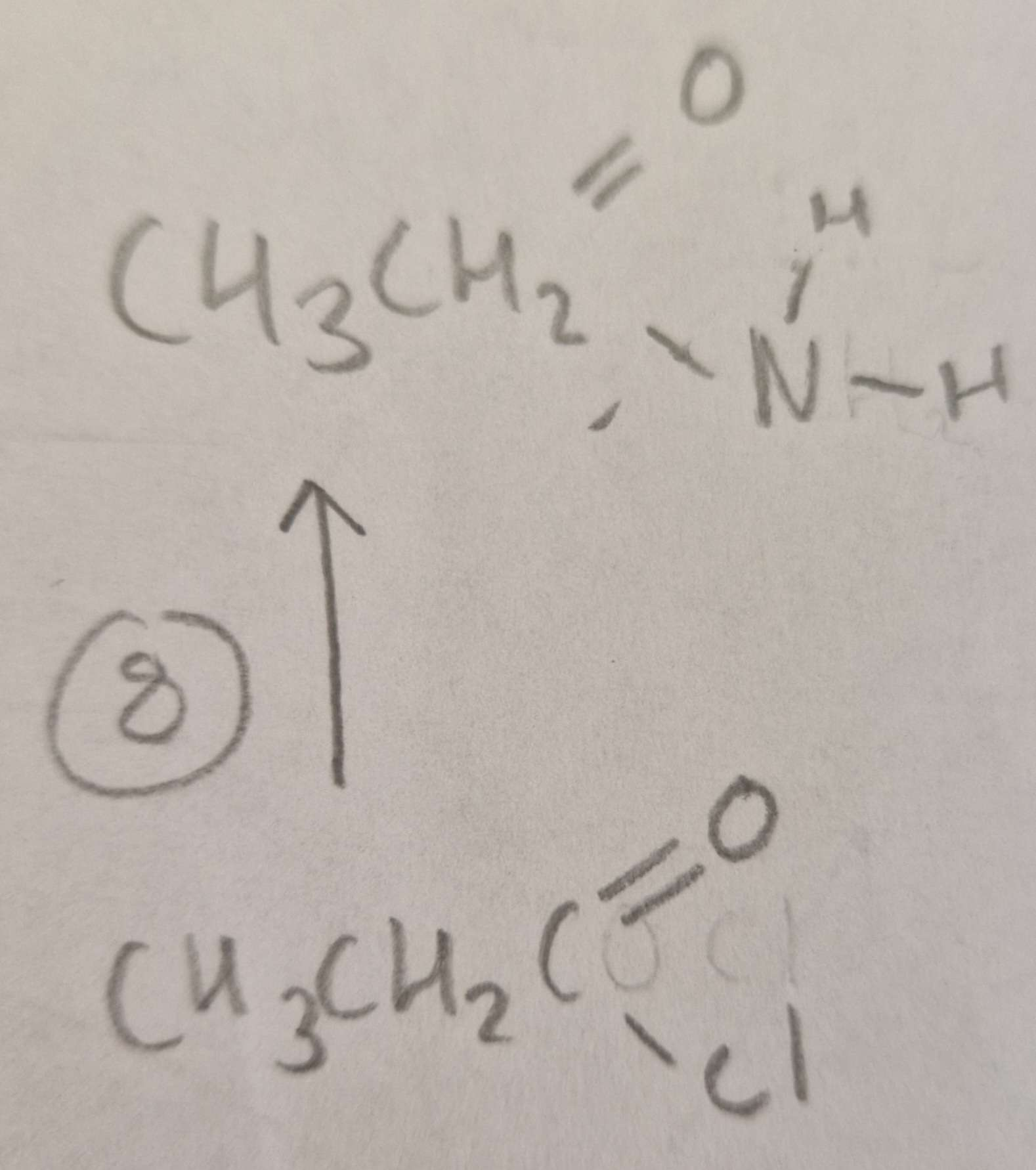
Acyl Chloride to Acid Amide Mechanism:
Nucleophilic
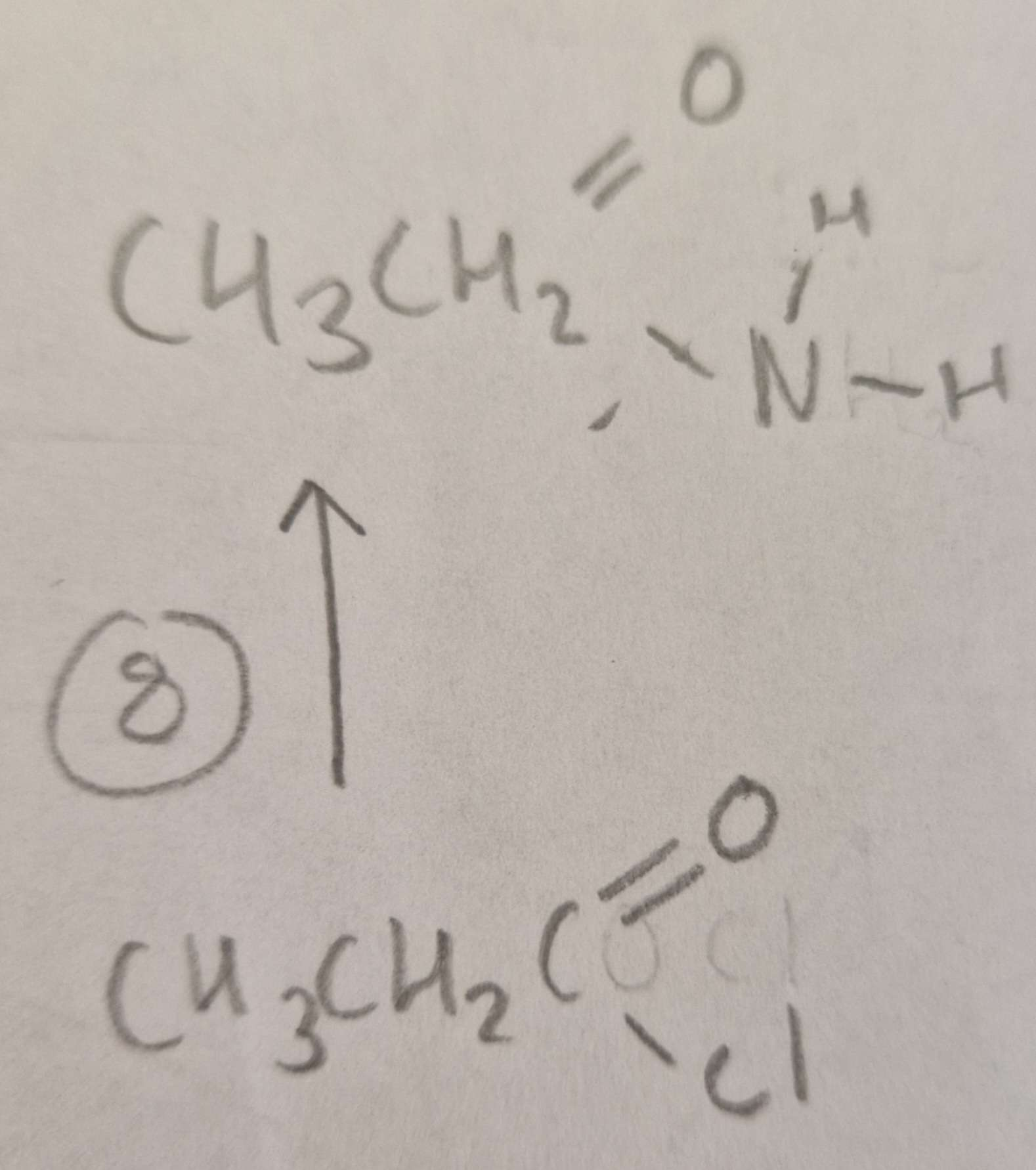
Acyl Chloride to Acid Amide Reagents:
NH3
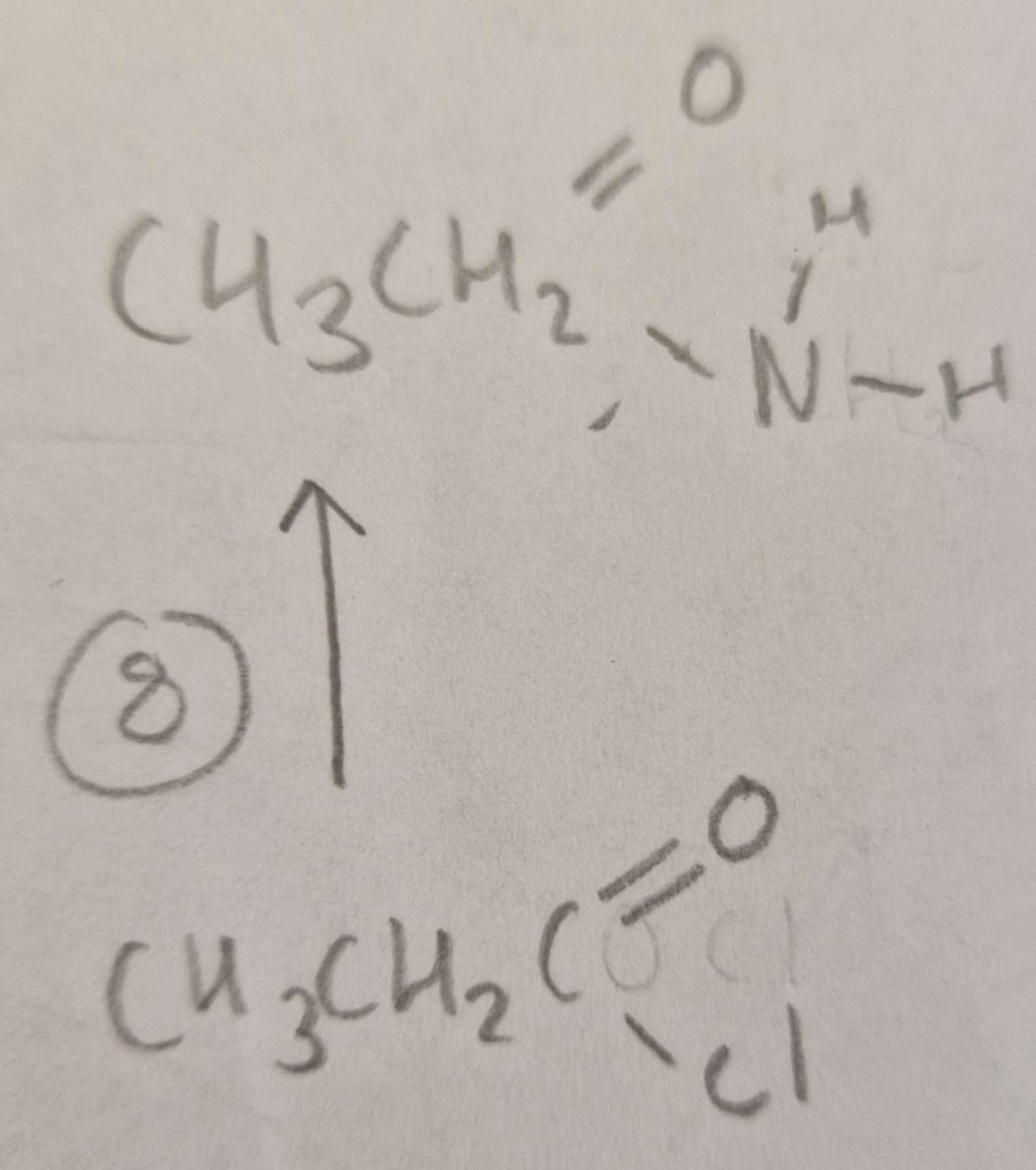
Acyl Chloride to Acid Amide Conditions:
R.T.P
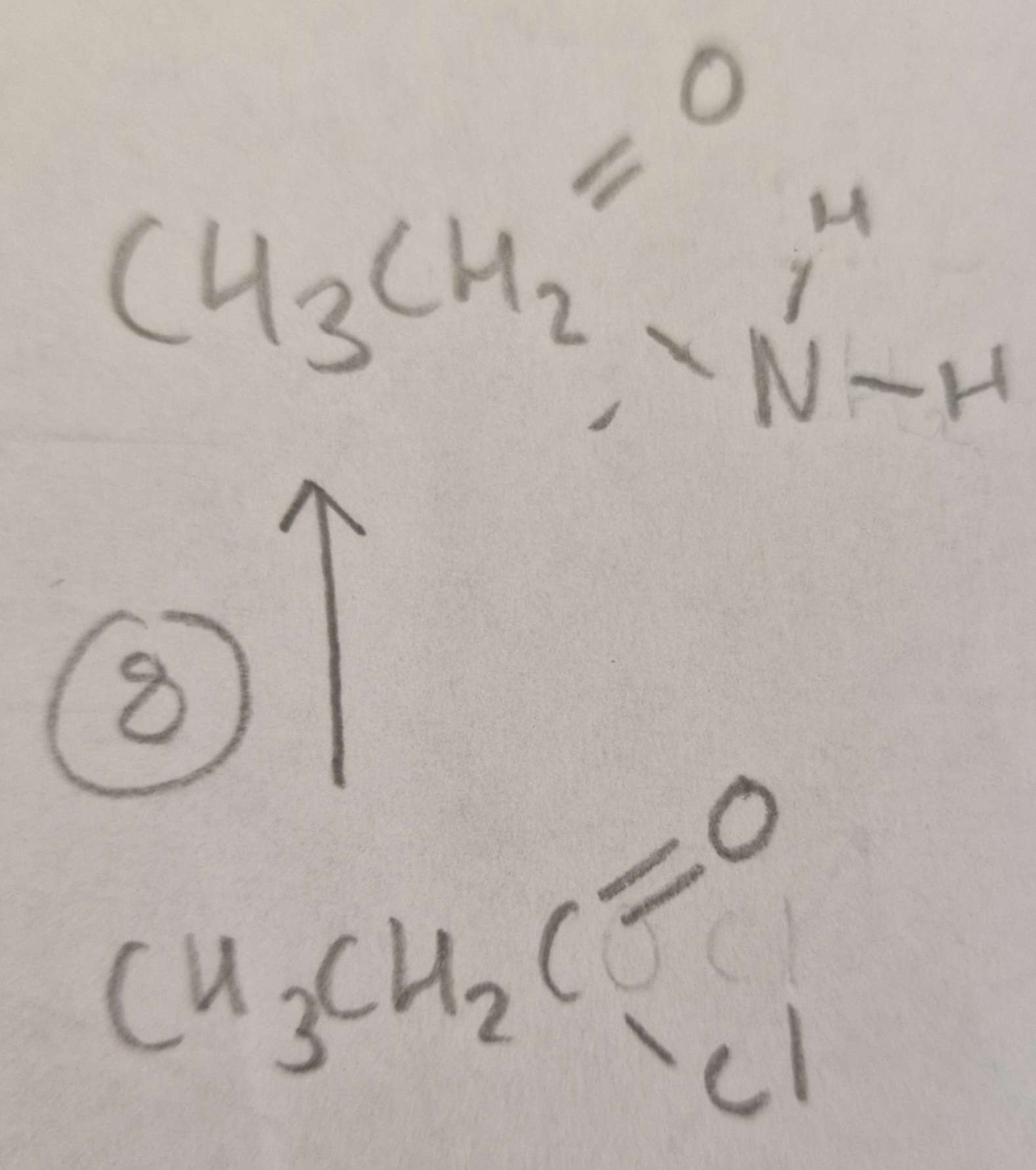
Acyl Chloride to Acid Amide Name of Product:
Ethanamide
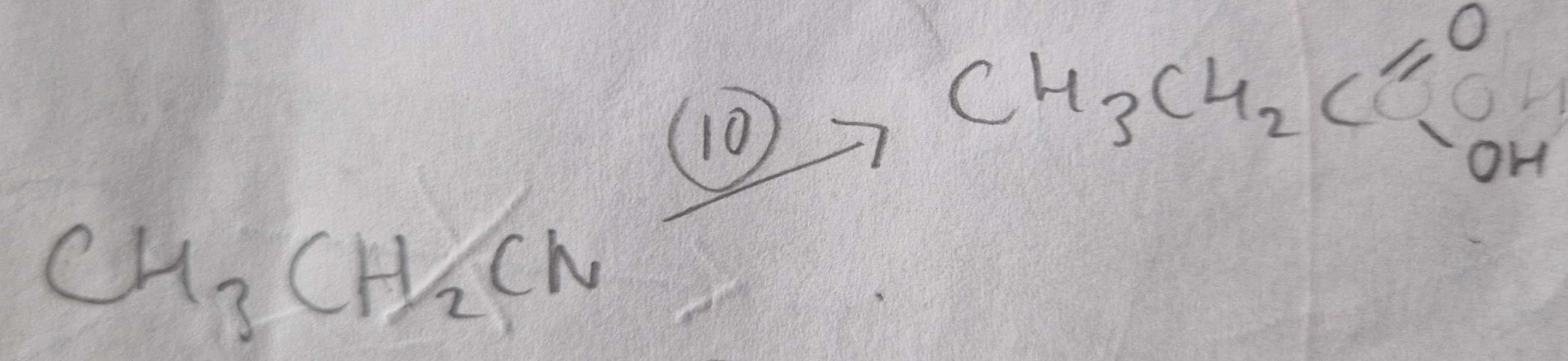
Nitrile to Carboxylic Acid Type of Reaction:
Hydrolosis
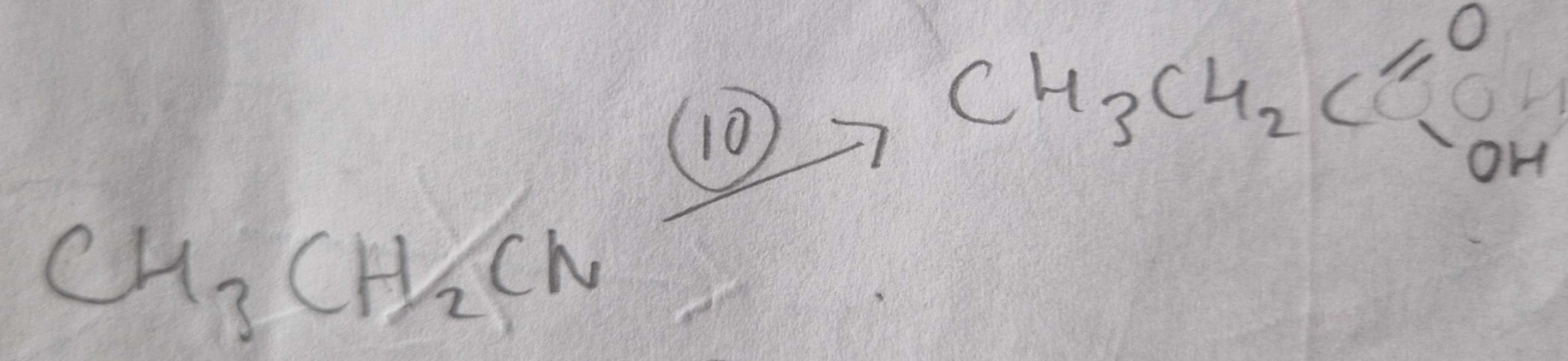
Nitrile to Carboxylic Acid Mechanism:
None
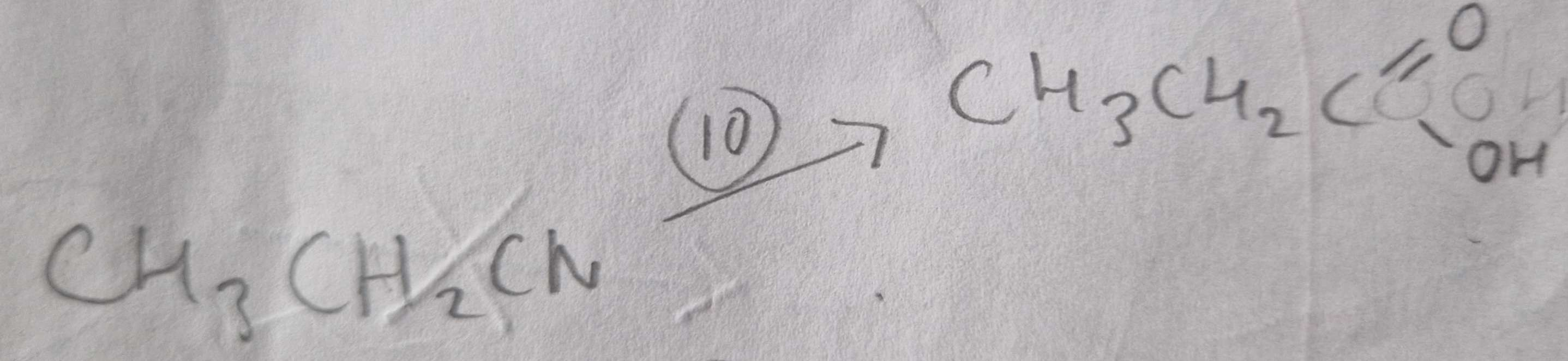
Nitrile to Carboxylic Acid Reagents:
HCl (aq)
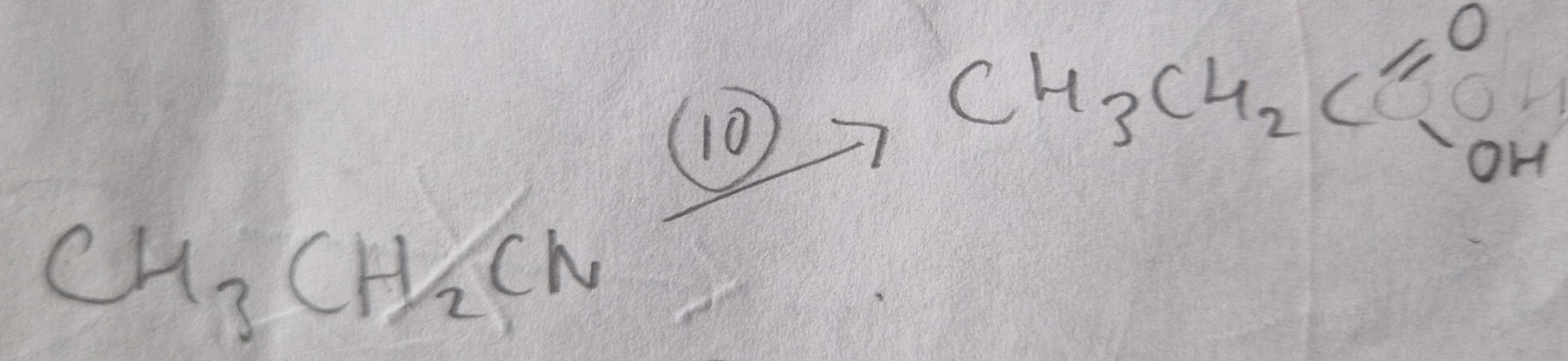
Nitrile to Carboxylic Acid Conditions:
H.U.R
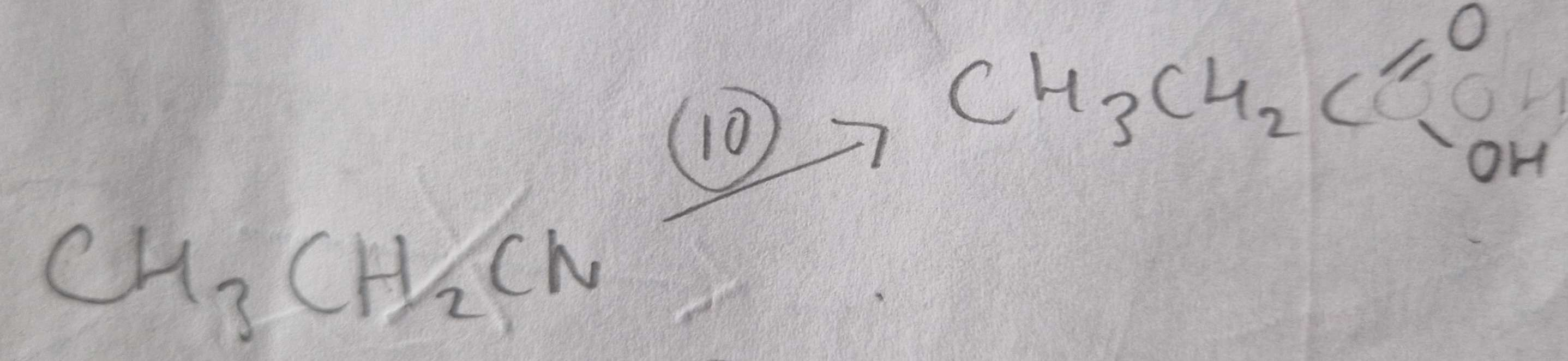
Nitrile to Carboxylic Acid Name of Products:
Propanoic Acid

Haloalkane to Alcohol Type of Reaction:
Substitution (know it’s Hydrolosis)
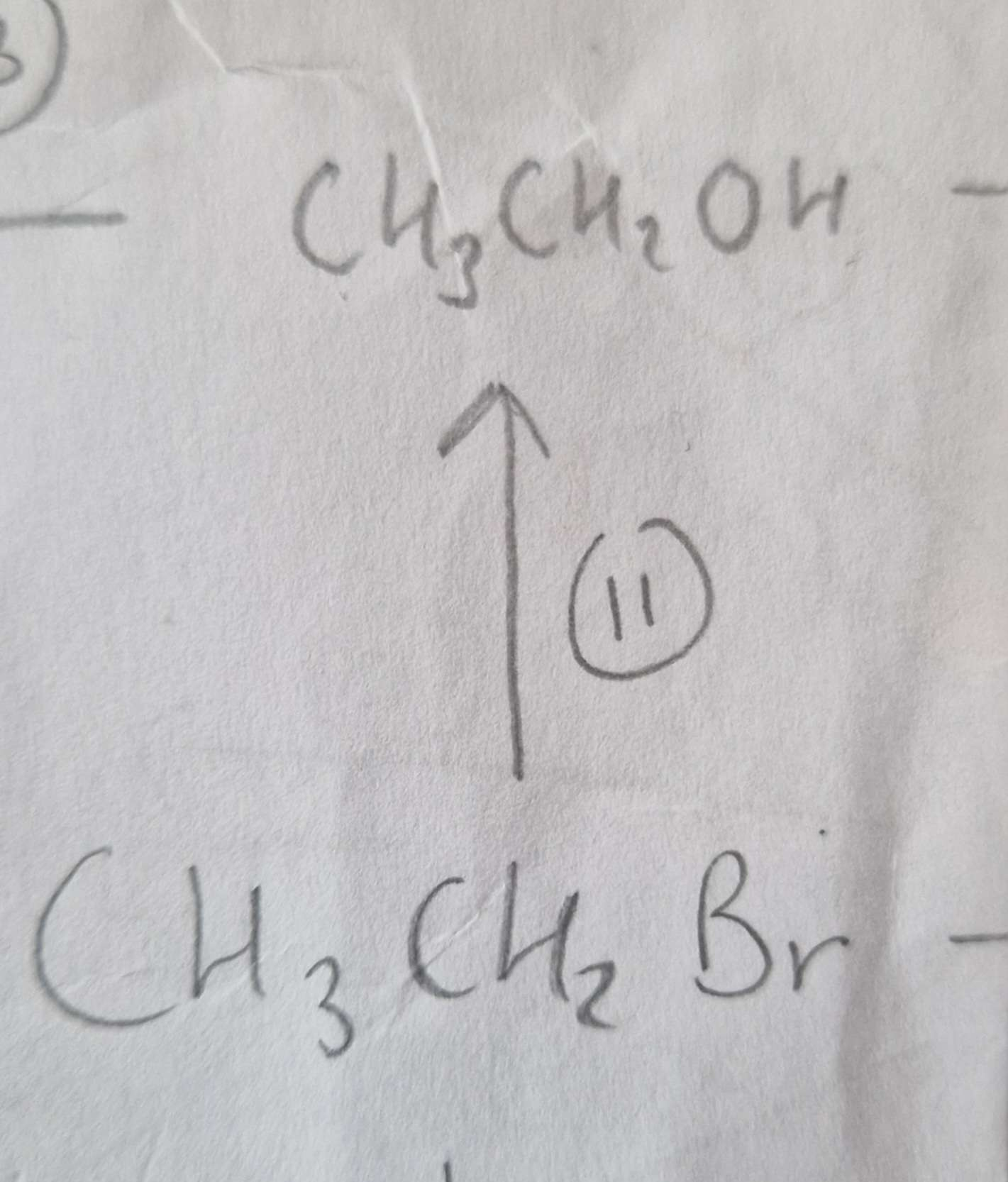
Haloalkane to Alcohol Mechanism:
Nucleophilic
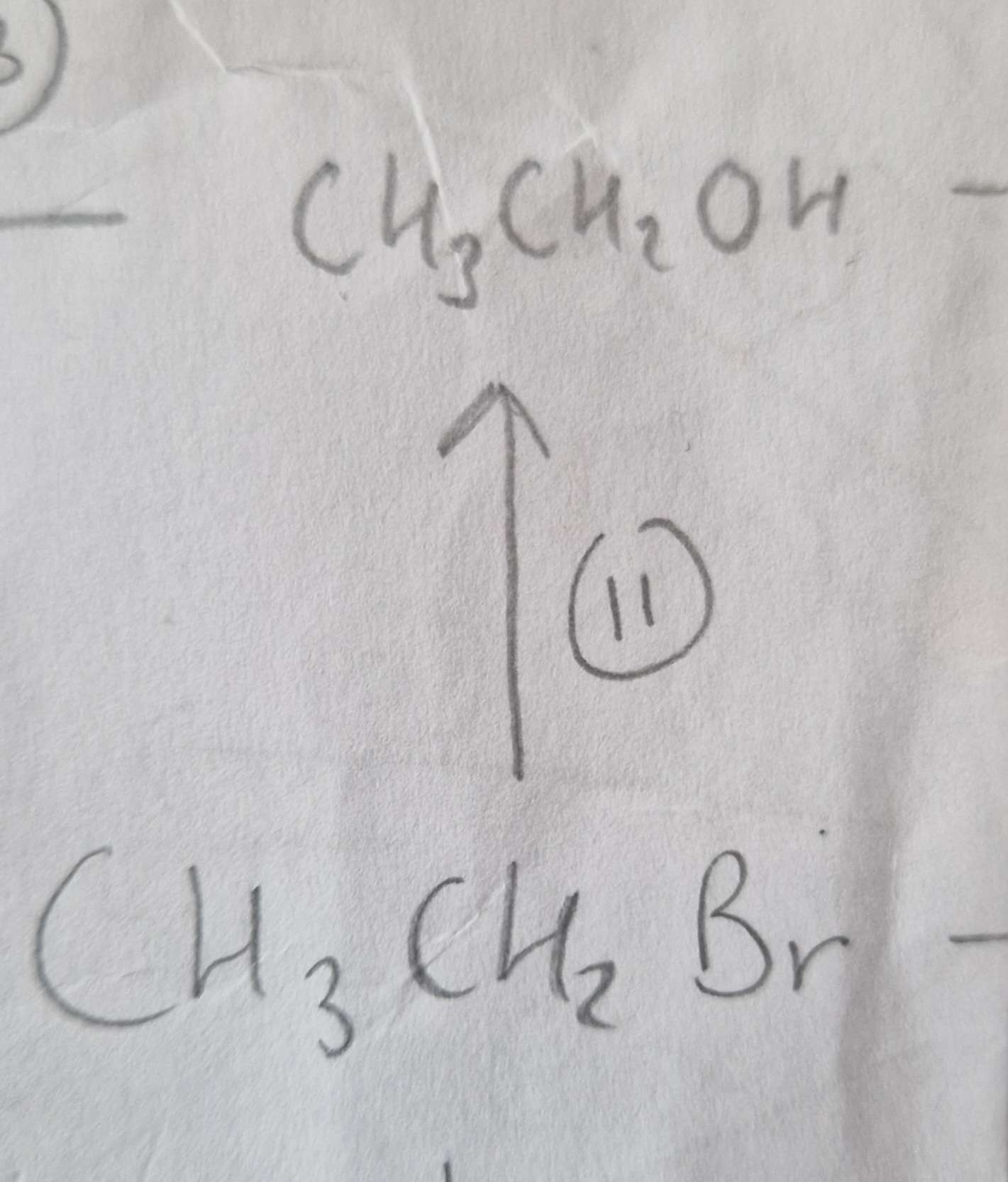
Haloalkane to Alcohol Reagents:
NaOH (aq)
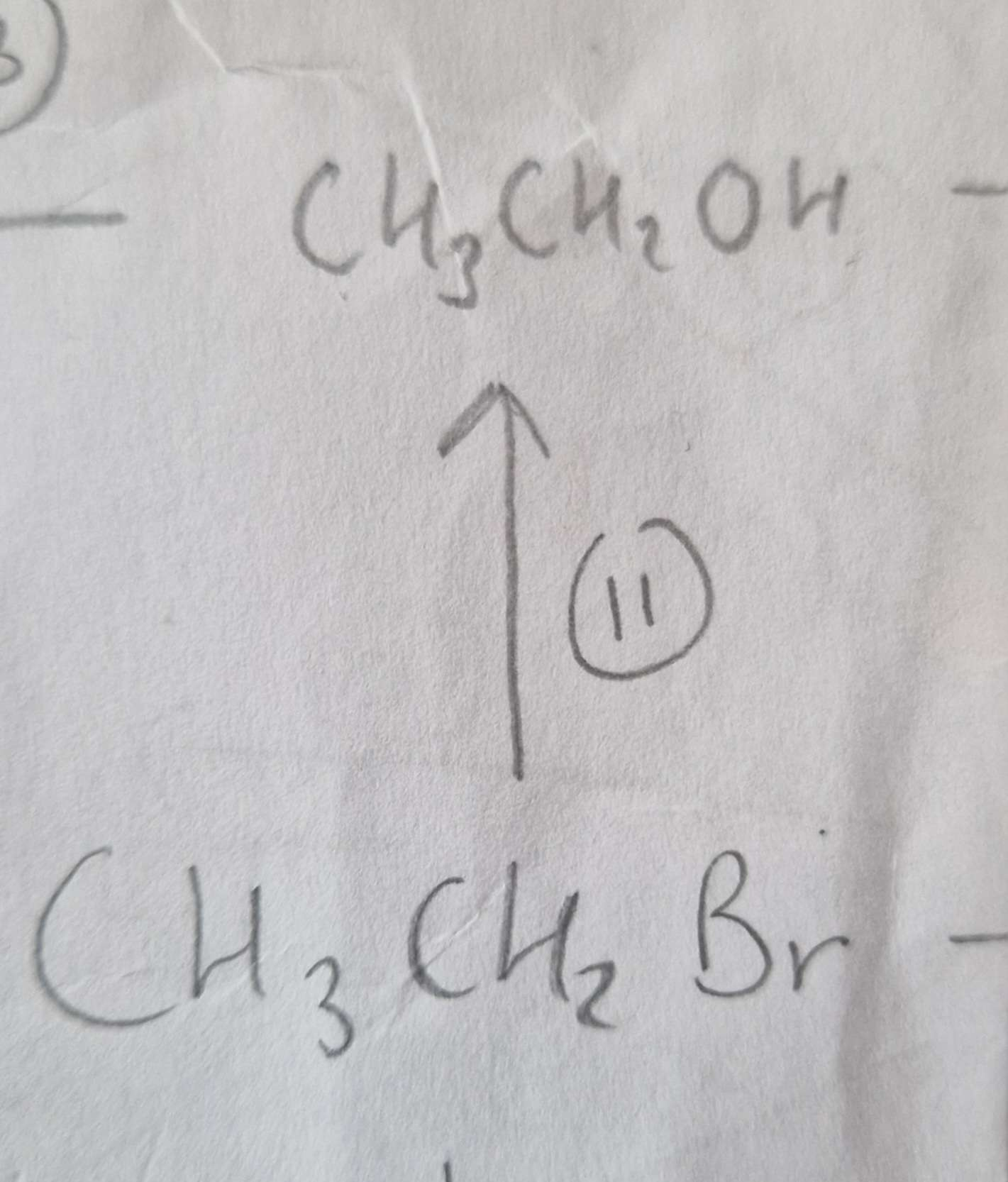
Haloalkane to Alcohol Conditions:
Warm
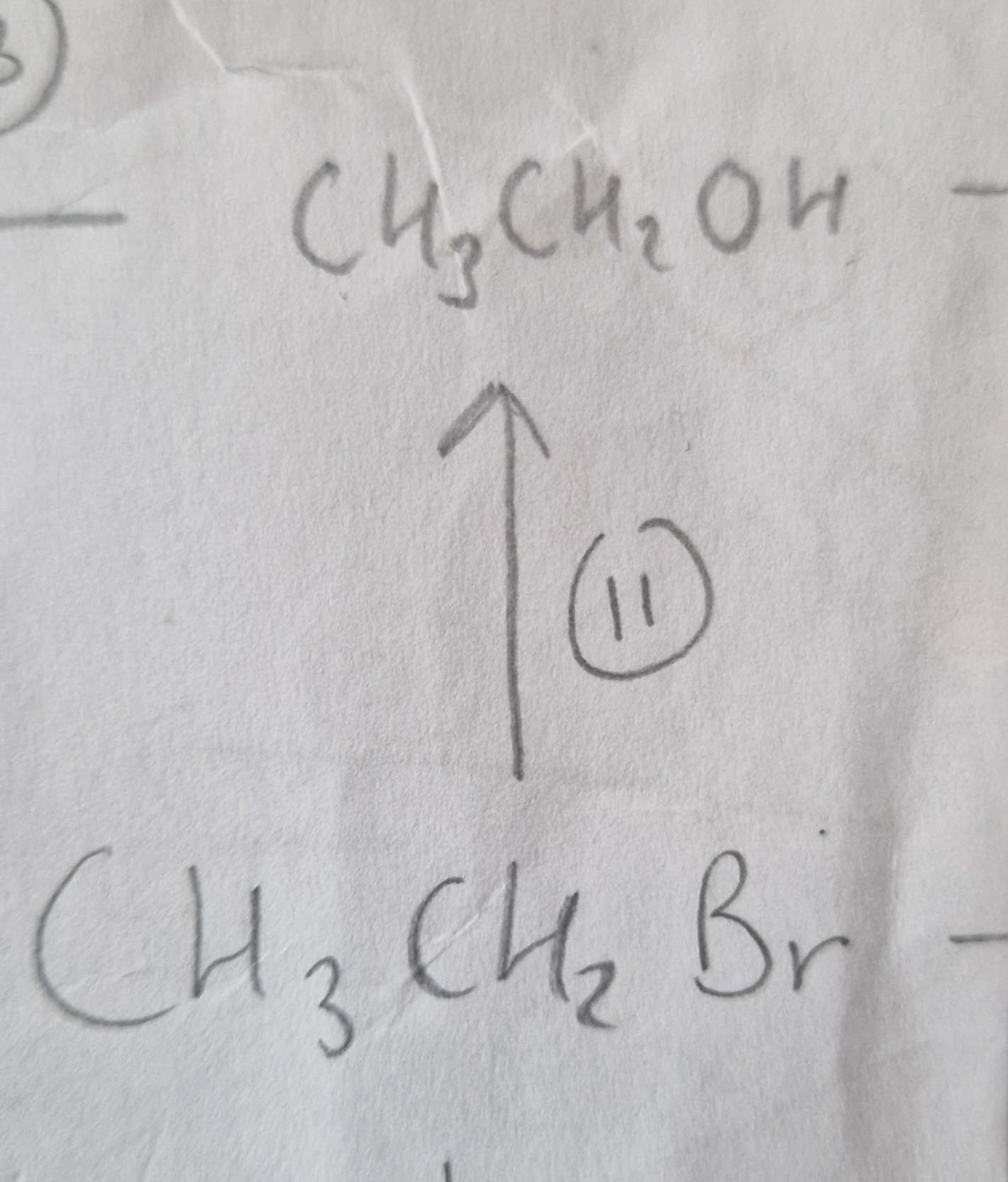
Haloalkane to Alcohol Name of Product:
Ethanol
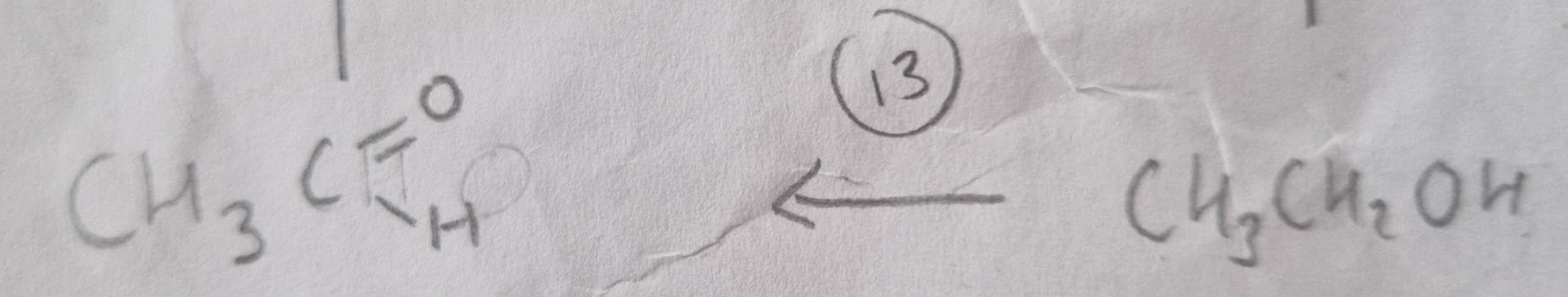
Alcohol to Aldehyde Type of Reaction:
Oxidation
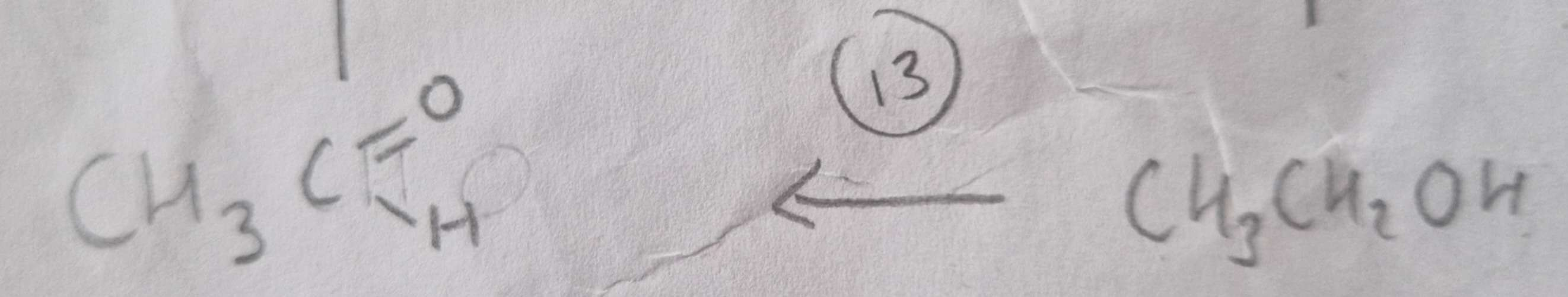
Alcohol to Aldehyde Mechanism:
None
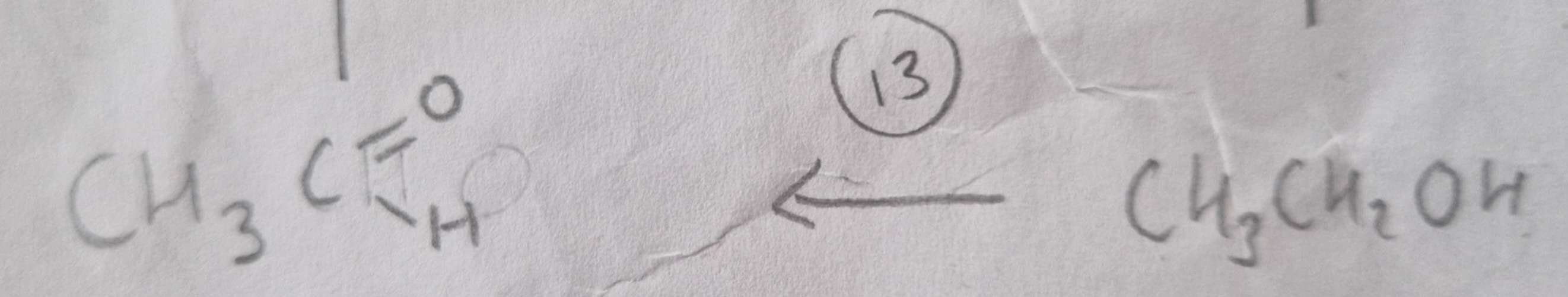
Alcohol to Aldehyde Reagents:
K2Cr2O7 (aq) + Dilute H2SO4
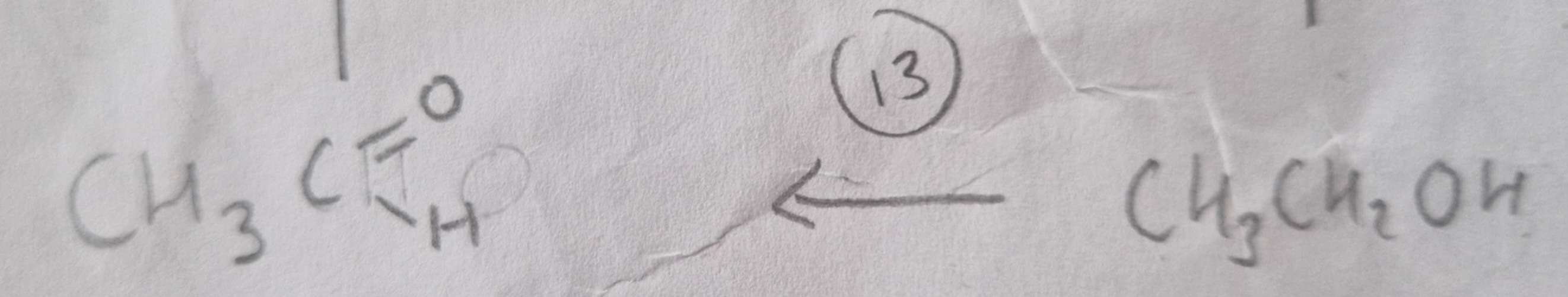
Alcohol to Aldehyde Conditions:
Warm and Distill
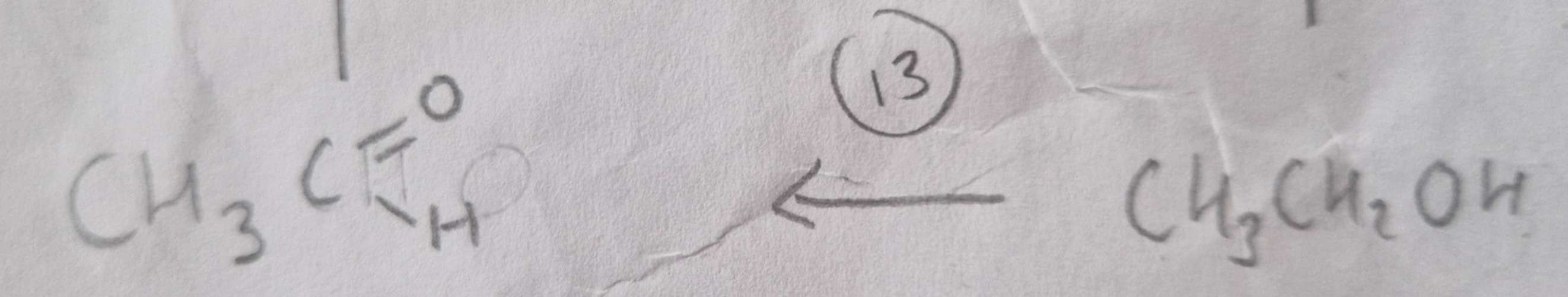
Alcohol to Aldehyde Name of Product:
Ethanal
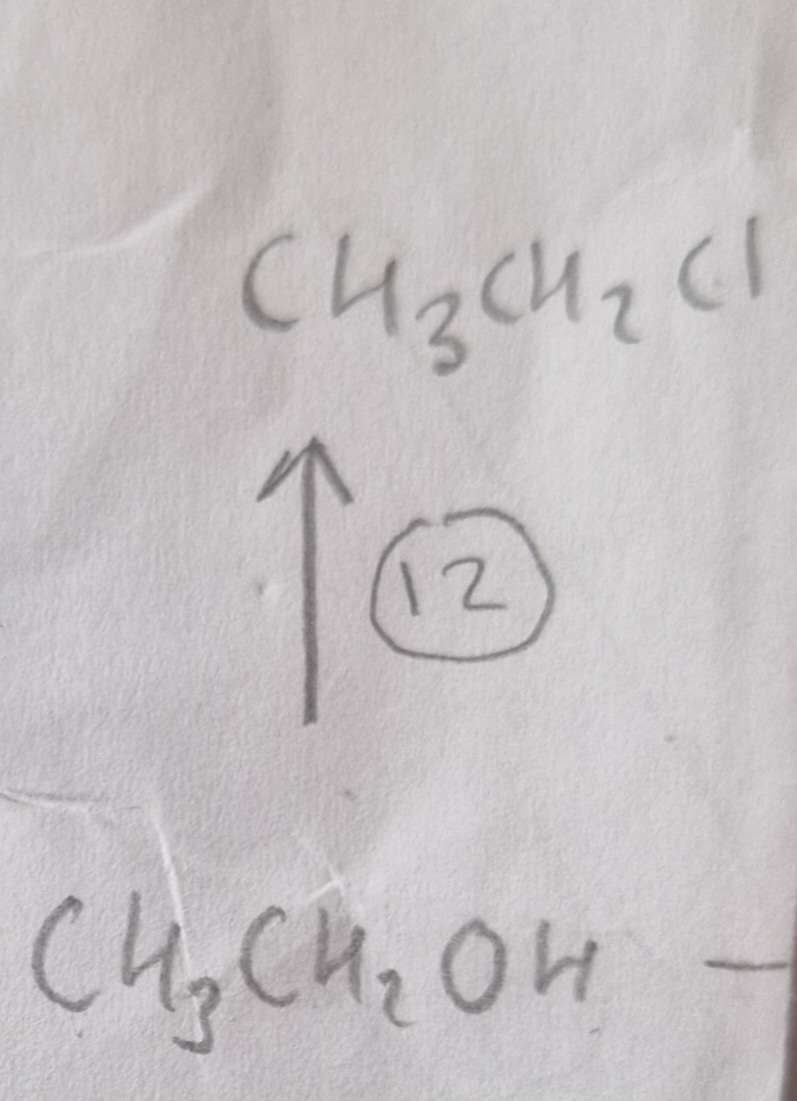
Alcohol to Haloalkane Type of Reaction:
Substitution
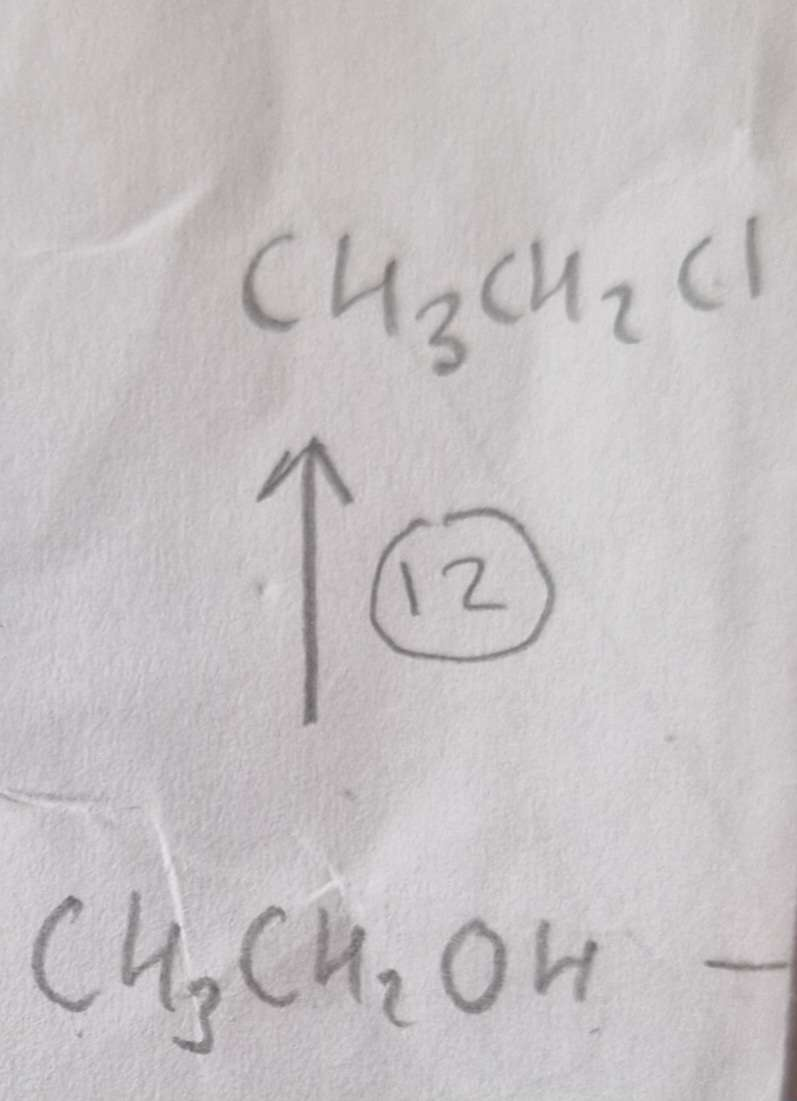
Alcohol to Haloalkane Mechanism:
None
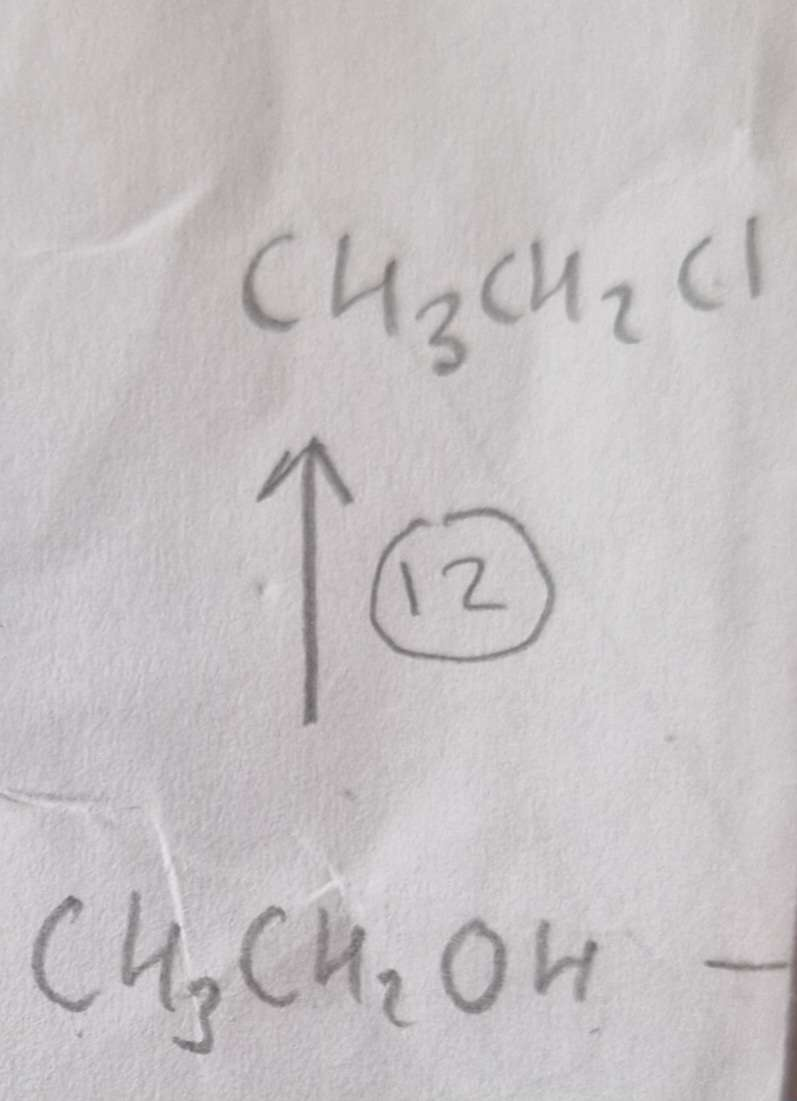
Alcohol to Haloalkane Reagents:
NaCl, conc H2SO4
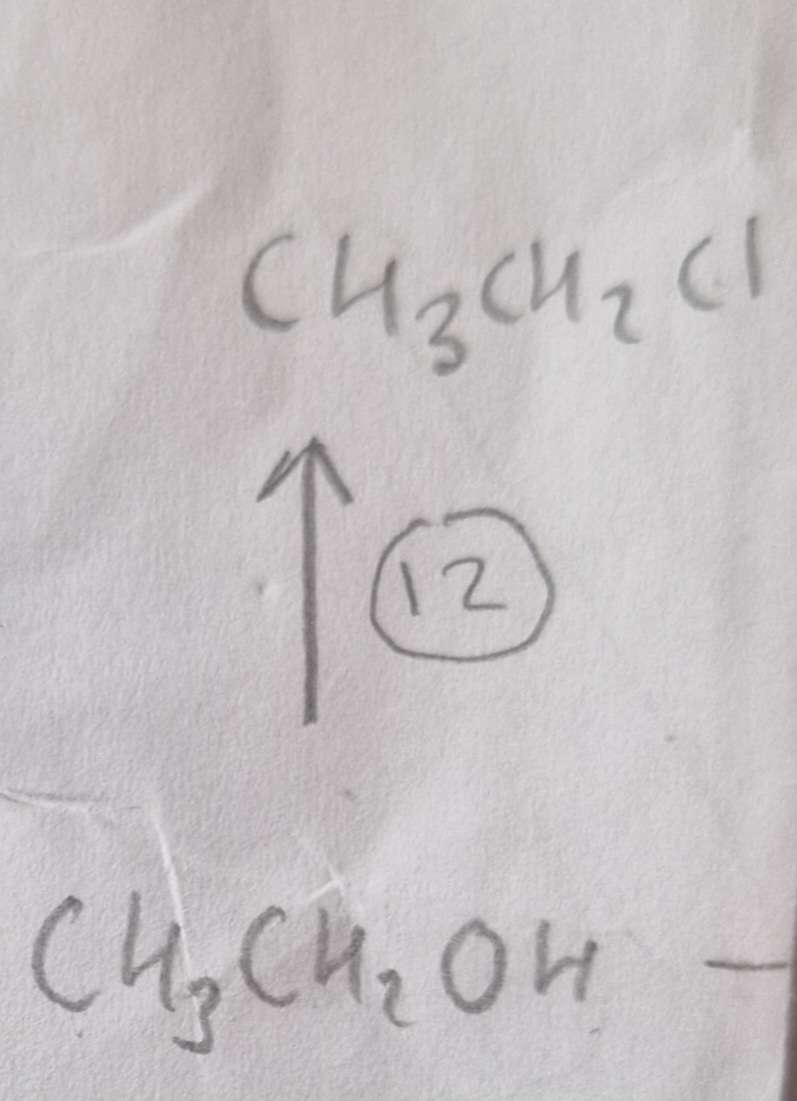
Alcohol to Haloalkane Conditions:
Warm
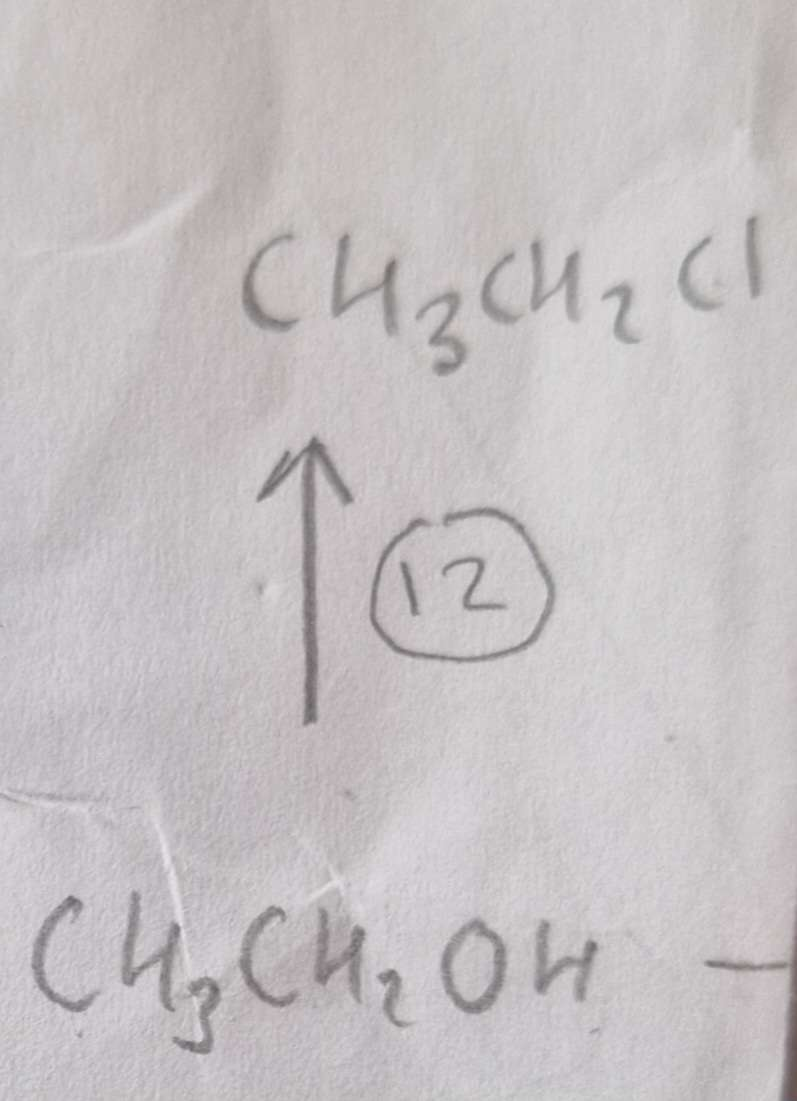
Alcohol to Haloalkane Name of Product:
Chloroethane
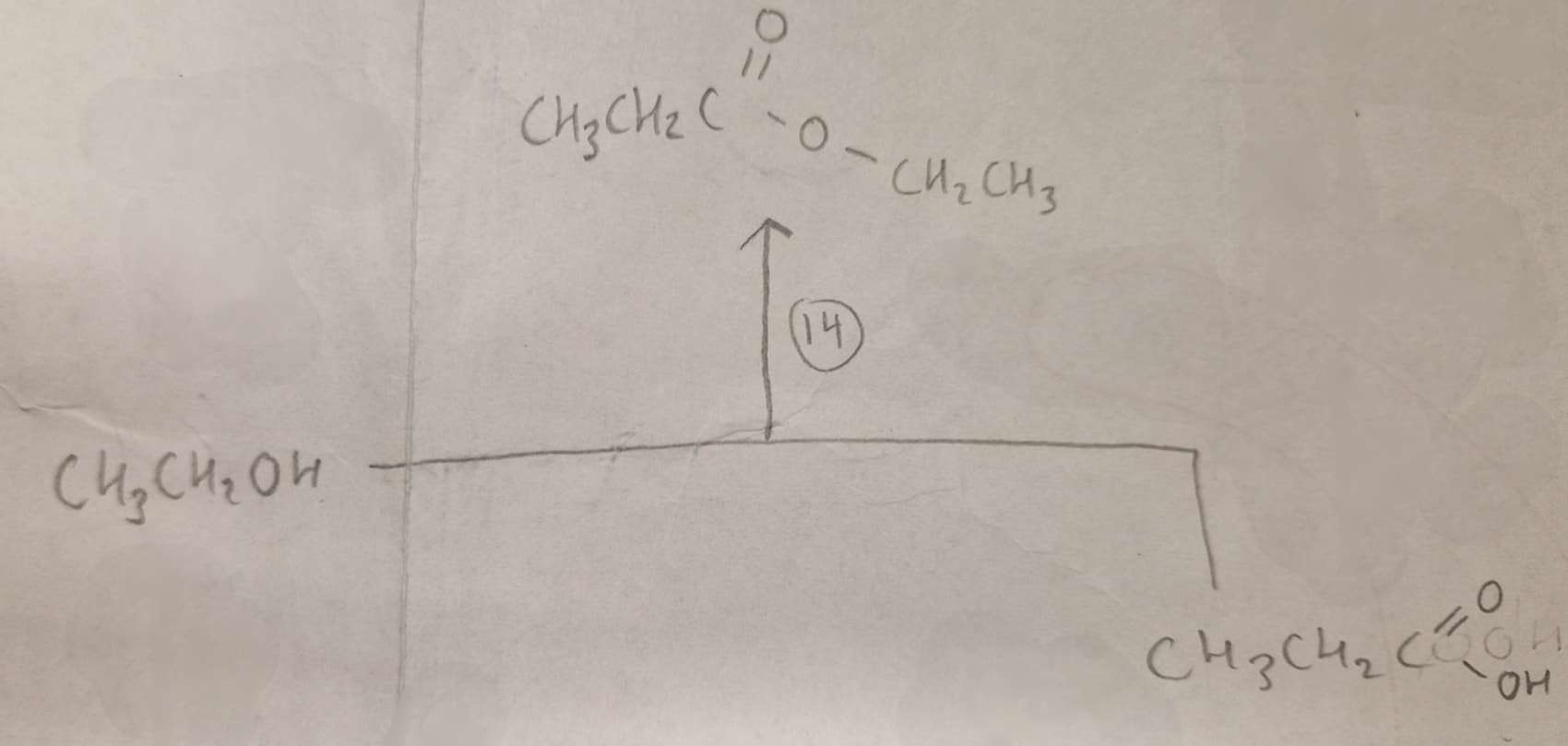
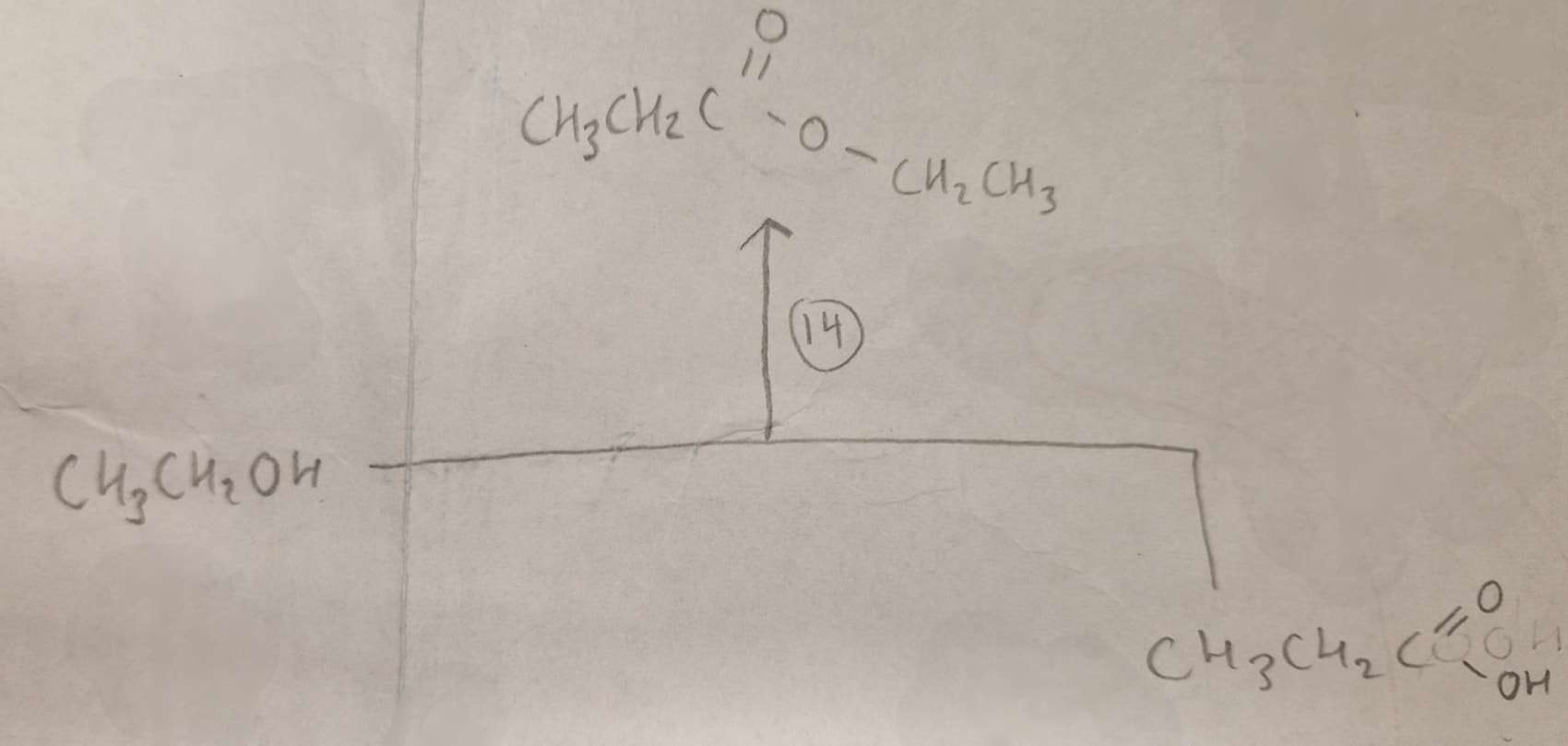
Alcohol + Carboxylic Acid to Ester Type of Reaction:
Esterification
Alcohol + Carboxylic Acid to Ester Mechanism:
None
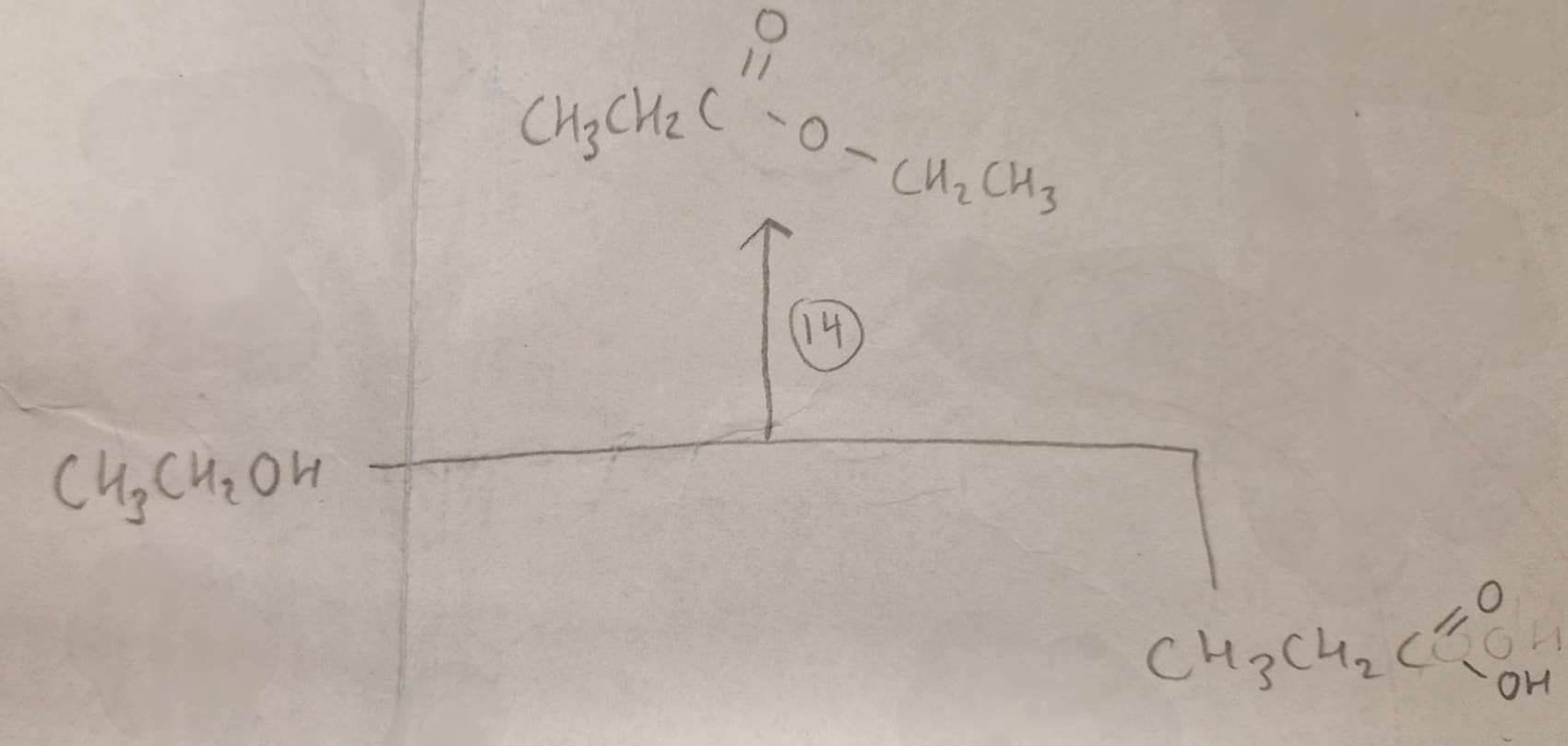
Alcohol + Carboxylic Acid to Ester Reagents:
conc H2SO4
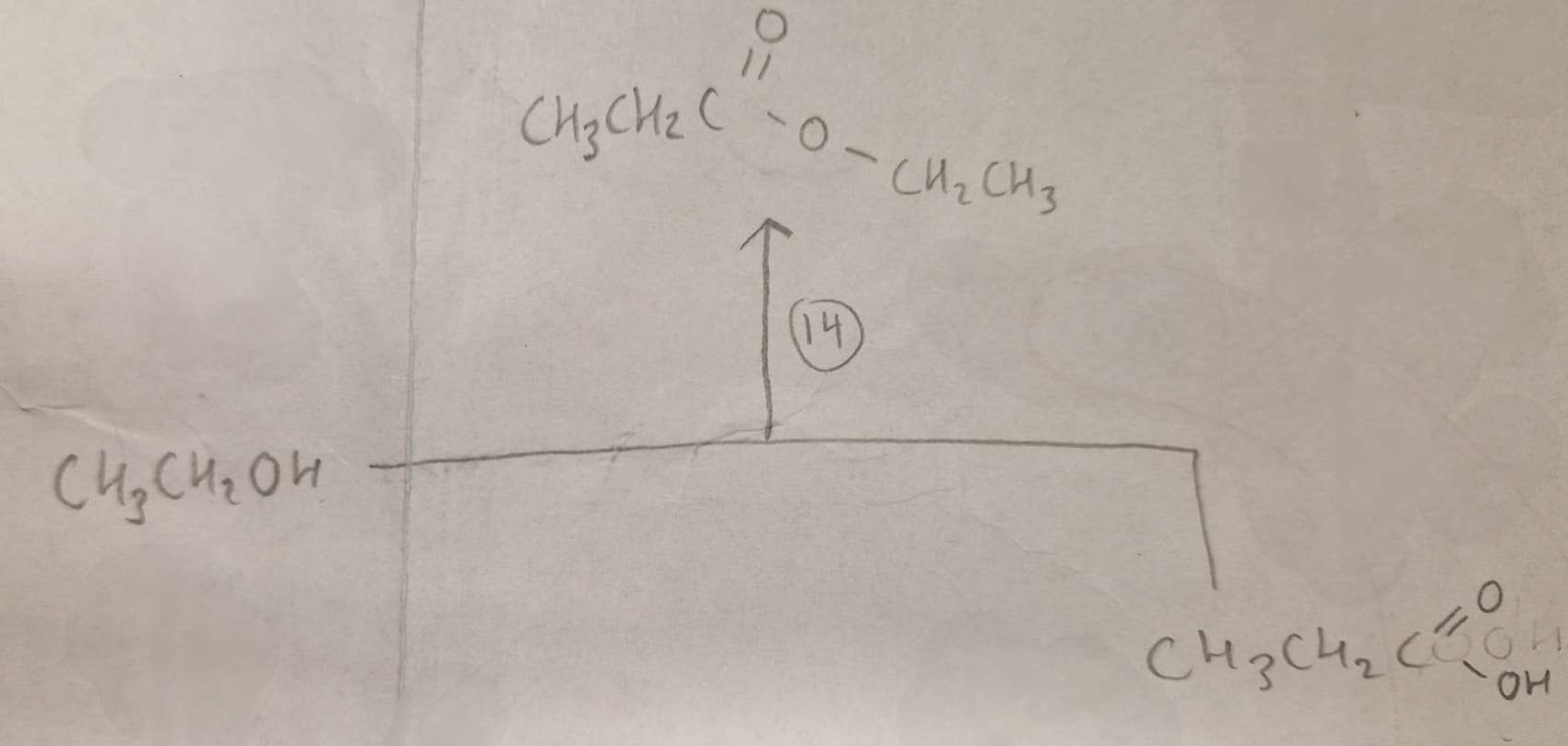
Alcohol + Carboxylic Acid to Ester Conditions:
H.U.R
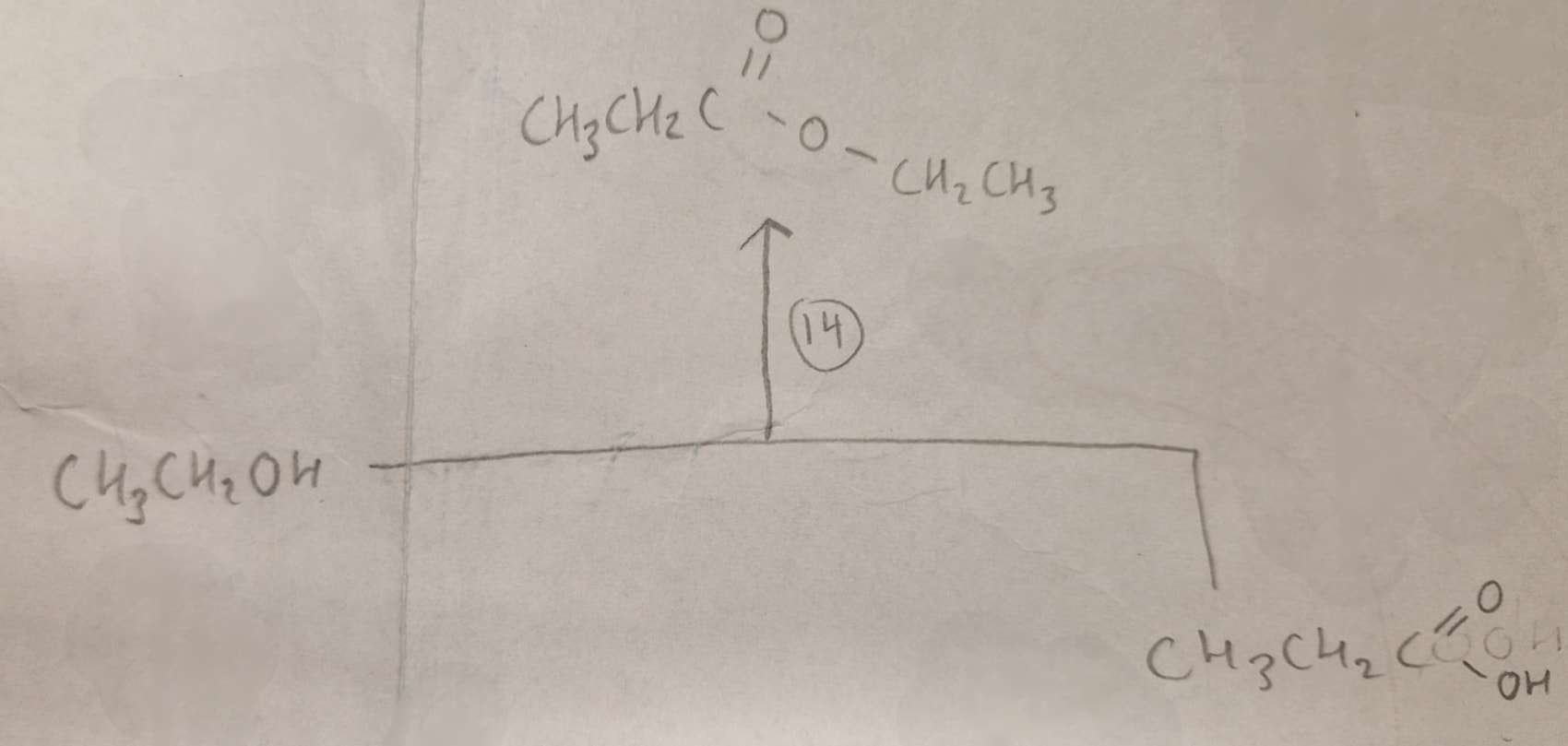
Alcohol + Carboxylic Acid to Ester Name of Product:
Ethyl Propanoate
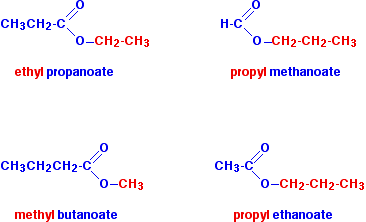
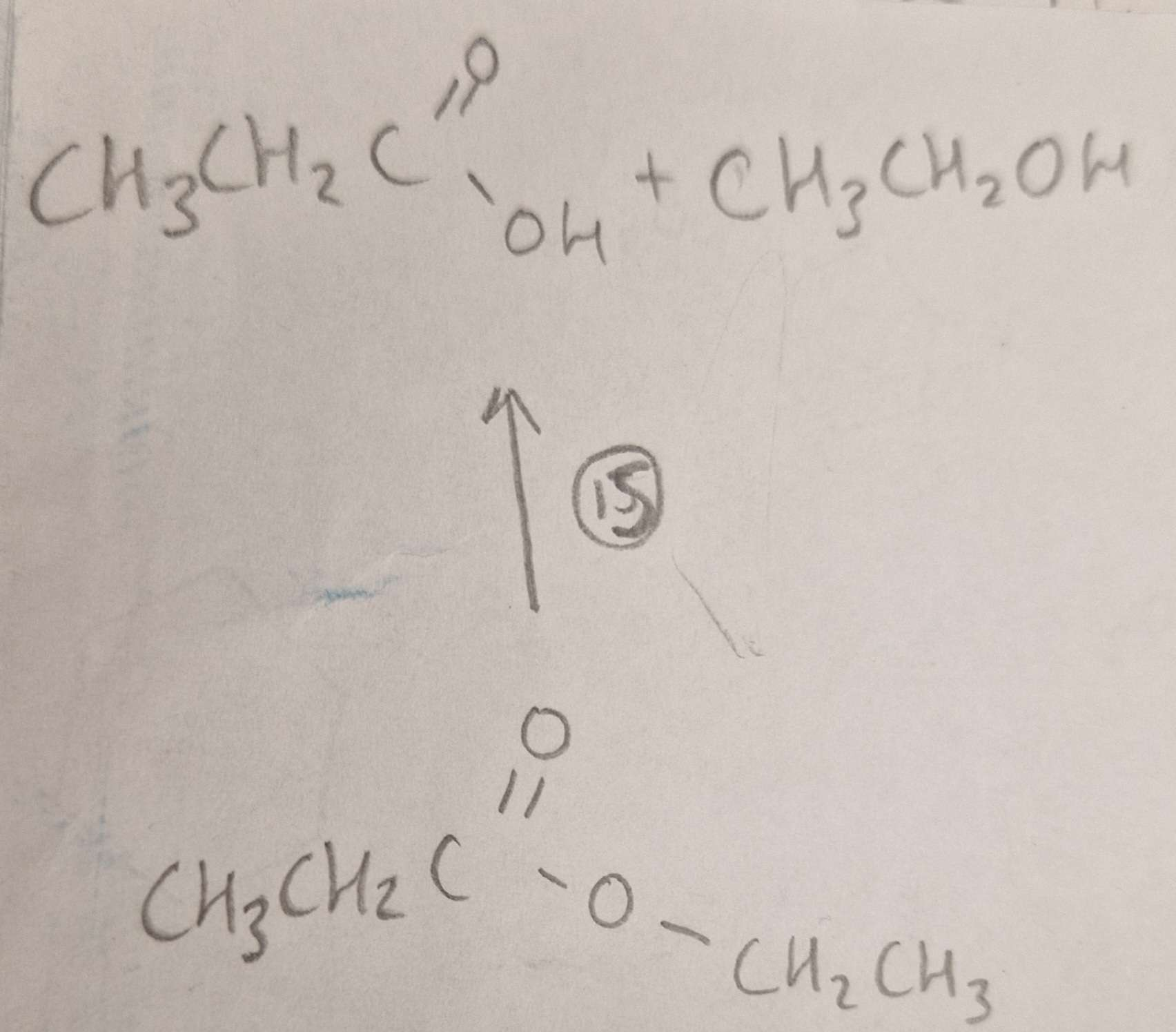
Ester to Carboxylic Acid and Alcohol Type of Reaction:
Hydrolysis
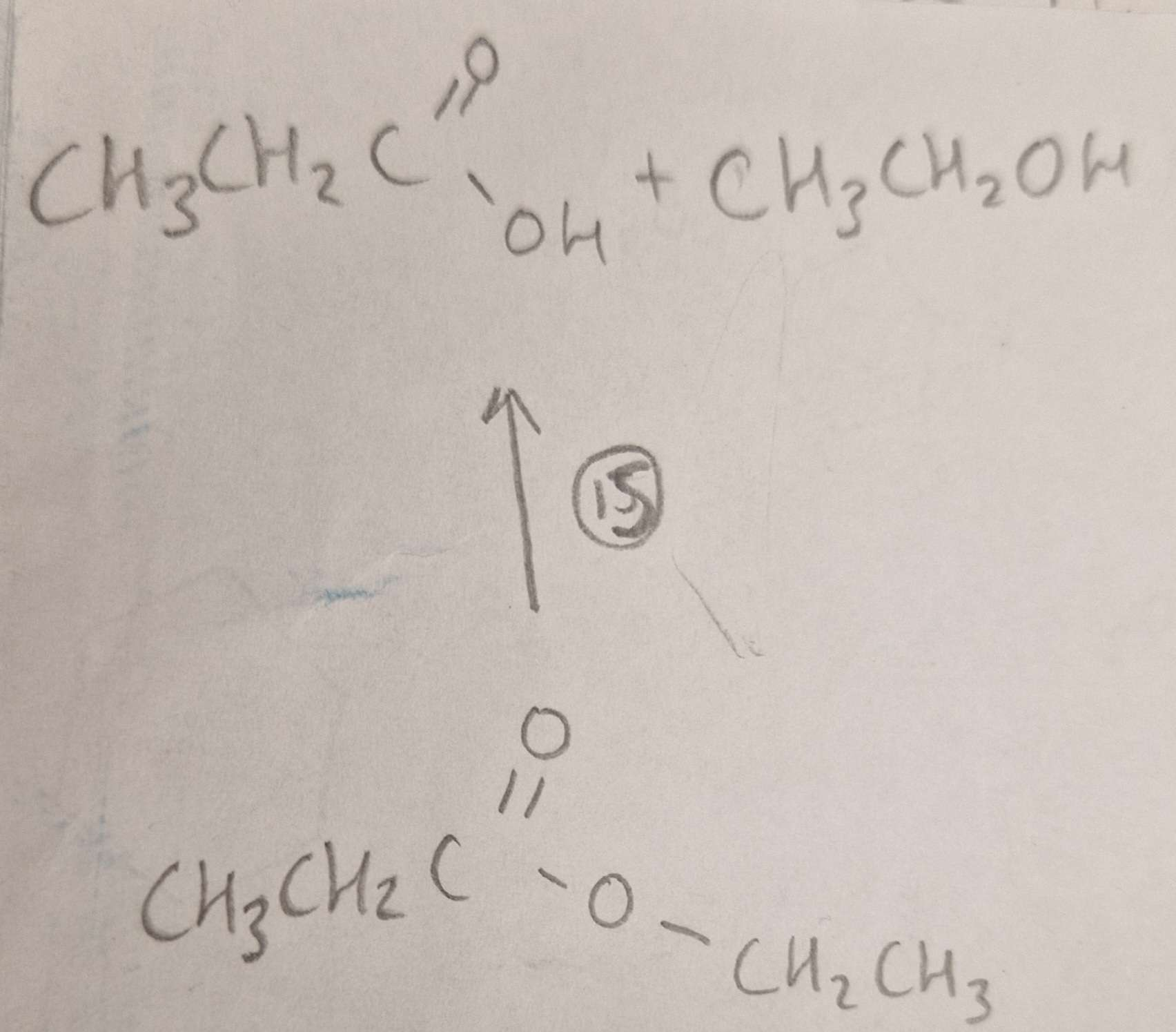
Ester to Carboxylic Acid and Alcohol Mechanism:
None
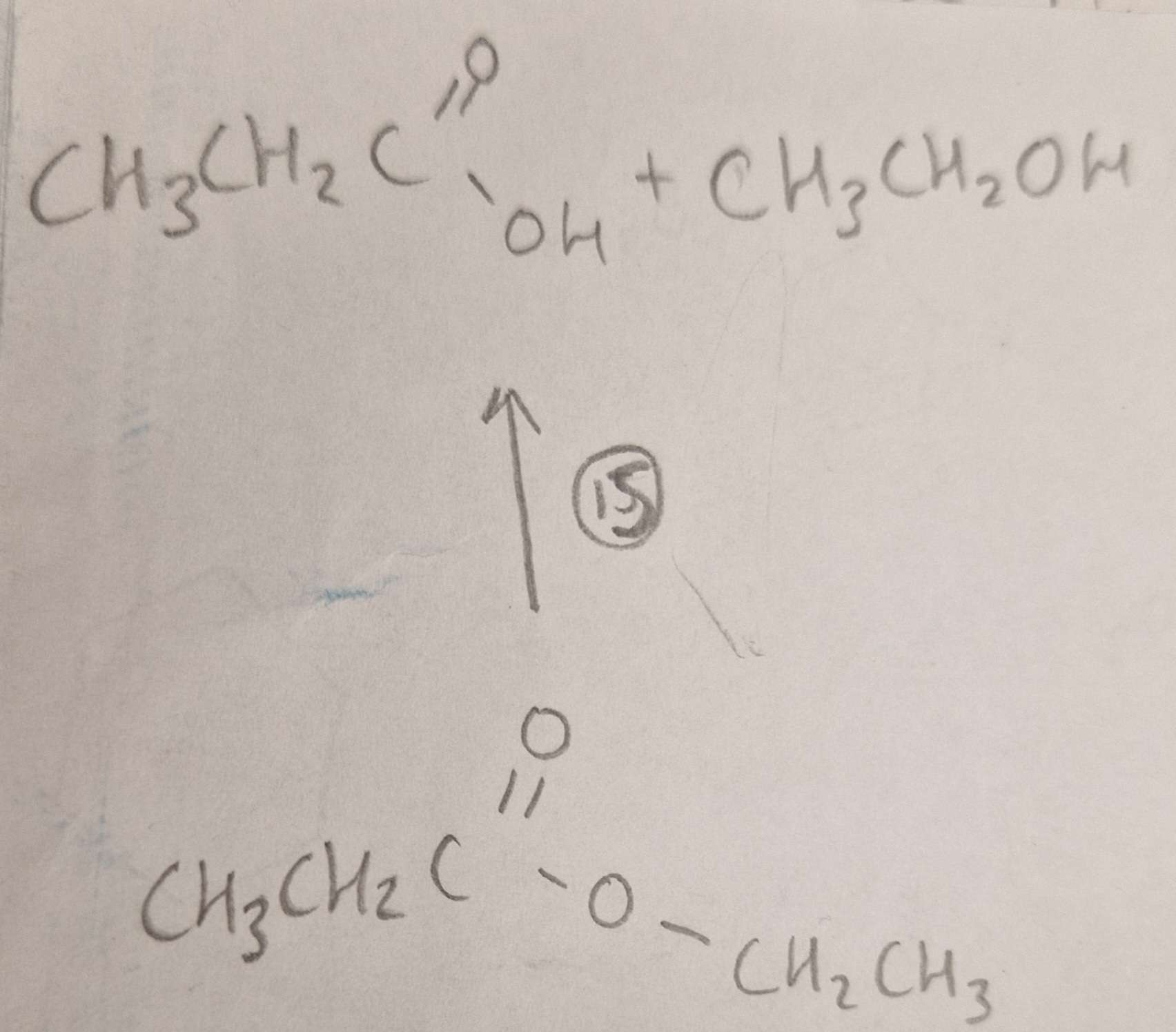
Ester to Carboxylic Acid and Alcohol Reagents:
H2SO4(aq)
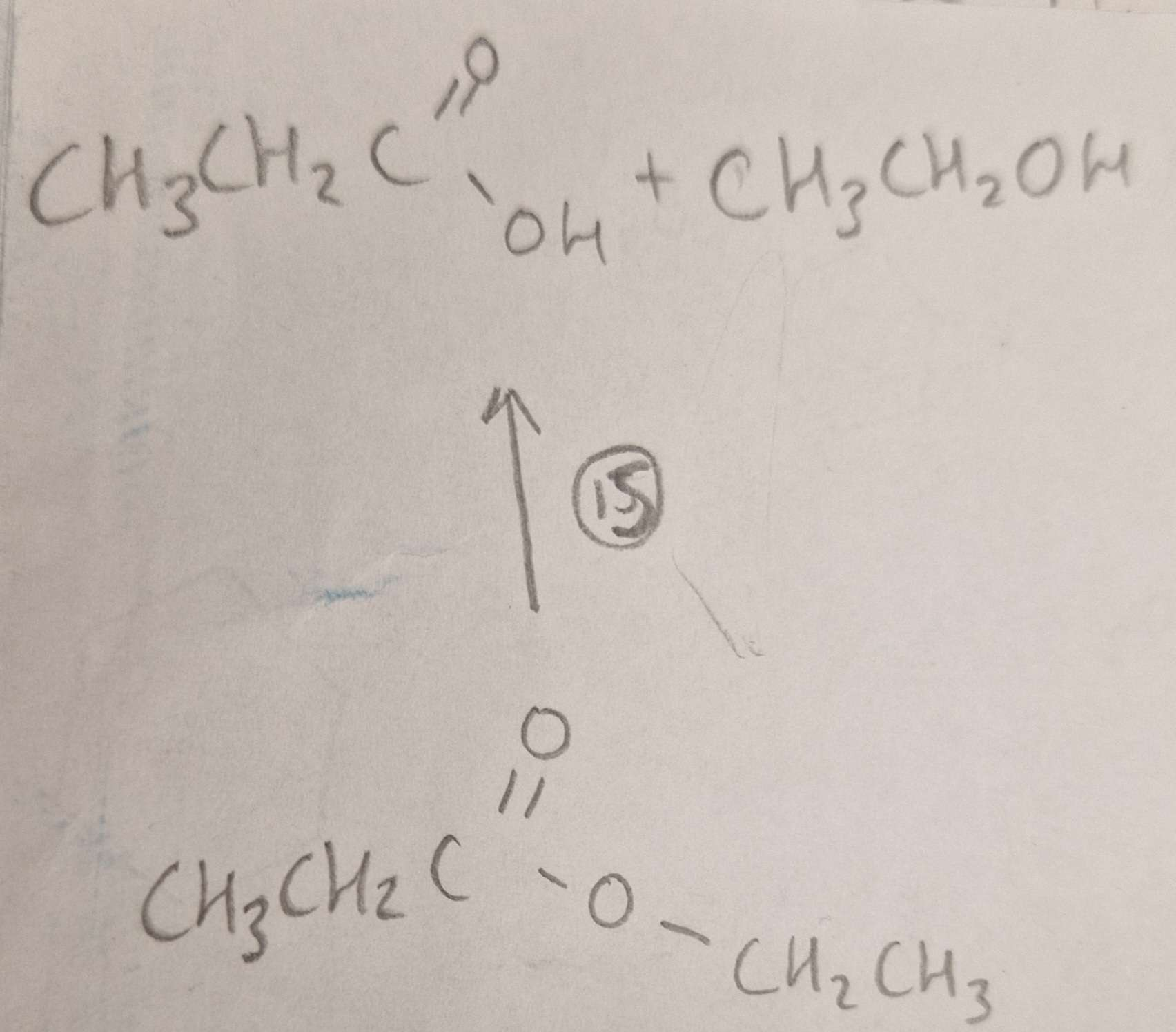
Ester to Carboxylic Acid and Alcohol Conditions:
H.U.R
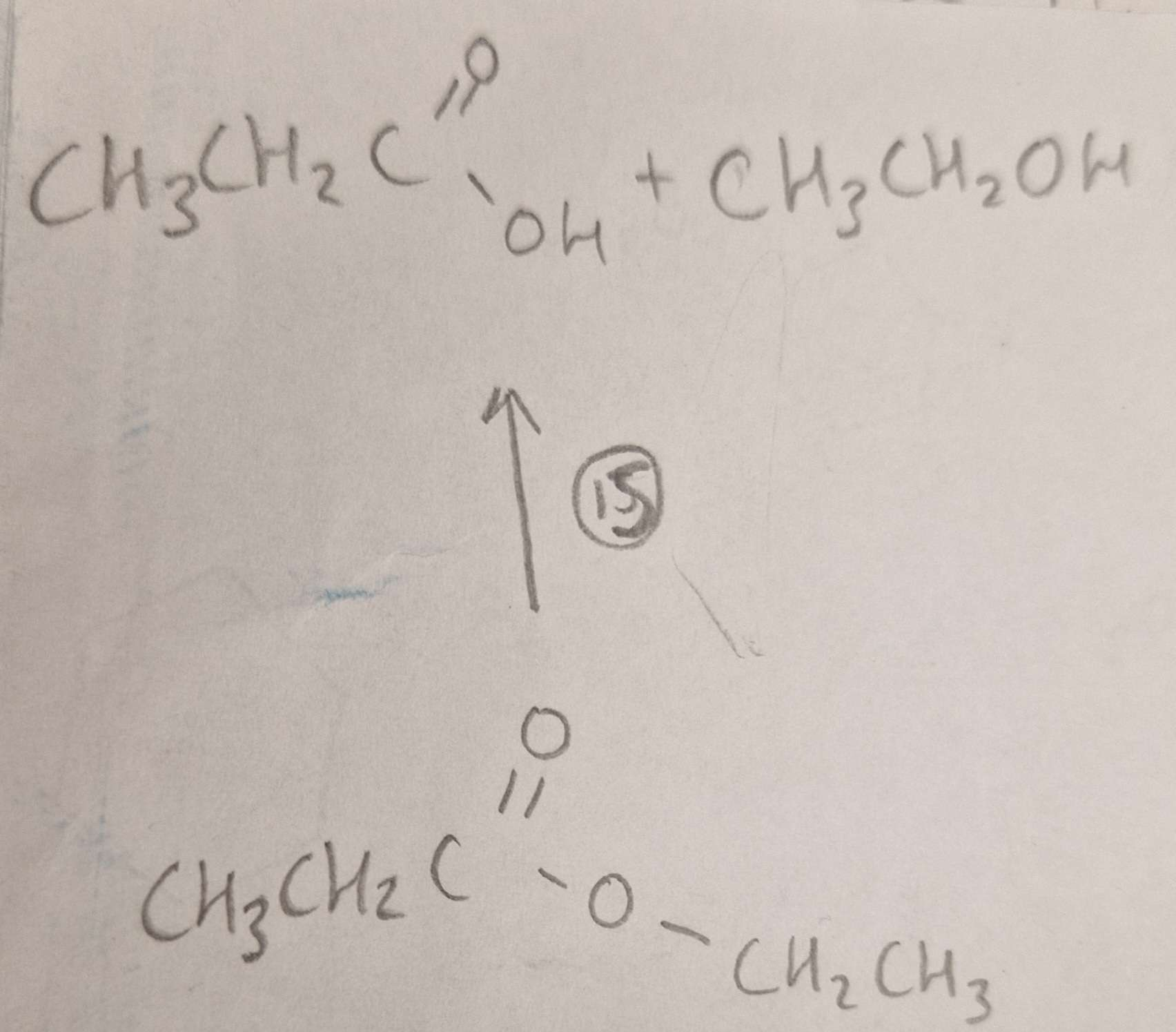
Ester to Carboxylic Acid and Alcohol Name of Product:
Propanoic Acid and Ethanol
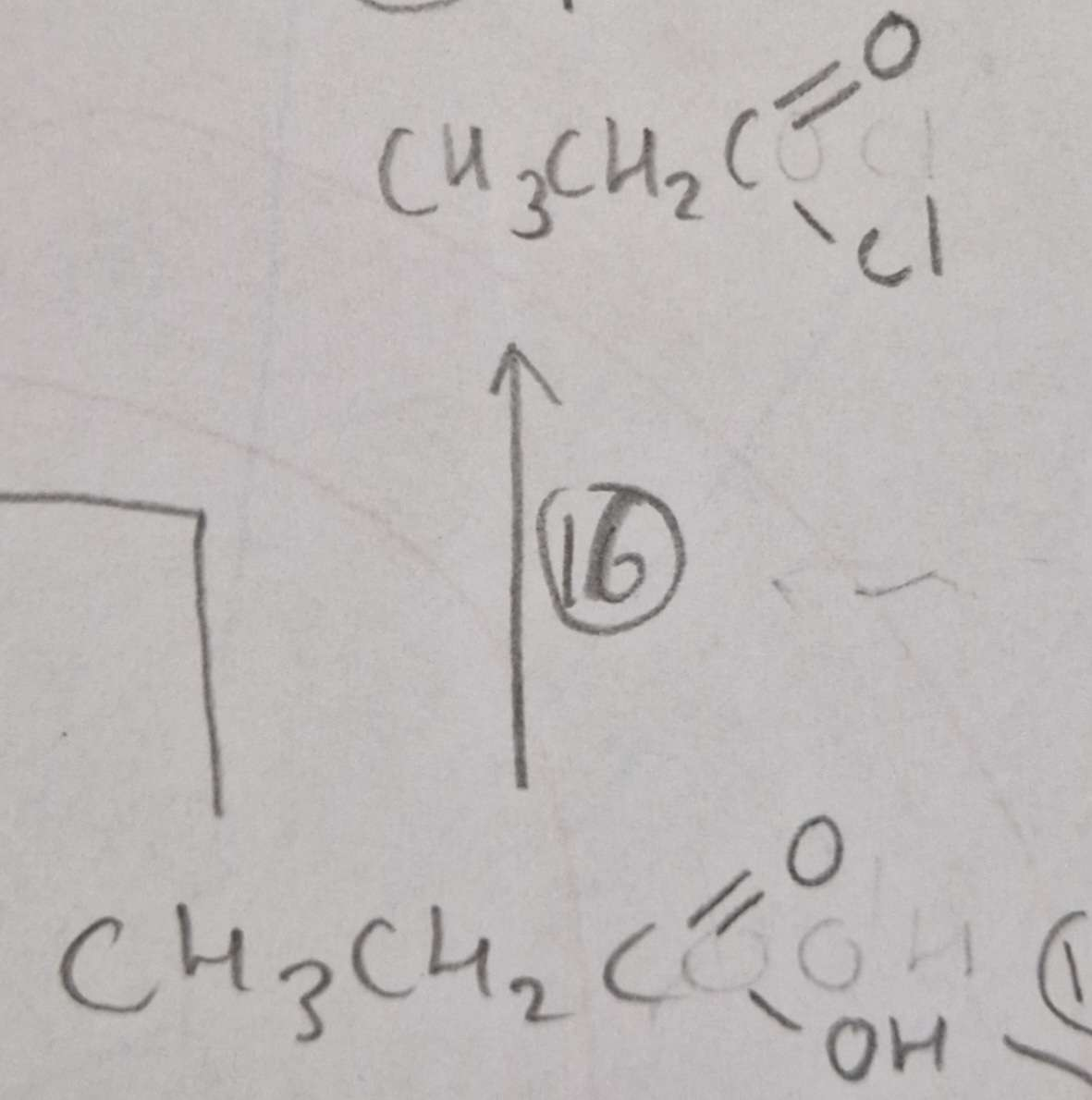
Carboxylic Acid to Acyl Chloride Type of Reaction:
Substitution
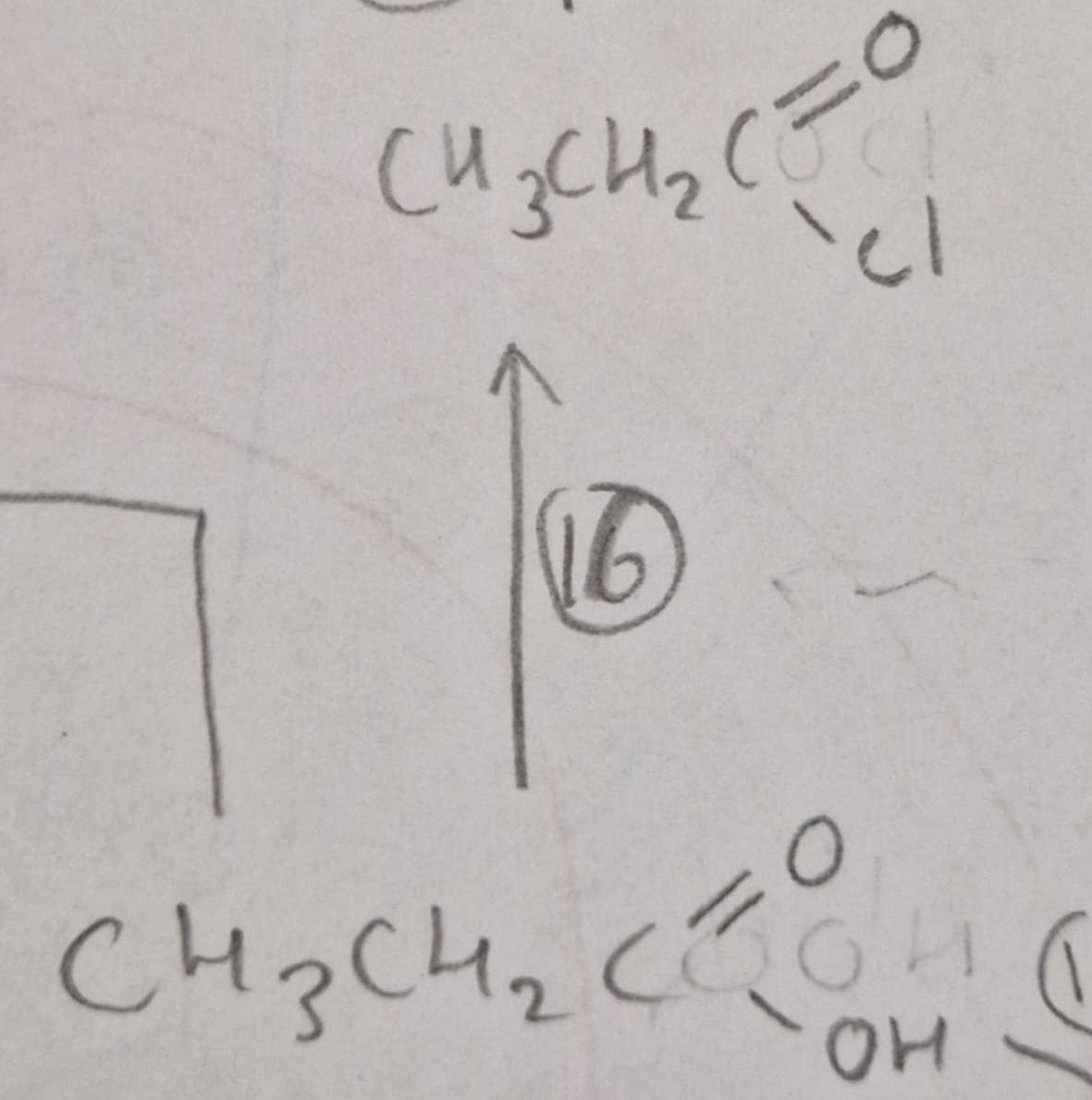
Carboxylic Acid to Acyl Chloride Mechanism:
None
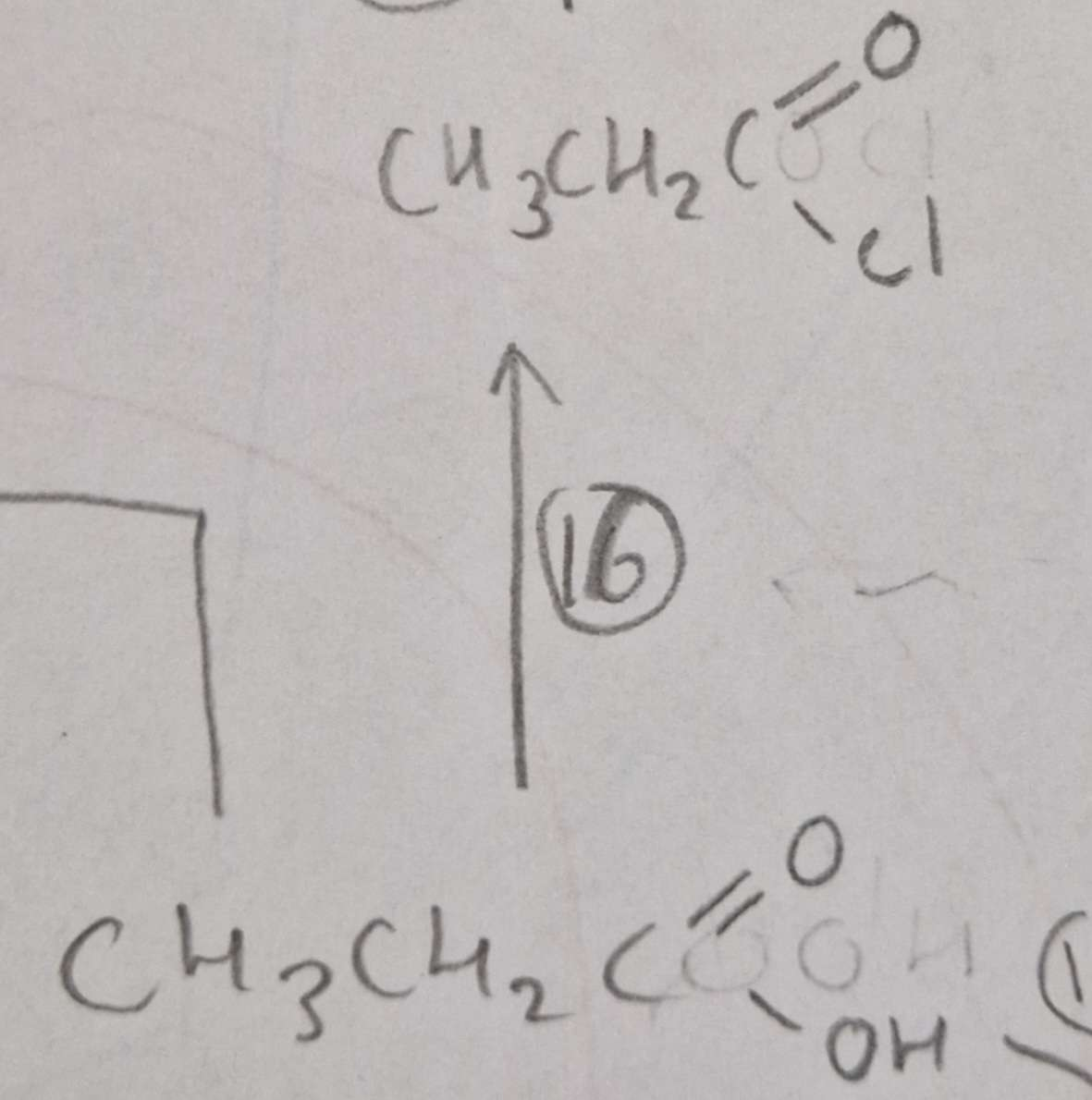
Carboxylic Acid to Acyl Chloride Reagents:
SOCl2
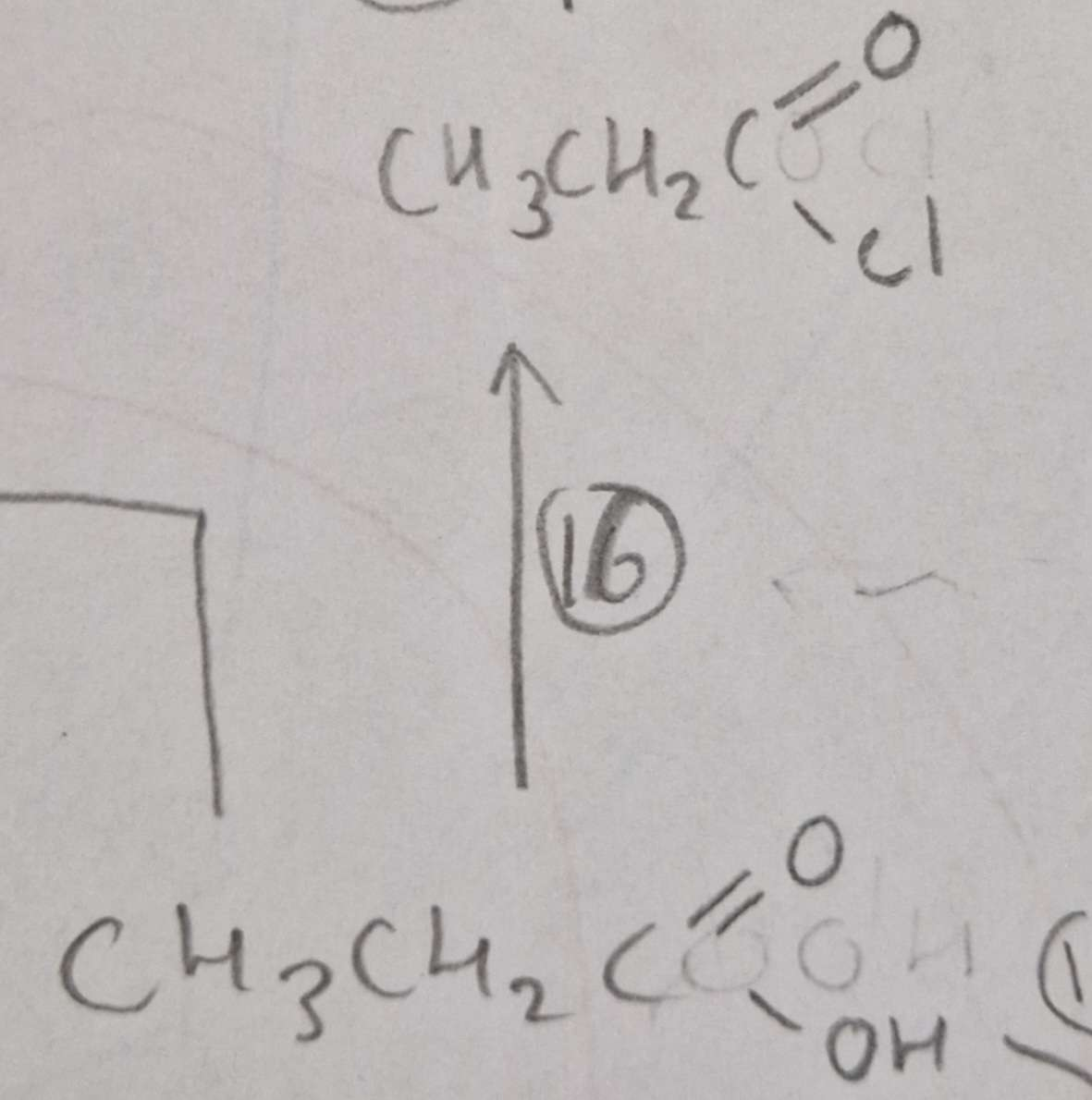
Carboxylic Acid to Acyl Chloride Conditions:
R.T.P
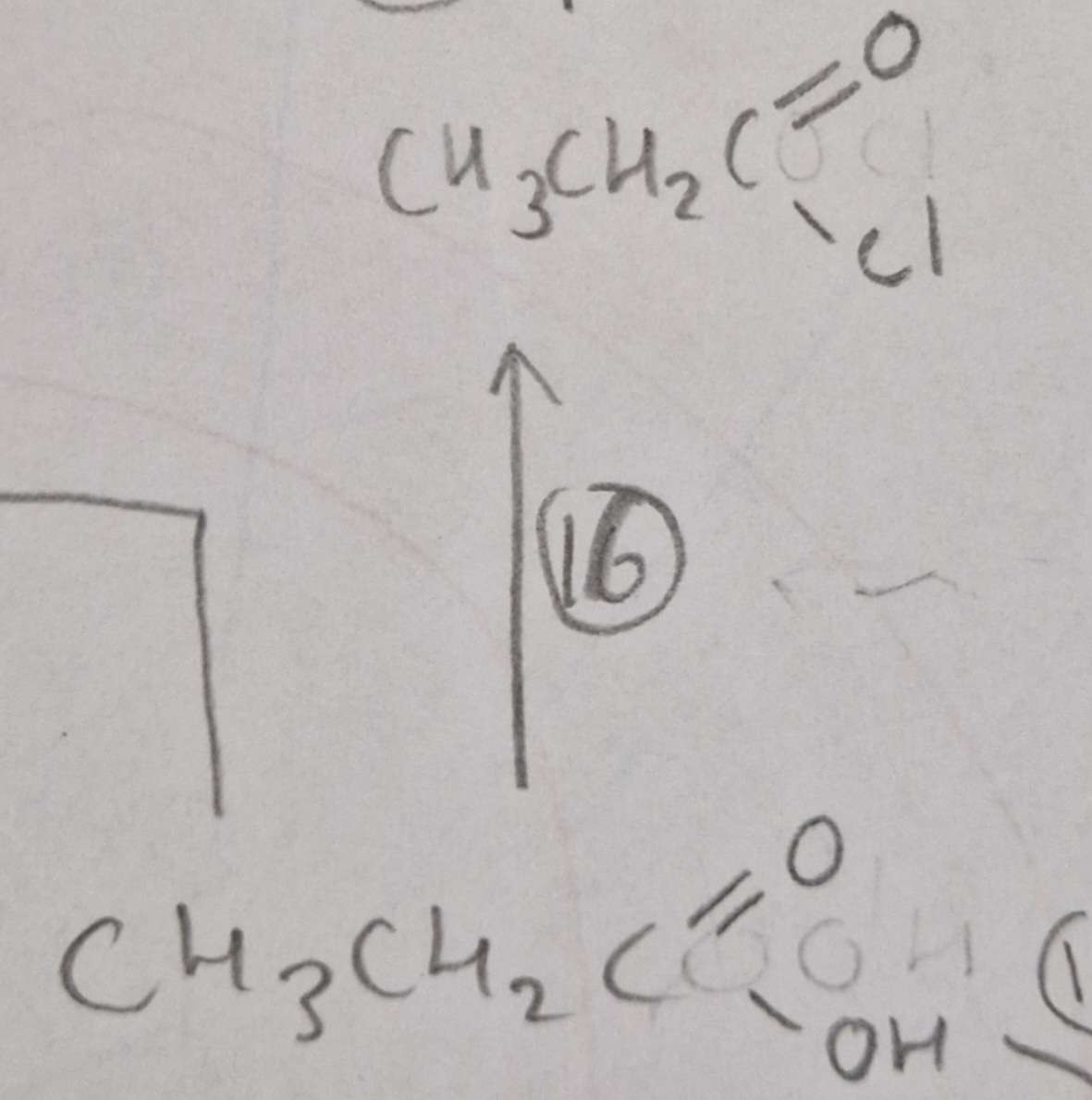
Carboxylic Acid to Acyl Chloride Name of Product:
Propanoyl Chloride
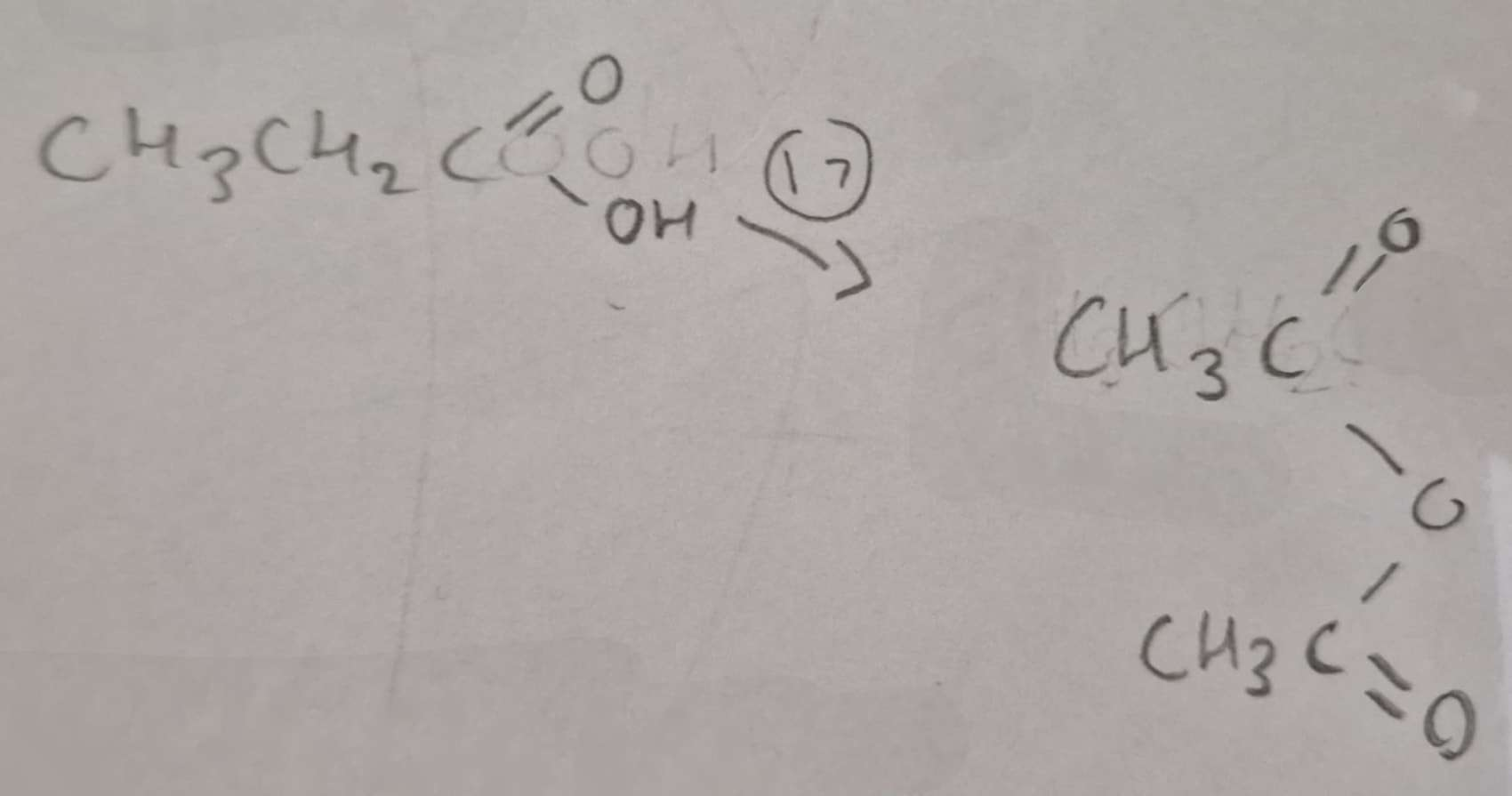
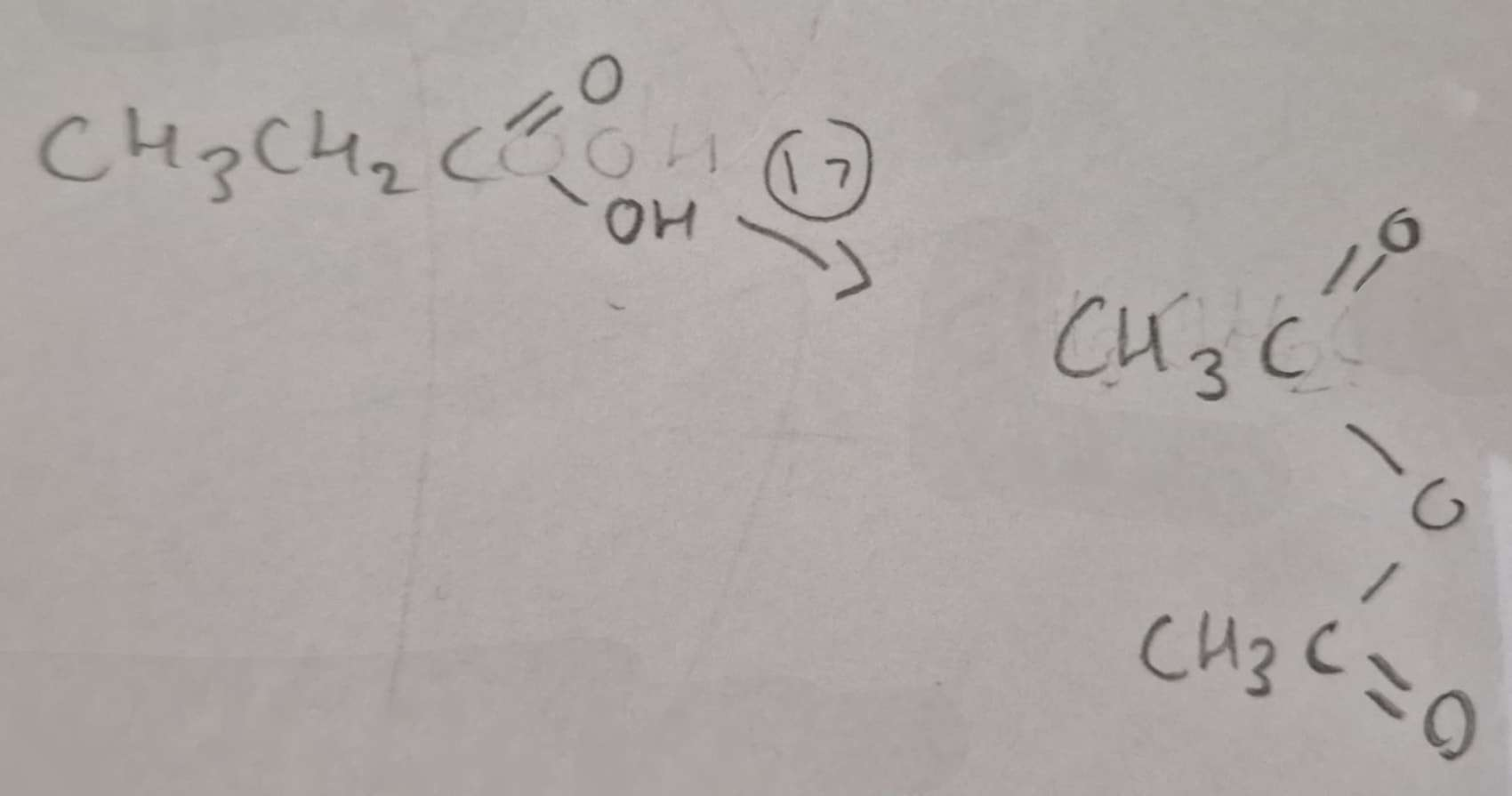
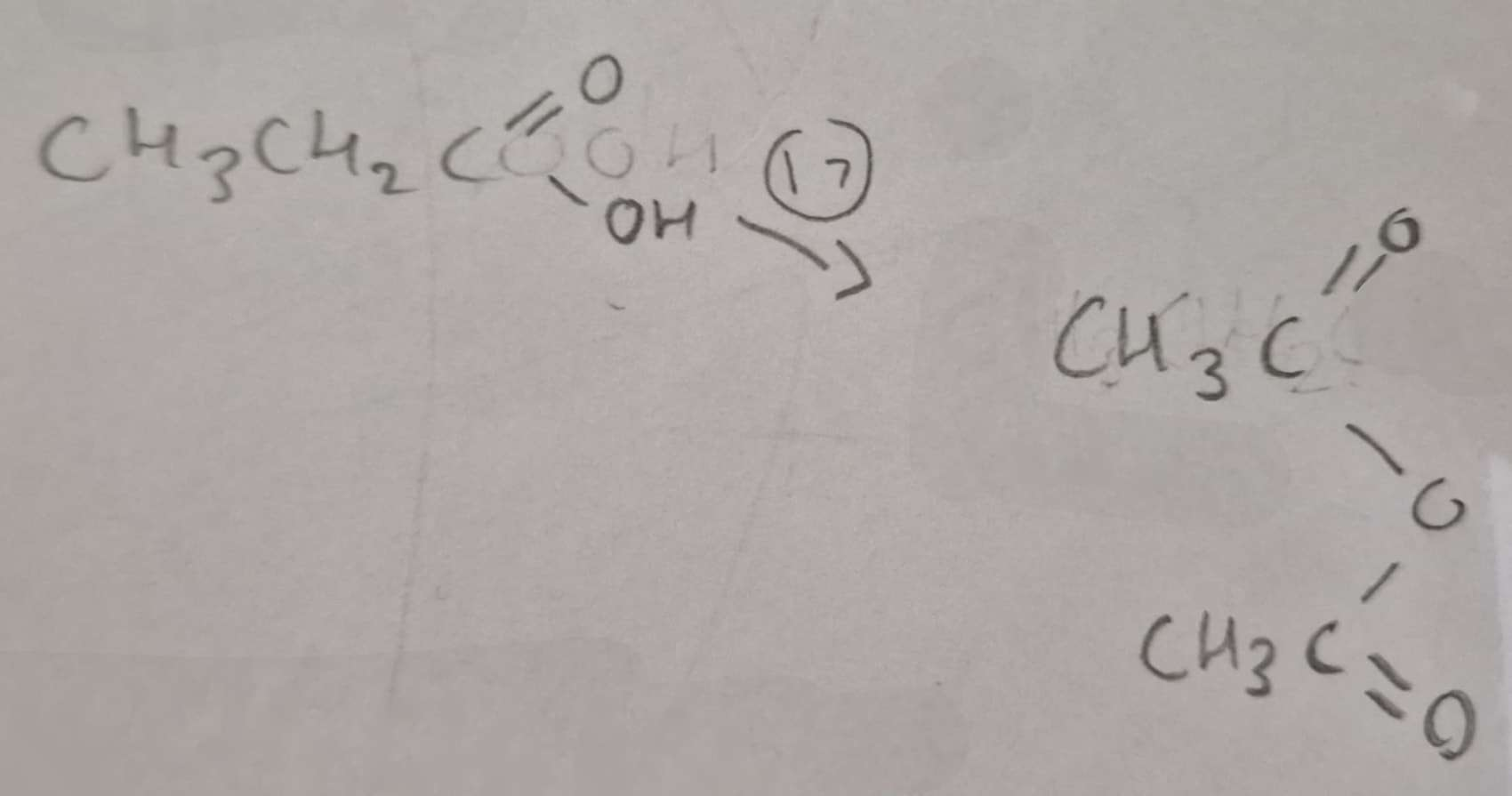
Carboxylic Acid to Acid Anhydride Type of Reaction:
Dehydration
Carboxylic Acid to Acid Anhydride Mechanism:
None
Carboxylic Acid to Acid Anhydride Reagents:
H3PO4
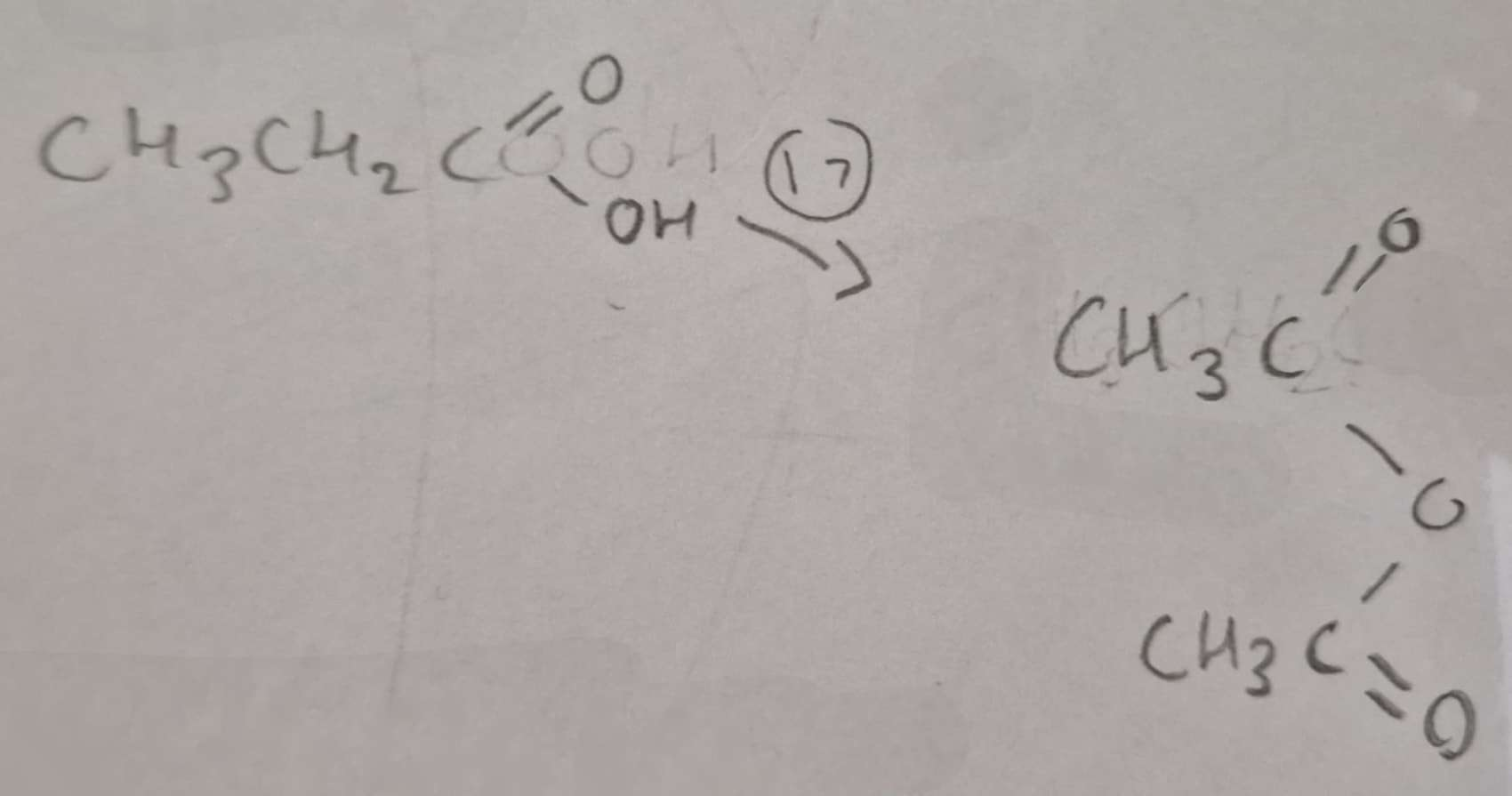
Carboxylic Acid to Acid Anhydride Conditions:
Heat
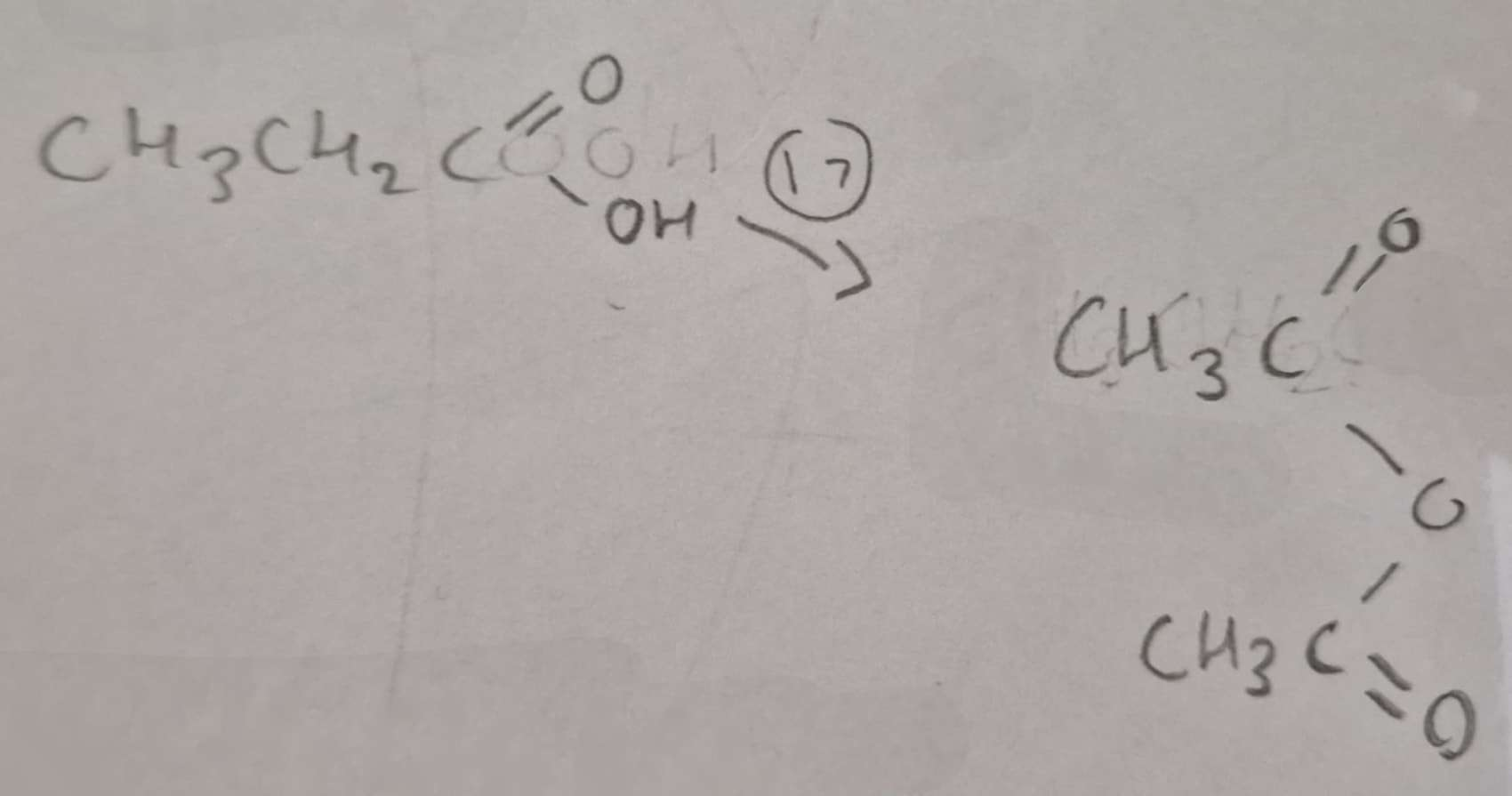
Carboxylic Acid to Acid Anhydride Name of Product:
Ethanoic Anhydride
REACTION 18 ammonium salt? what is the reaction even supposed to be
(Question for James)
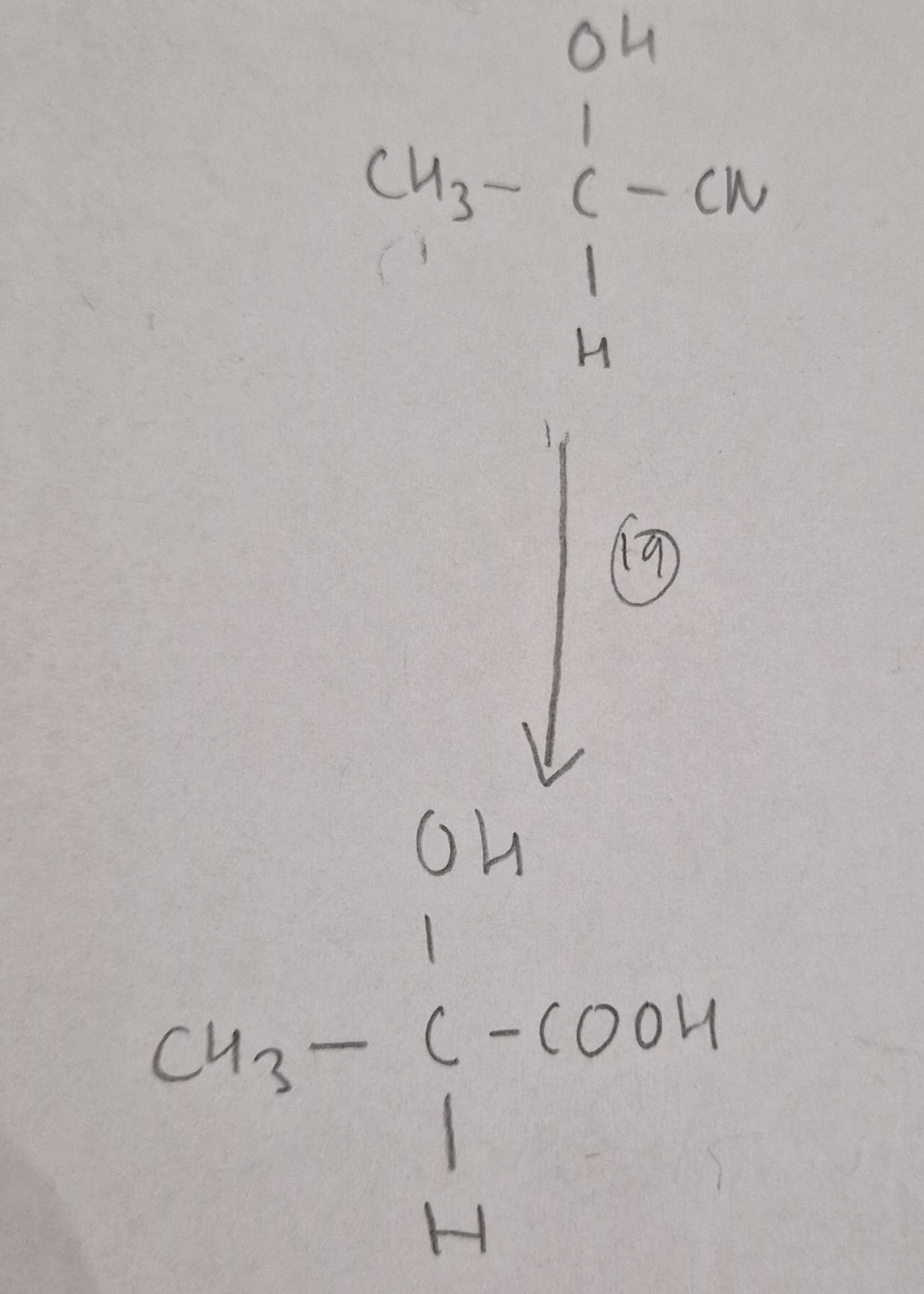
Hydroxynitrile to Hydroxycarboxylic Acid Type of Reaction:
Hydrolysis
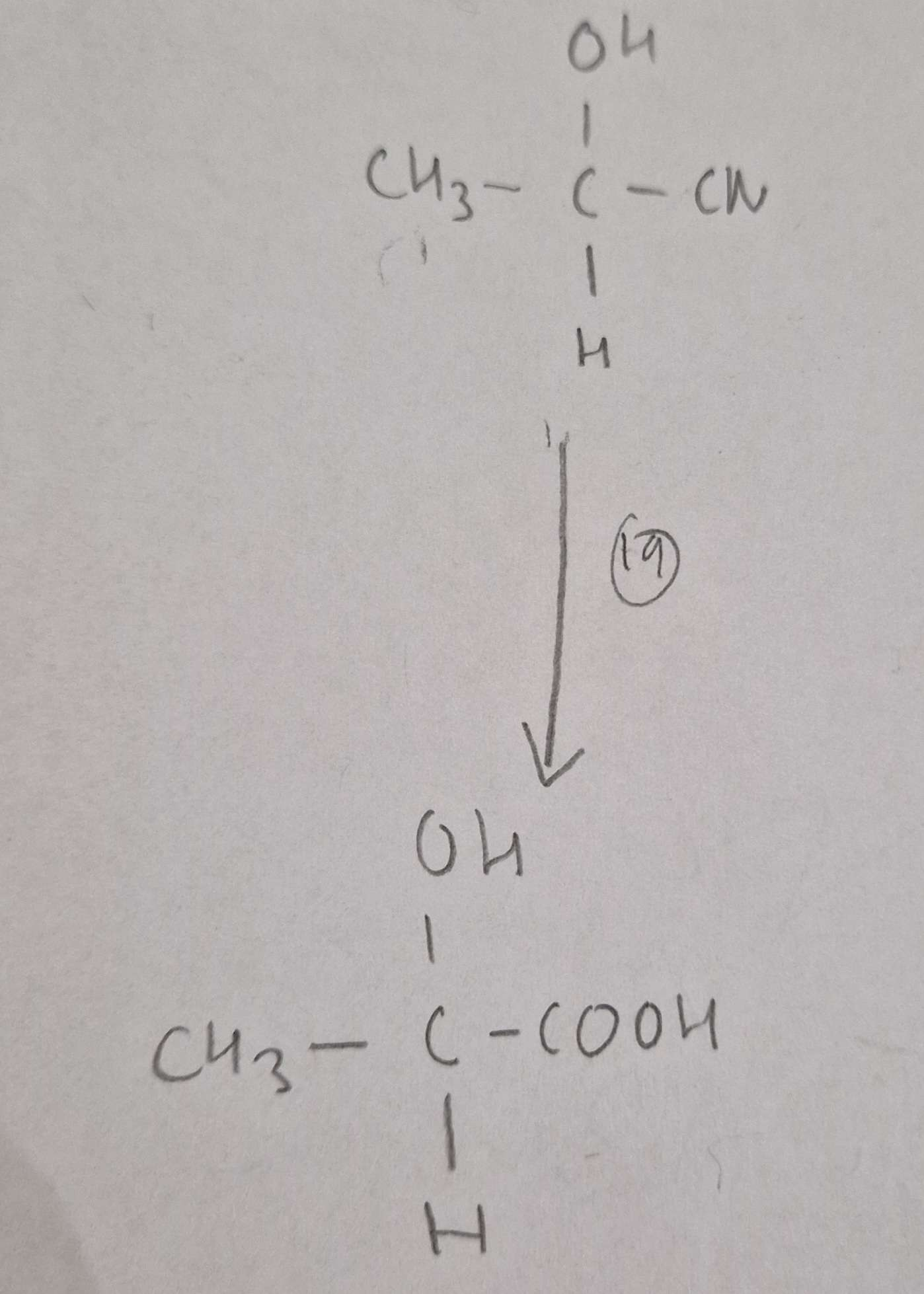
Hydroxynitrile to Hydroxycarboxylic Acid Mechanism:
None
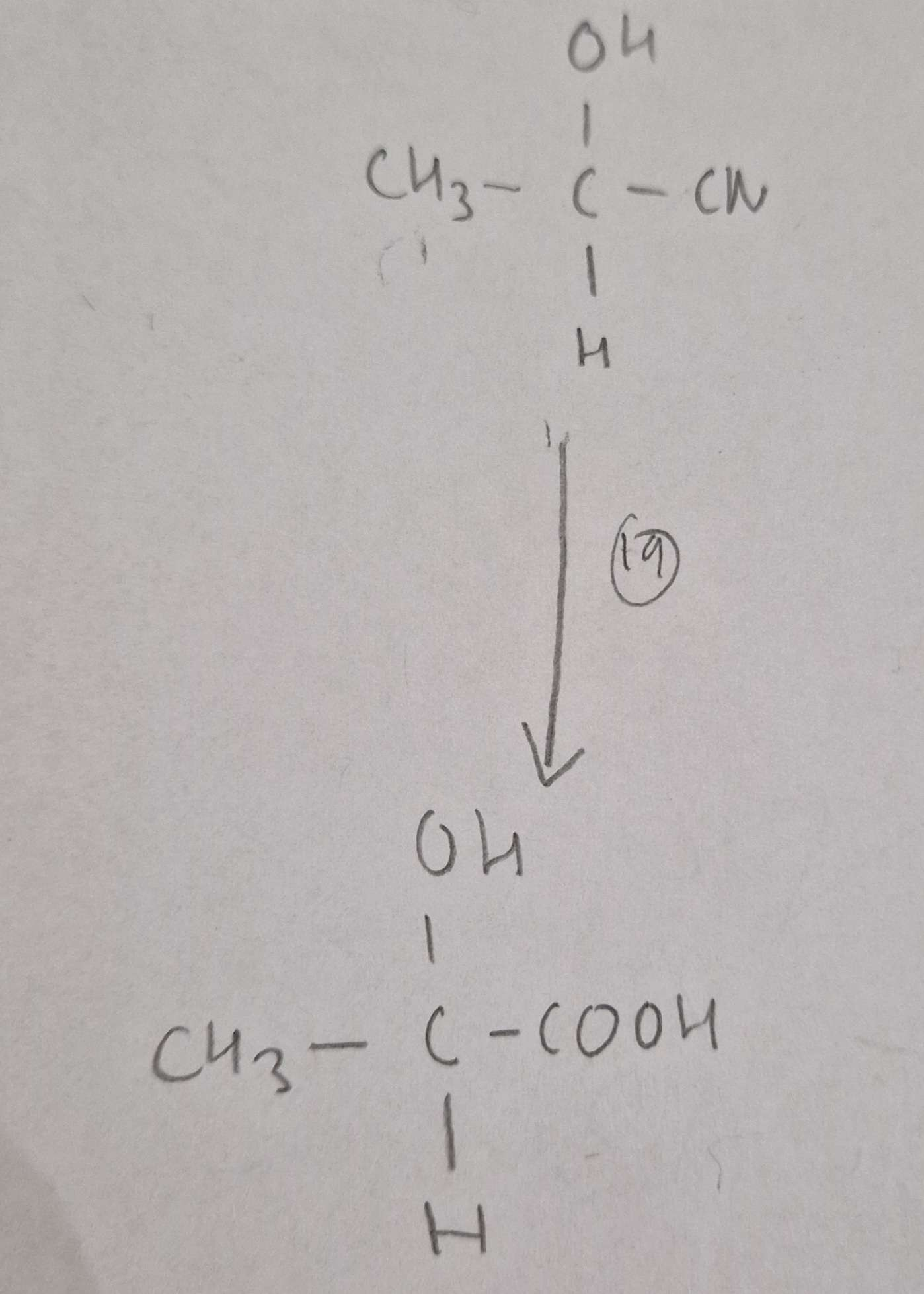
Hydroxynitrile to Hydroxycarboxylic Acid Reagents:
HCl(aq)
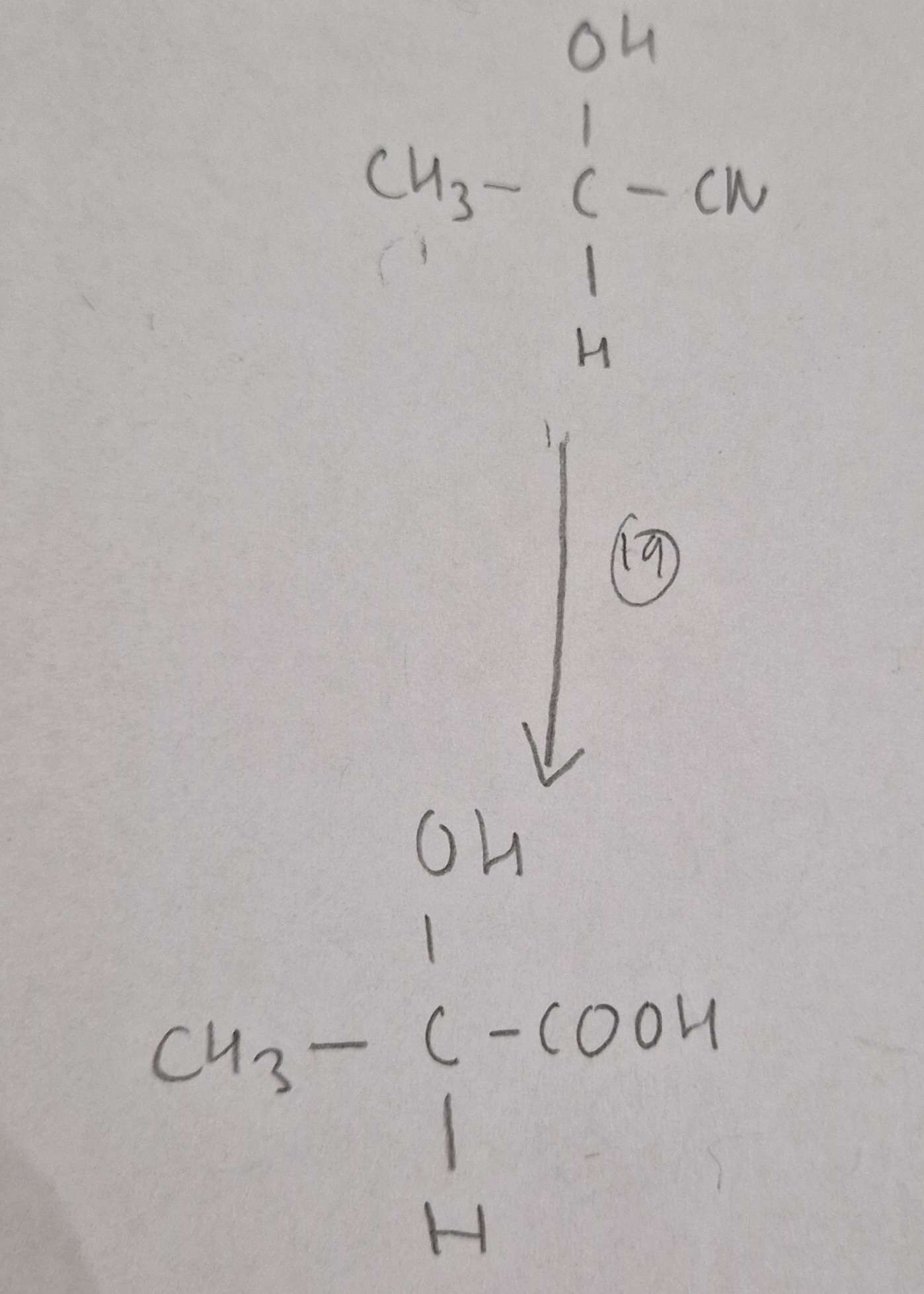
Hydroxynitrile to Hydroxycarboxylic Acid Conditions:
H.U.R
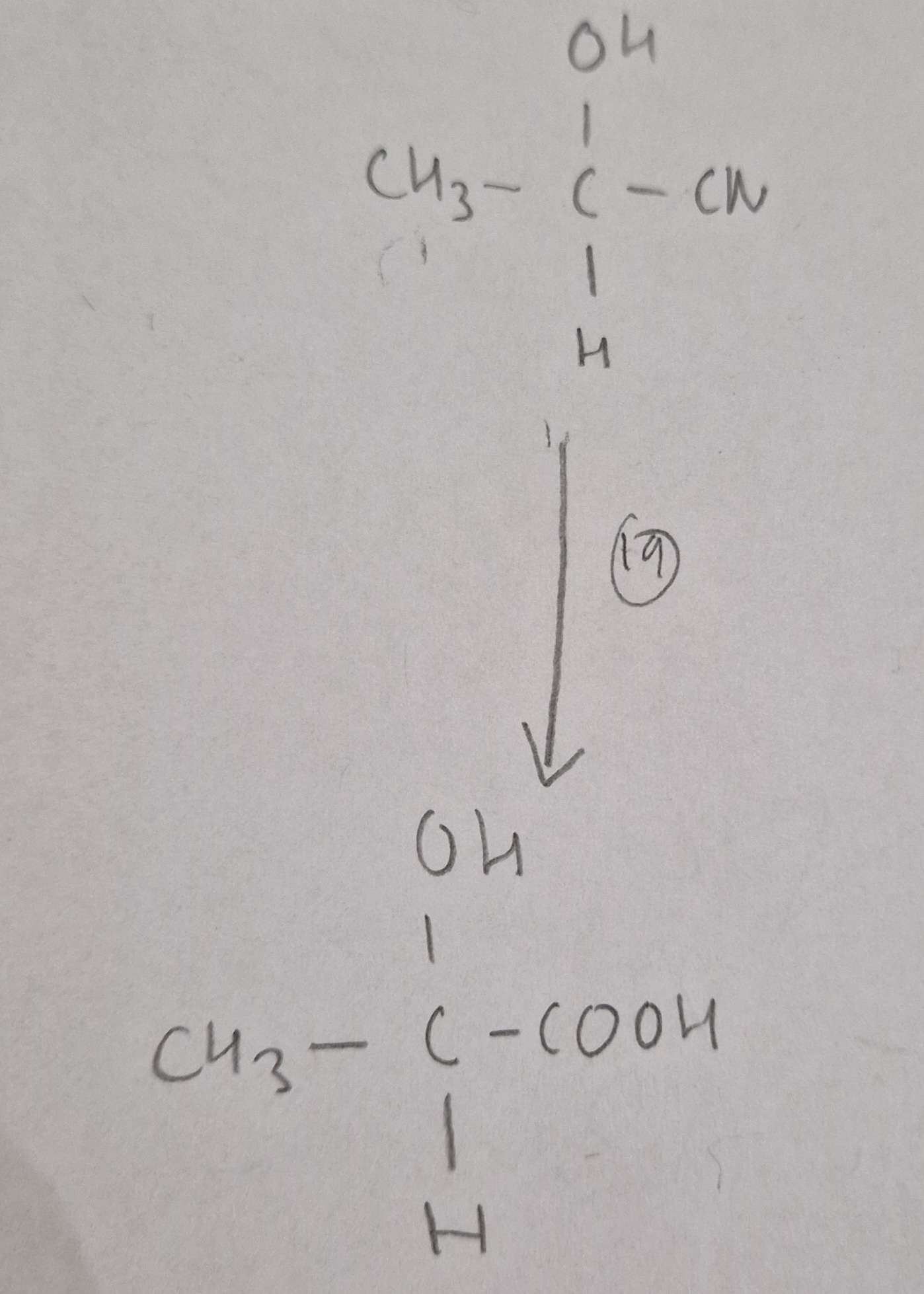
Hydroxynitrile to Hydroxycarboxylic Acid Name of Product:
2-Hydroxypropanoic Acid (Latic Acid)
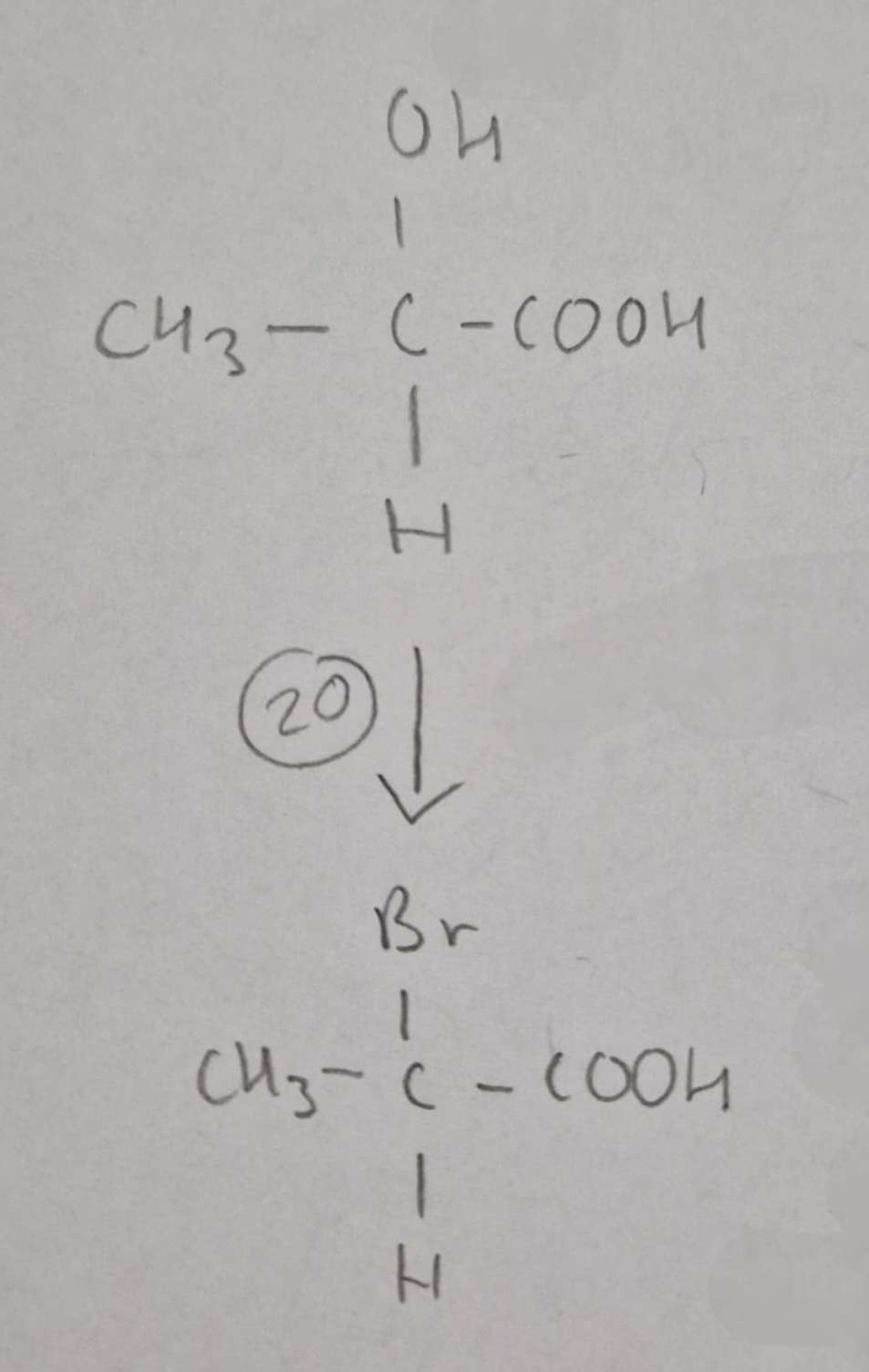
Hydroxycarboxylic Acid to Haloalkanic Acid Type of Reaction:
Substitution
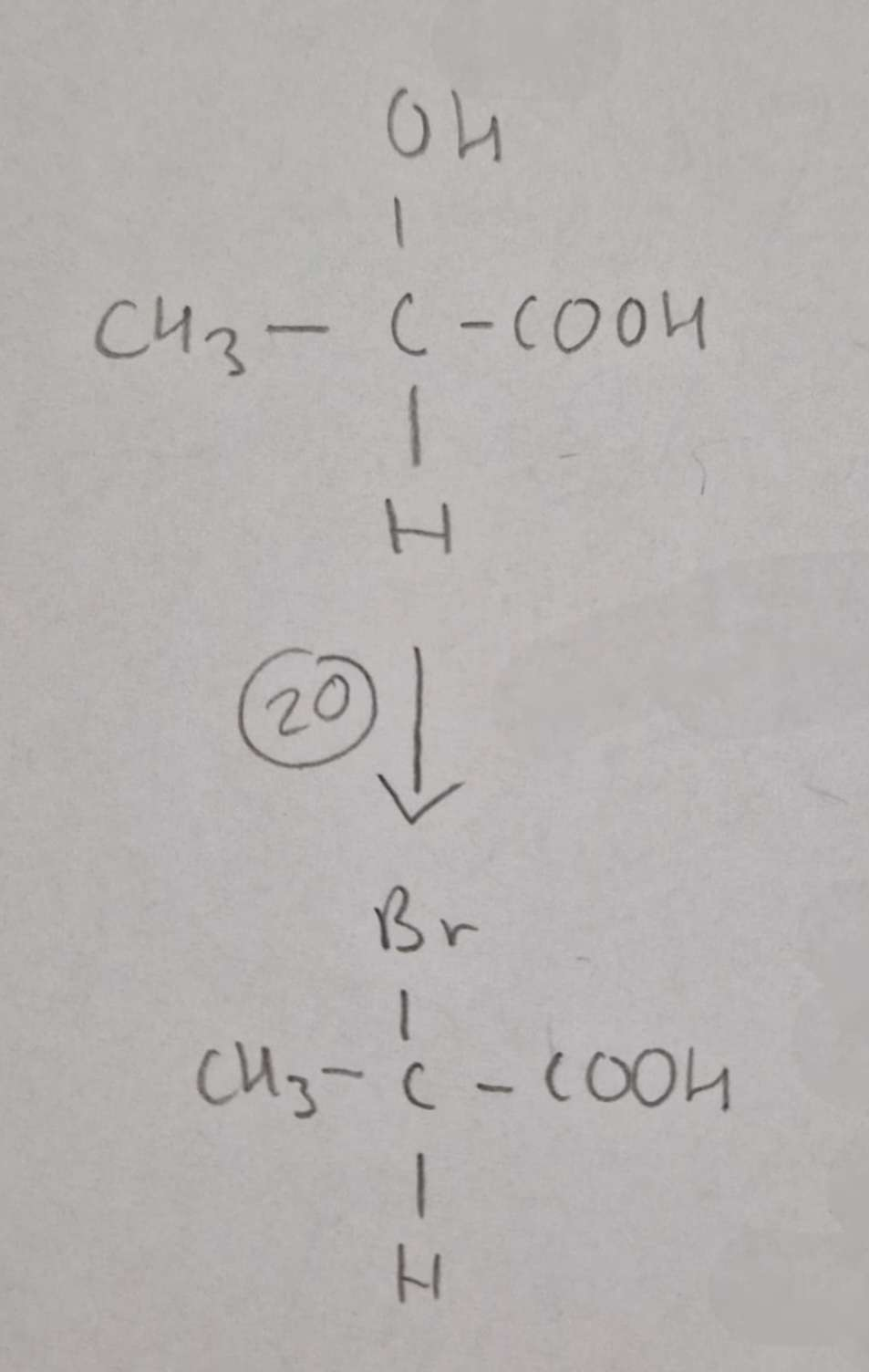
Hydroxycarboxylic Acid to Haloalkanic Acid Mechanism:
None
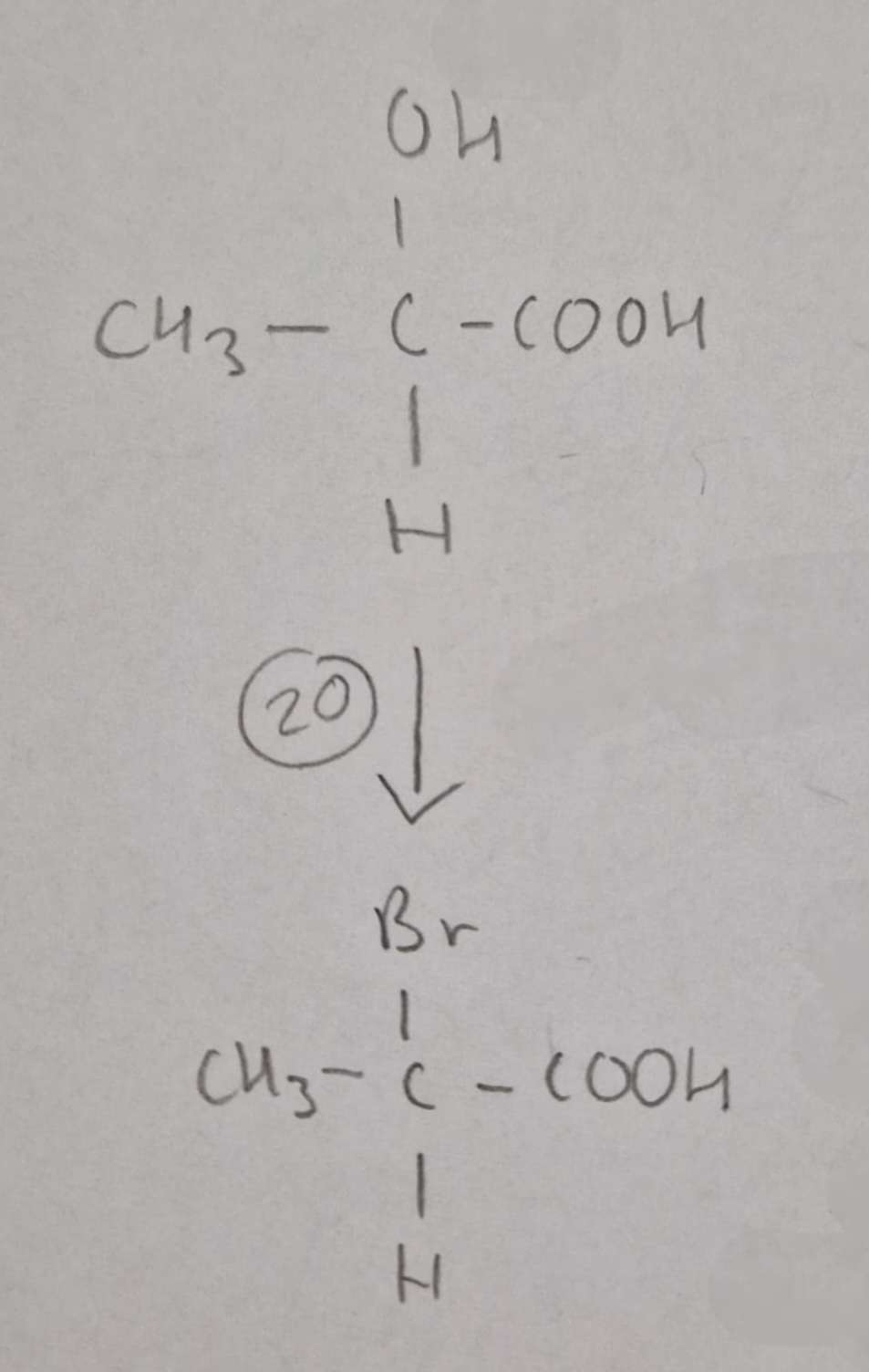
Hydroxycarboxylic Acid to Haloalkanic Acid Reagents:
NaBr + conc H2SO4
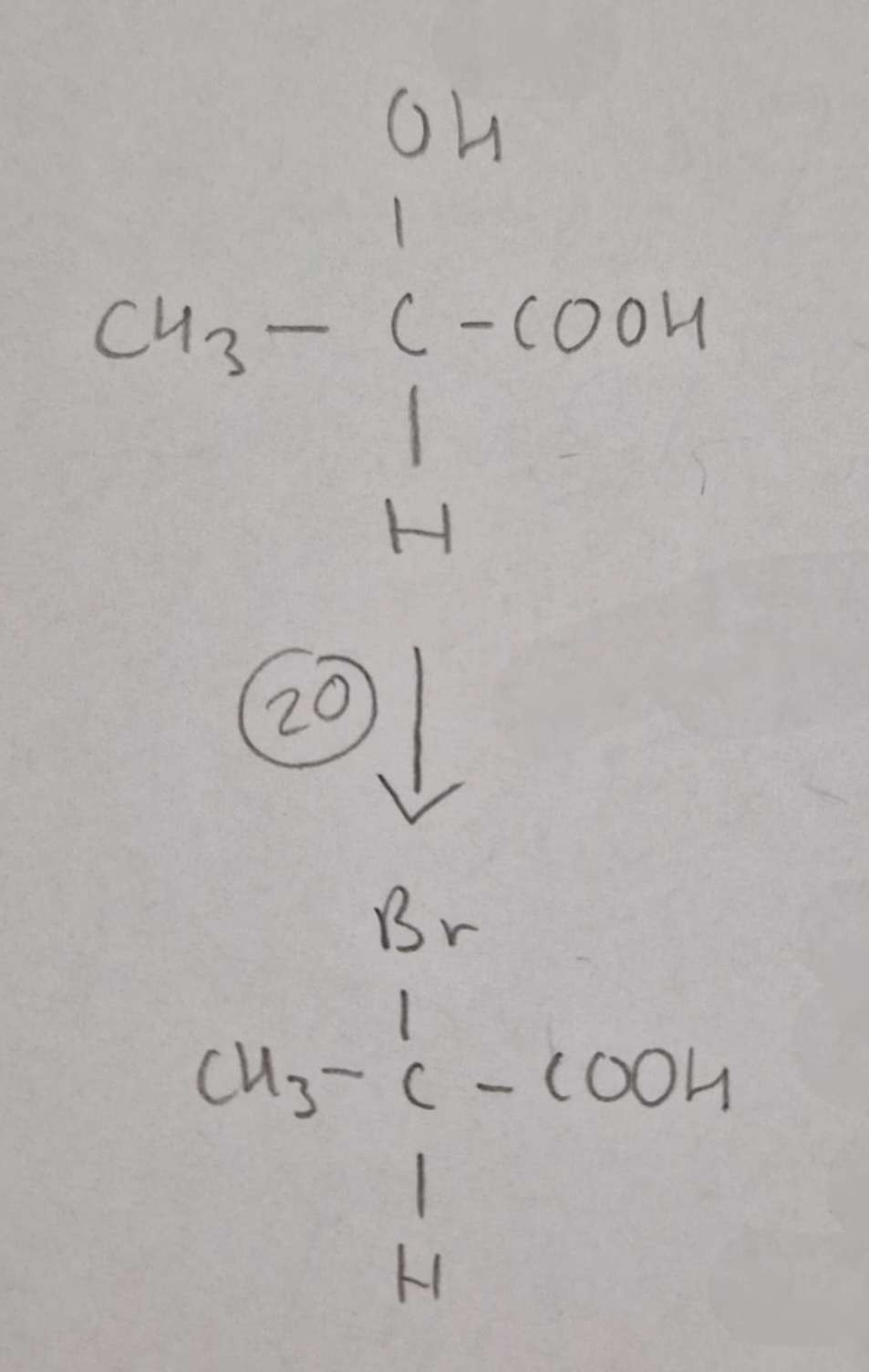
Hydroxycarboxylic Acid to Haloalkanic Acid Conditions:
H.U.R
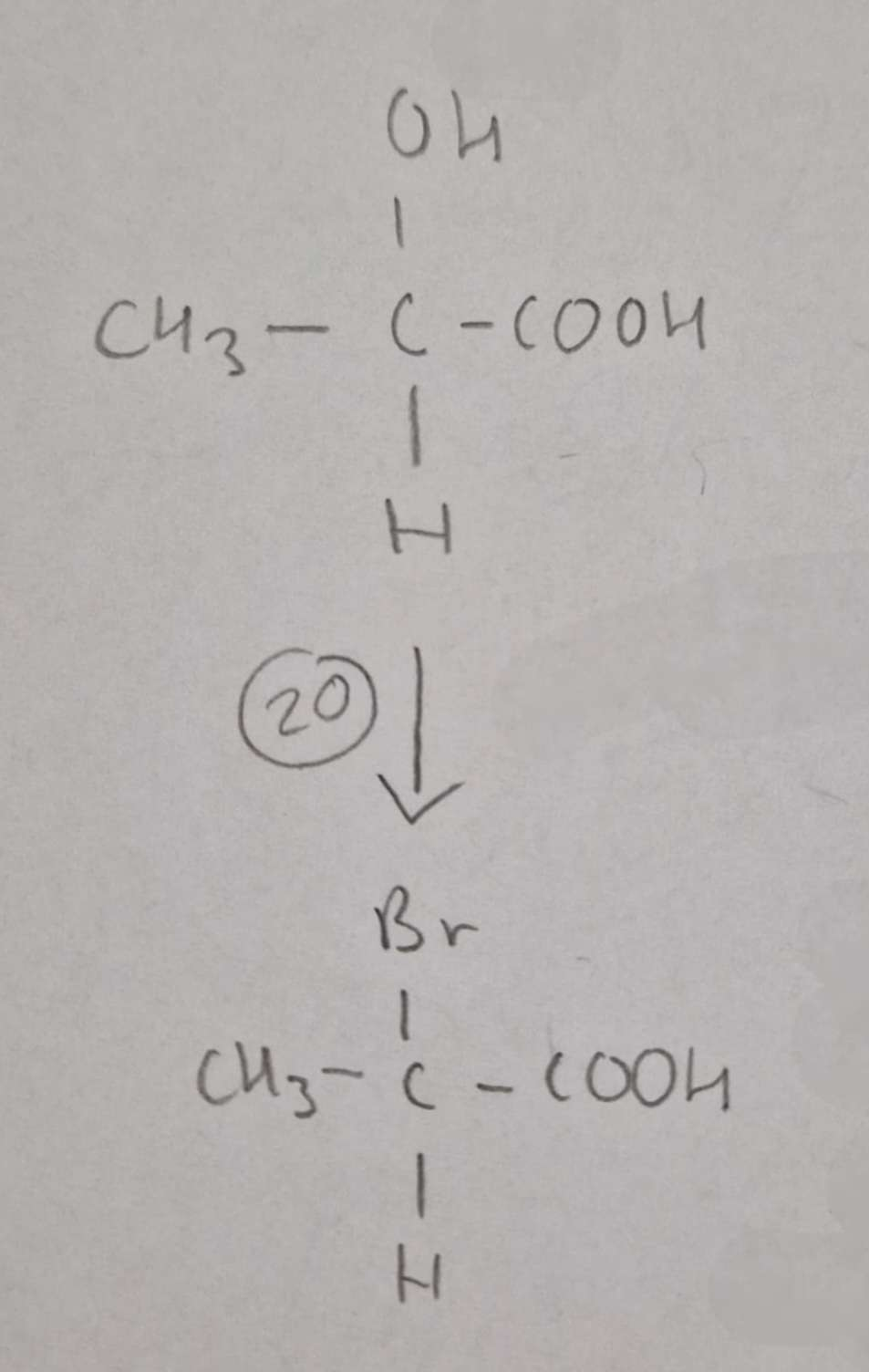
Hydroxycarboxylic Acid to 2-Bromopropanoic Acid Name of Product:
2-Bromopropanoic Acid
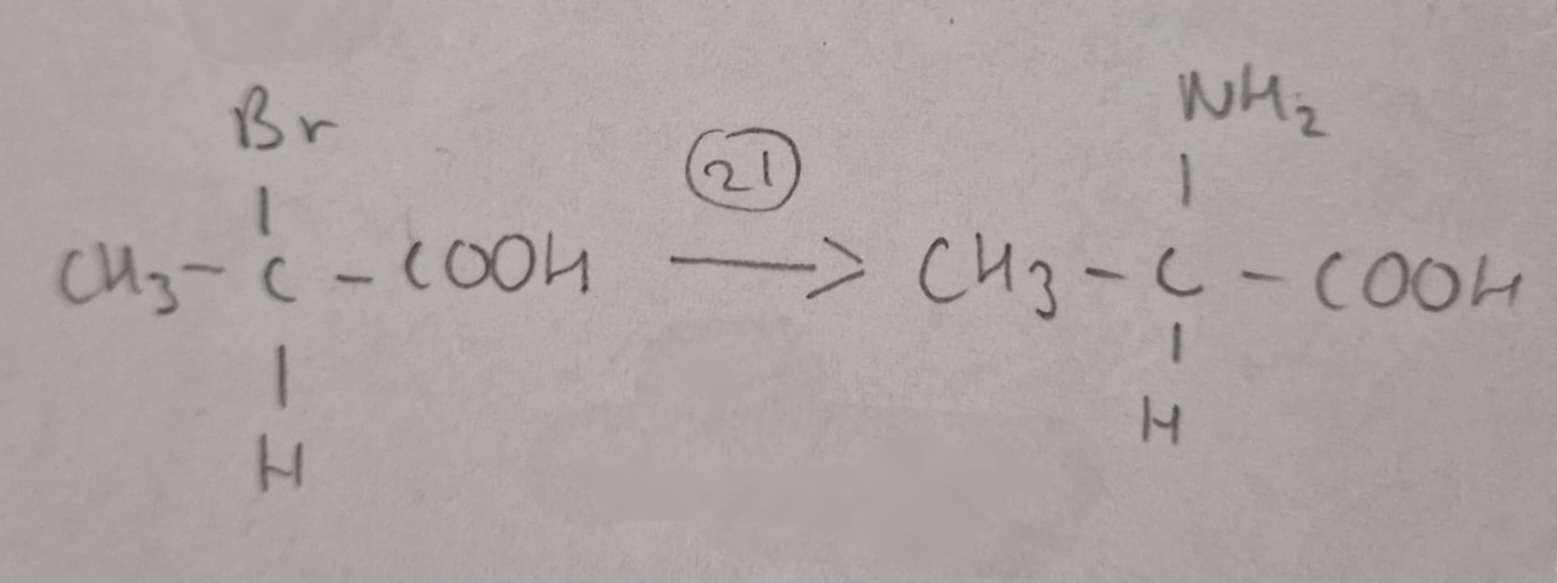
Haloalkanic Acid to Amino Acid Type of Reaction:
Substitution
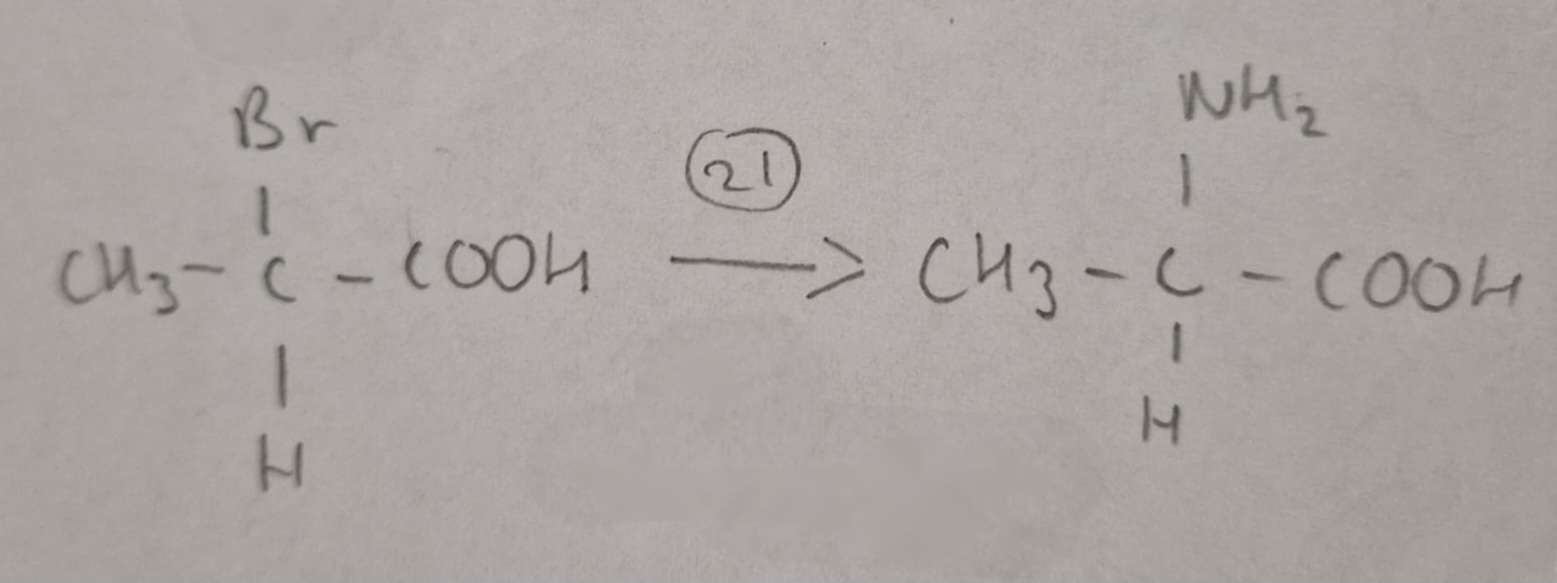
Haloalkanic Acid to Amino Acid Mechanism:
None
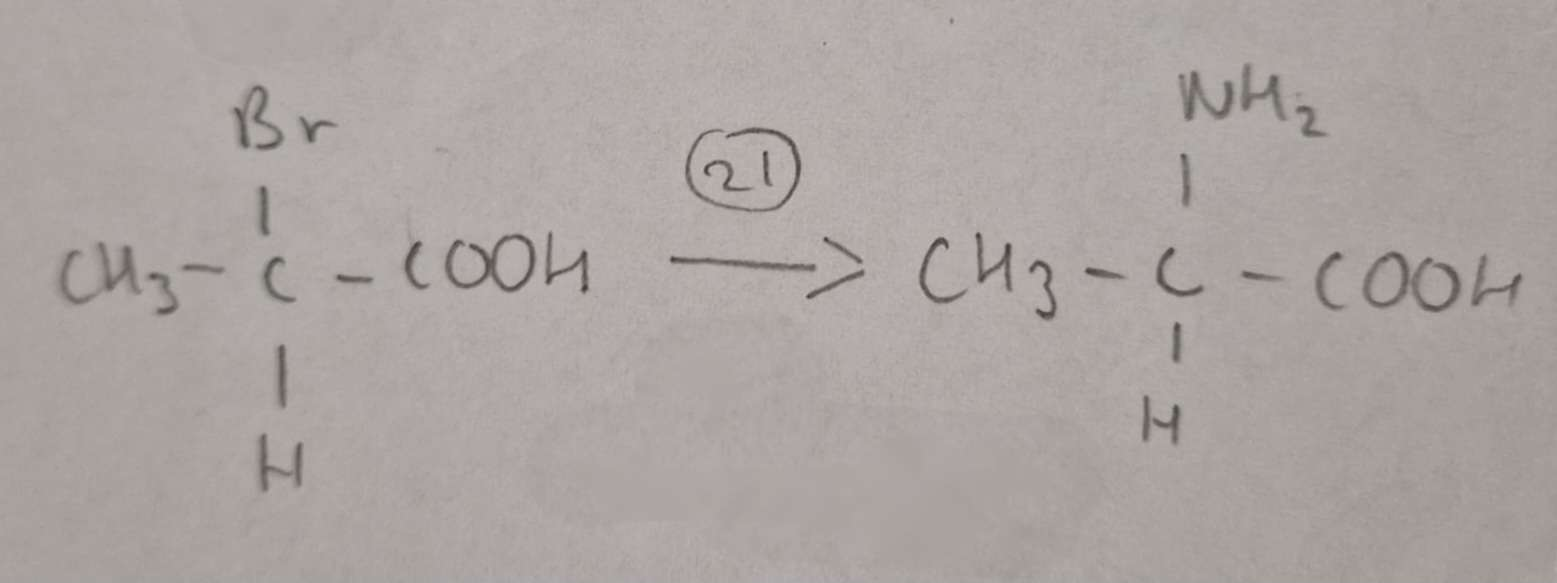
Haloalkanic Acid to Amino Acid Reagents:
NH3 in excess Ethanol

Haloalkanic Acid to Amino Acid Conditions:
H.U.P
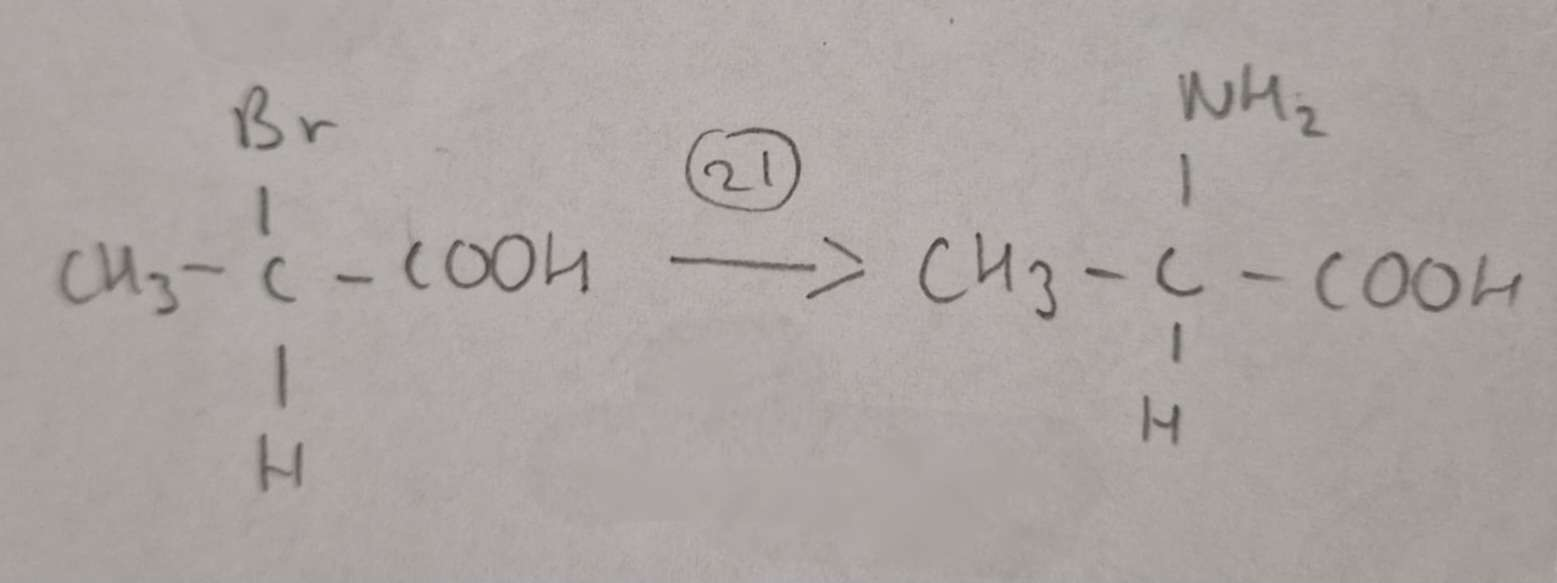
Haloalkanic Acid to Amino Acid Name of Product:
2-Aminopropanoic Acid
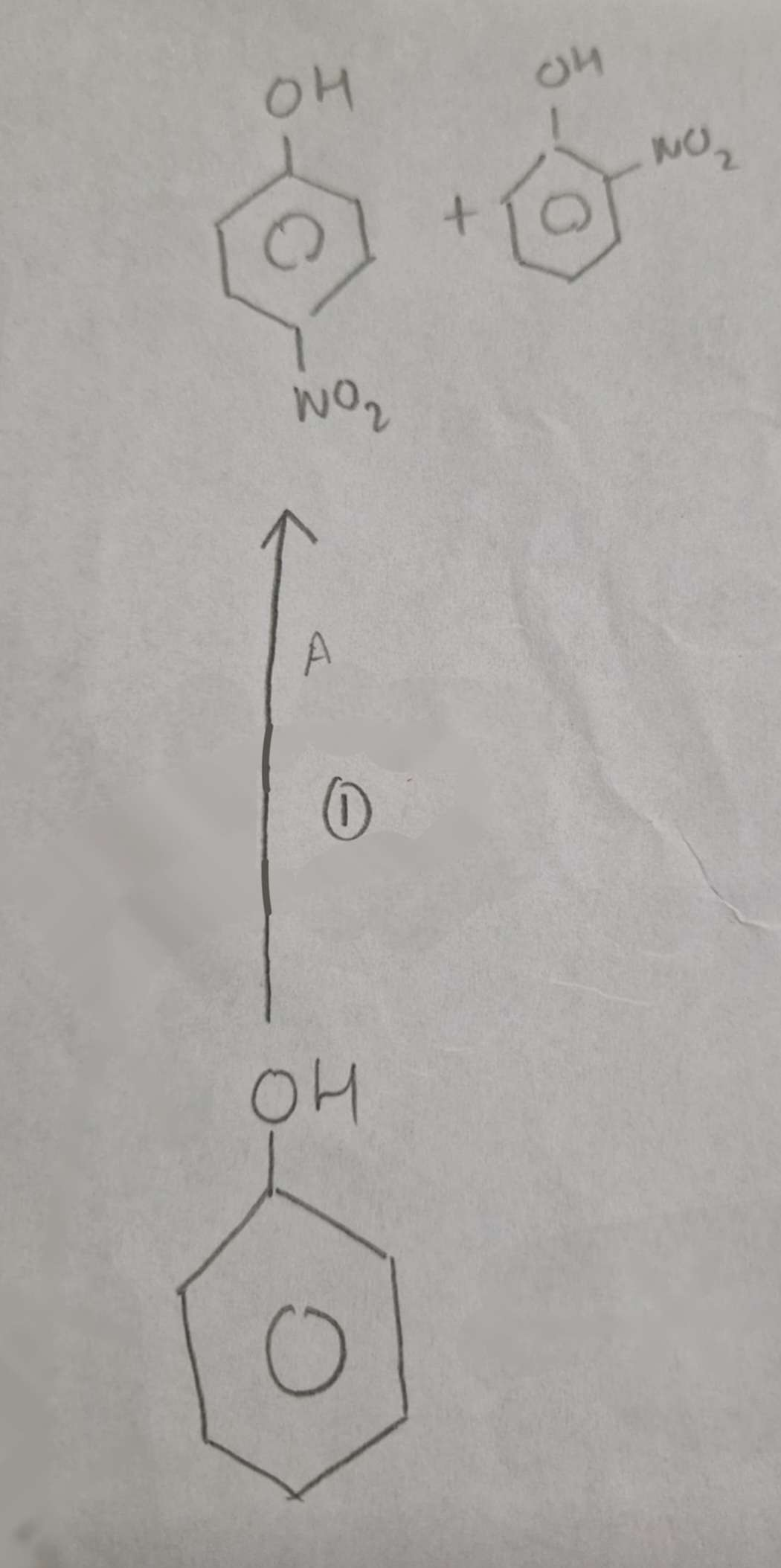
Phenol to Nitrophenols Type of Reaction:
Substitution (Nitration)
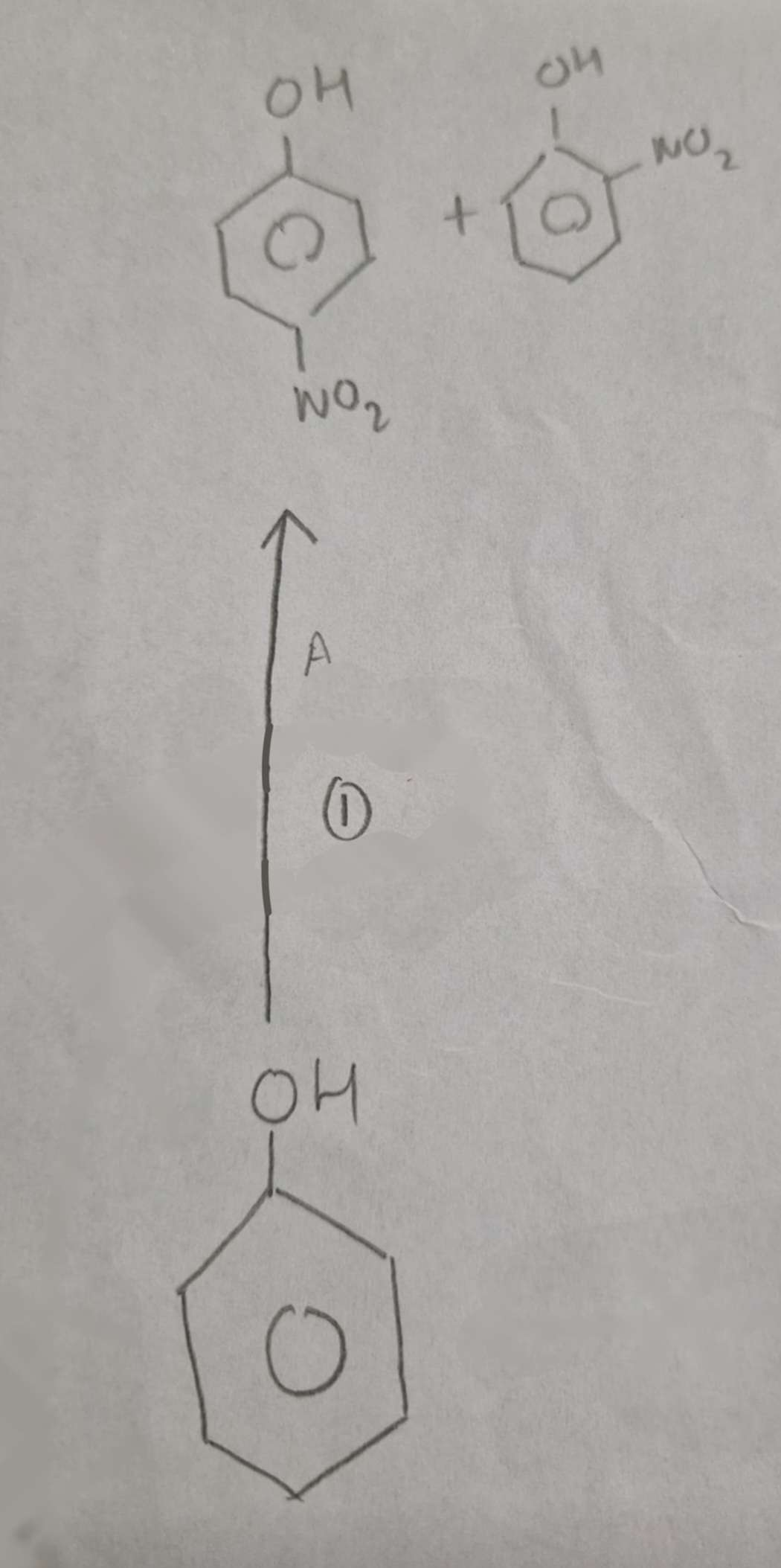
Phenol to Nitrophenols Mechanism:
Electrophilic Substitution
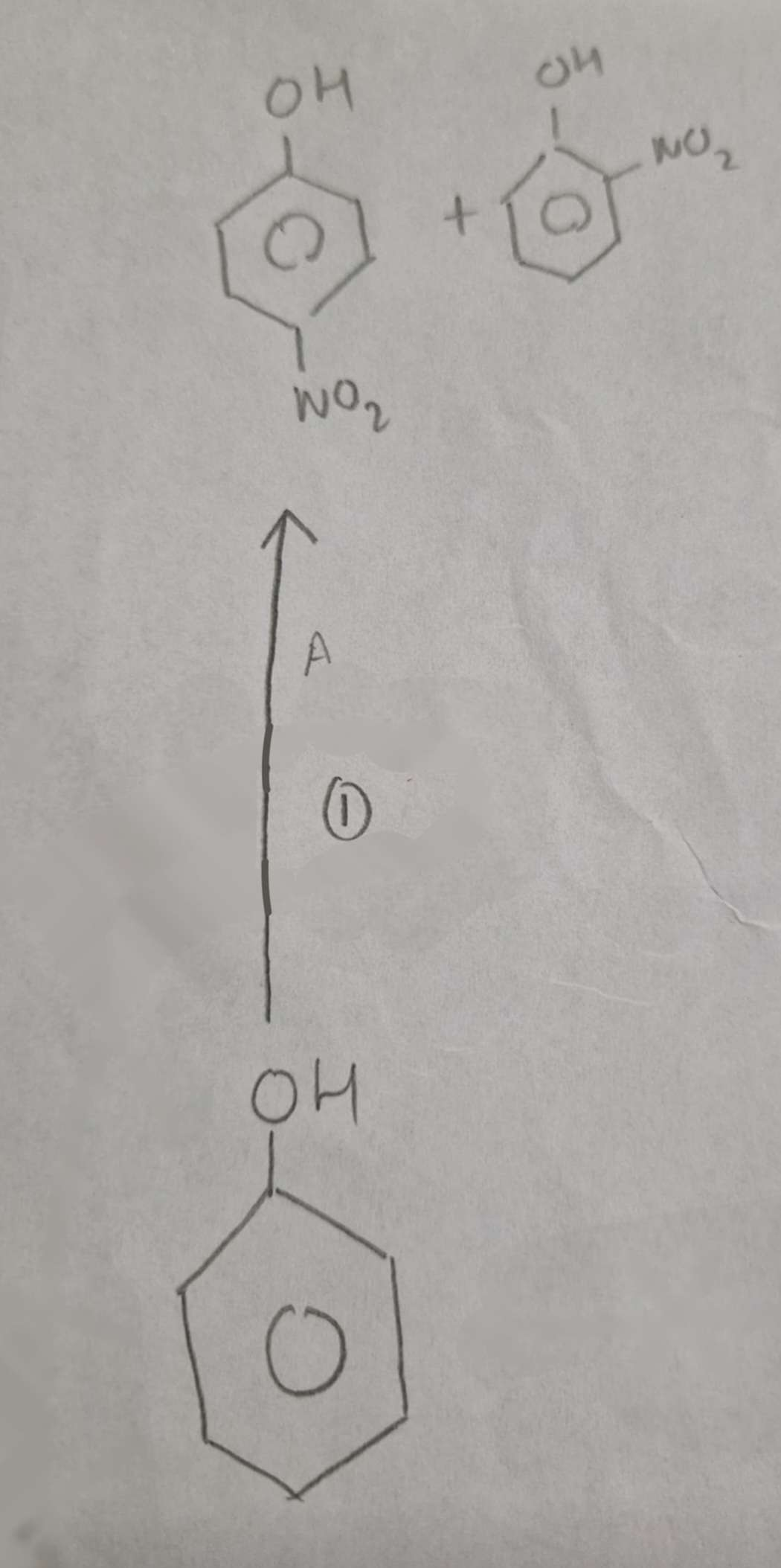
Phenol to Nitrophenols Reagents:
Dilute HNO3
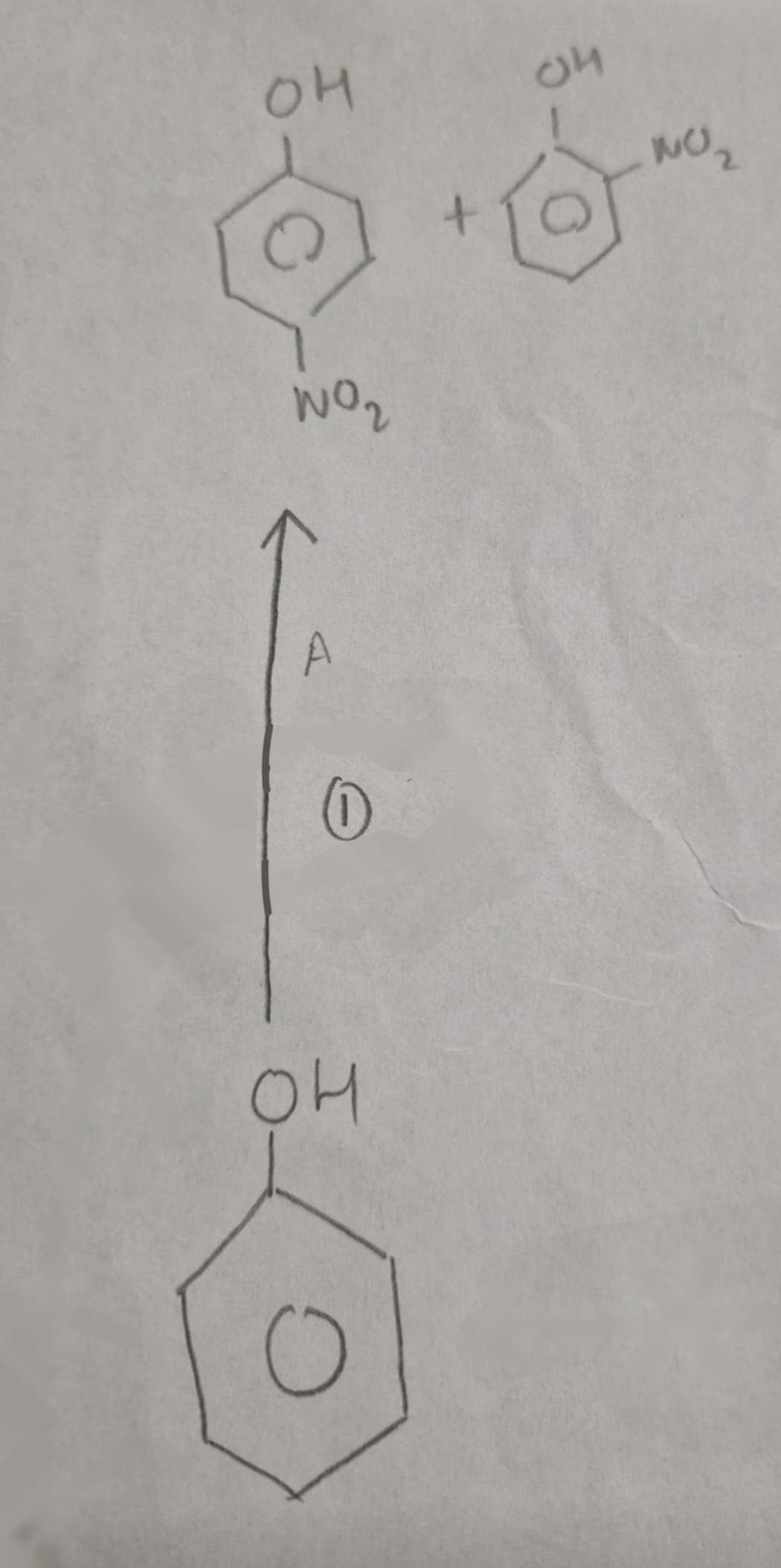
Phenol to Nitrophenols Conditions:
Warm
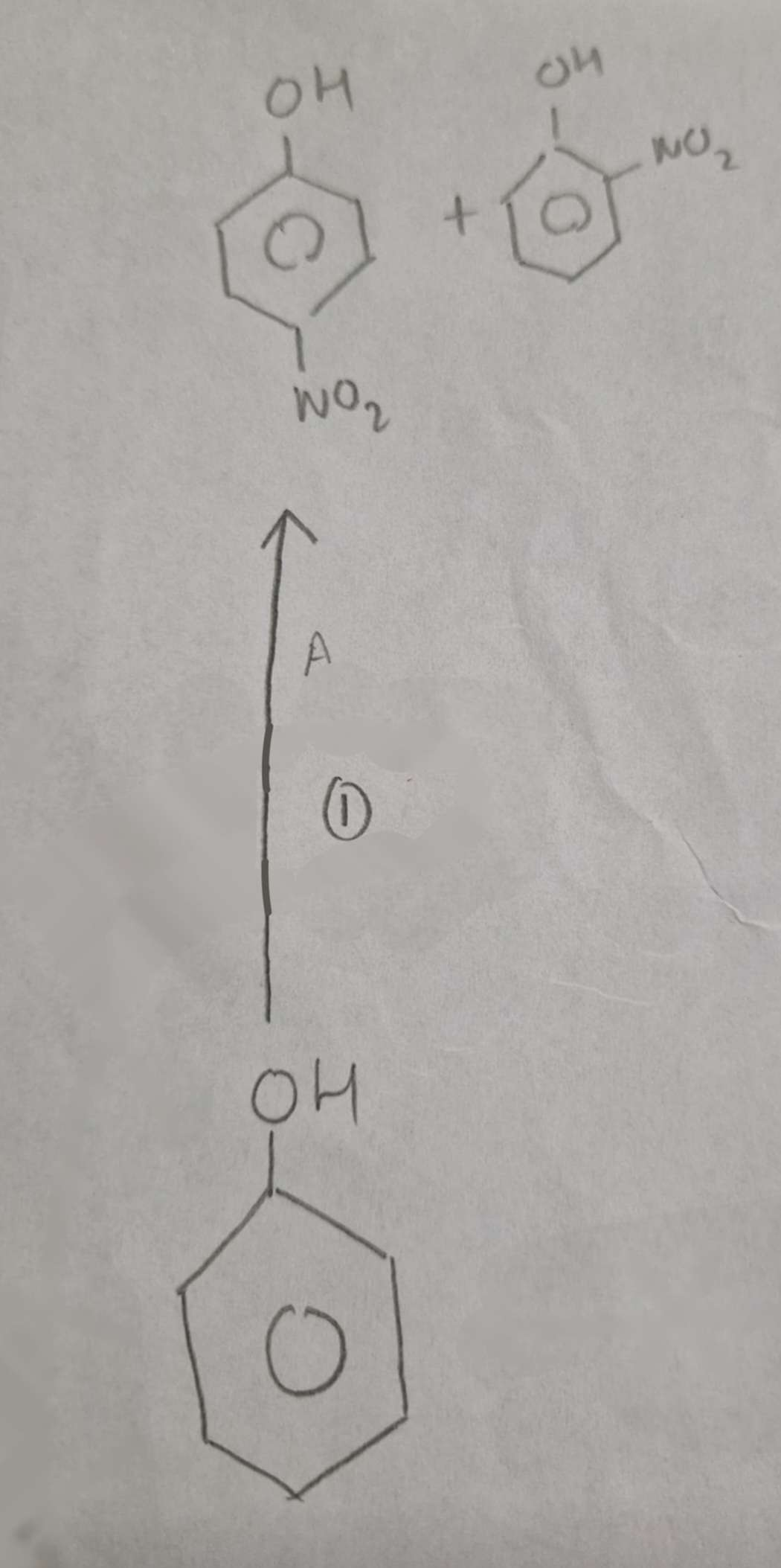
Phenol to Nitrophenols Name of Products:
2-Nitrophenol and 4-Nitrophenol
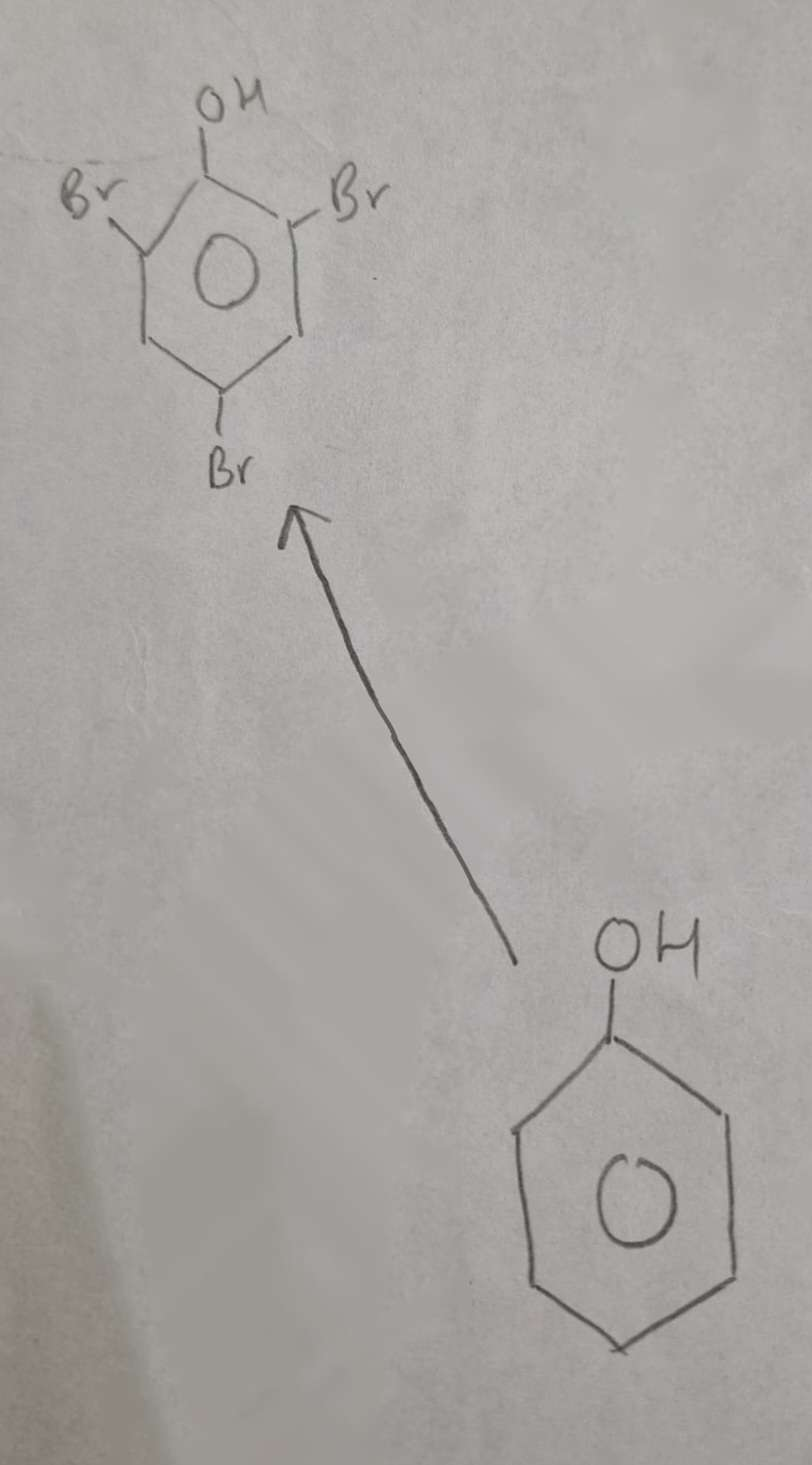
Phenol to Halophenol Type of Reaction:
Substitution (Halogenation)
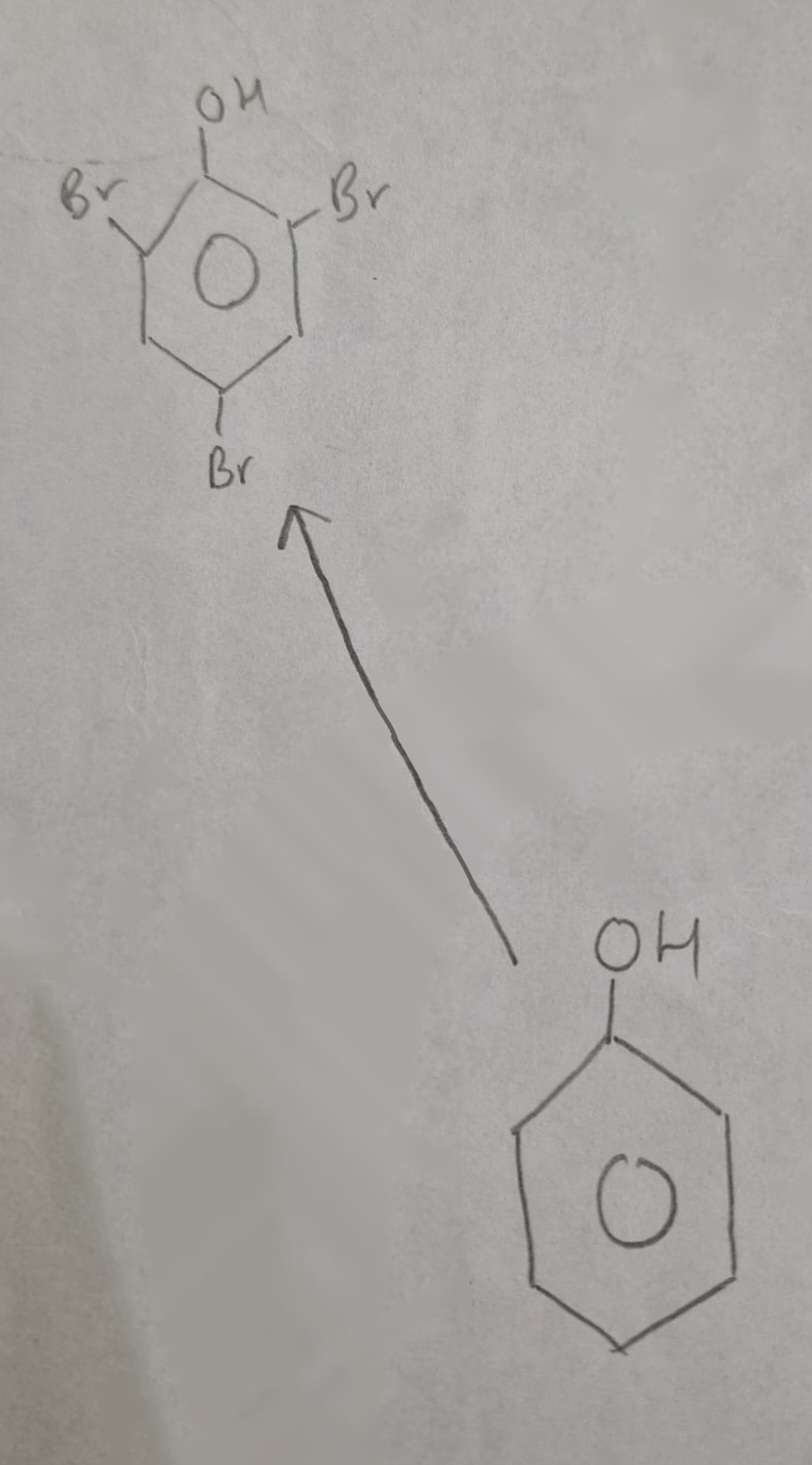
Phenol to Halophenol Mechanism:
Electrophilic Substitution
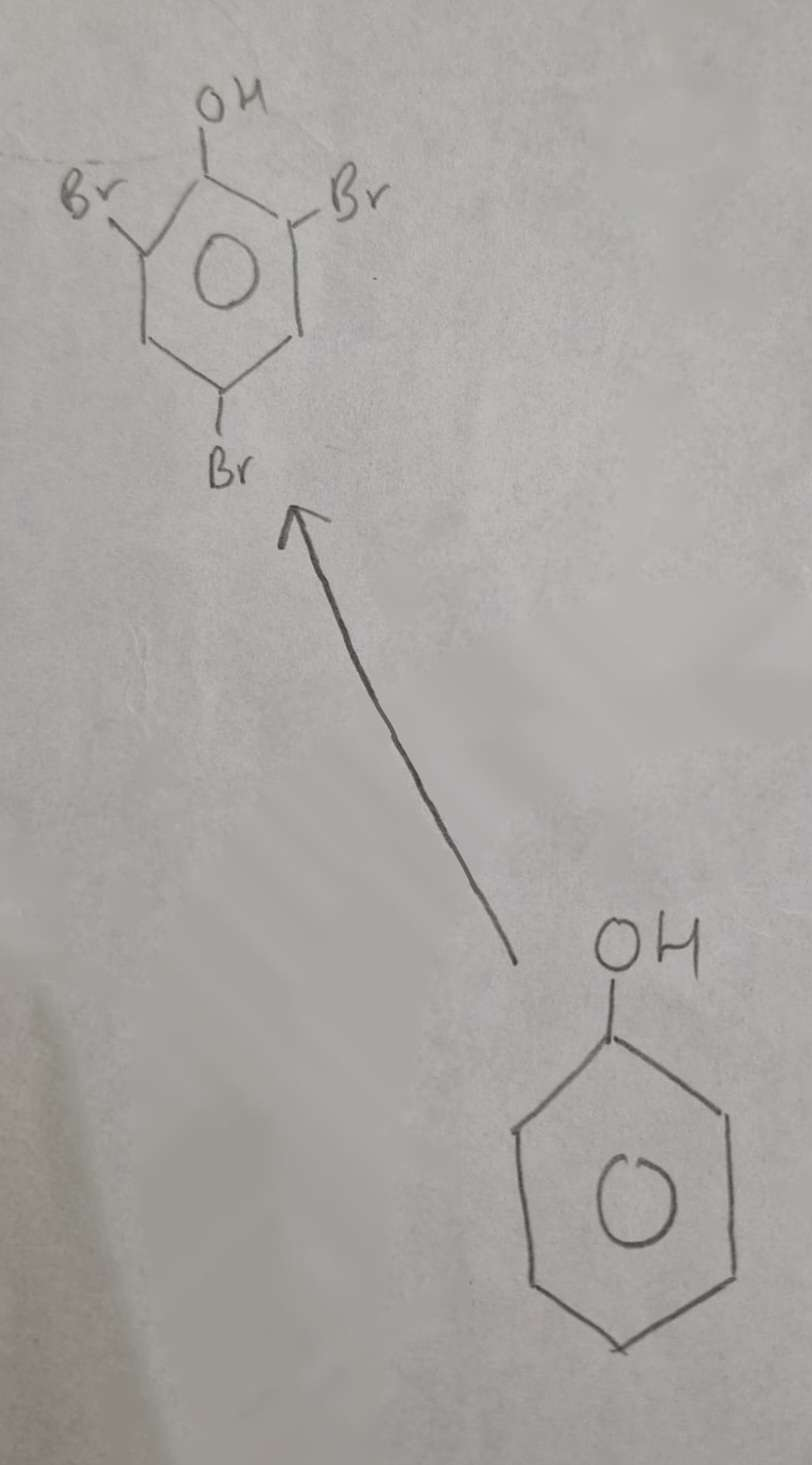
Phenol to Halophenol Reagents:
Br2 (aq)
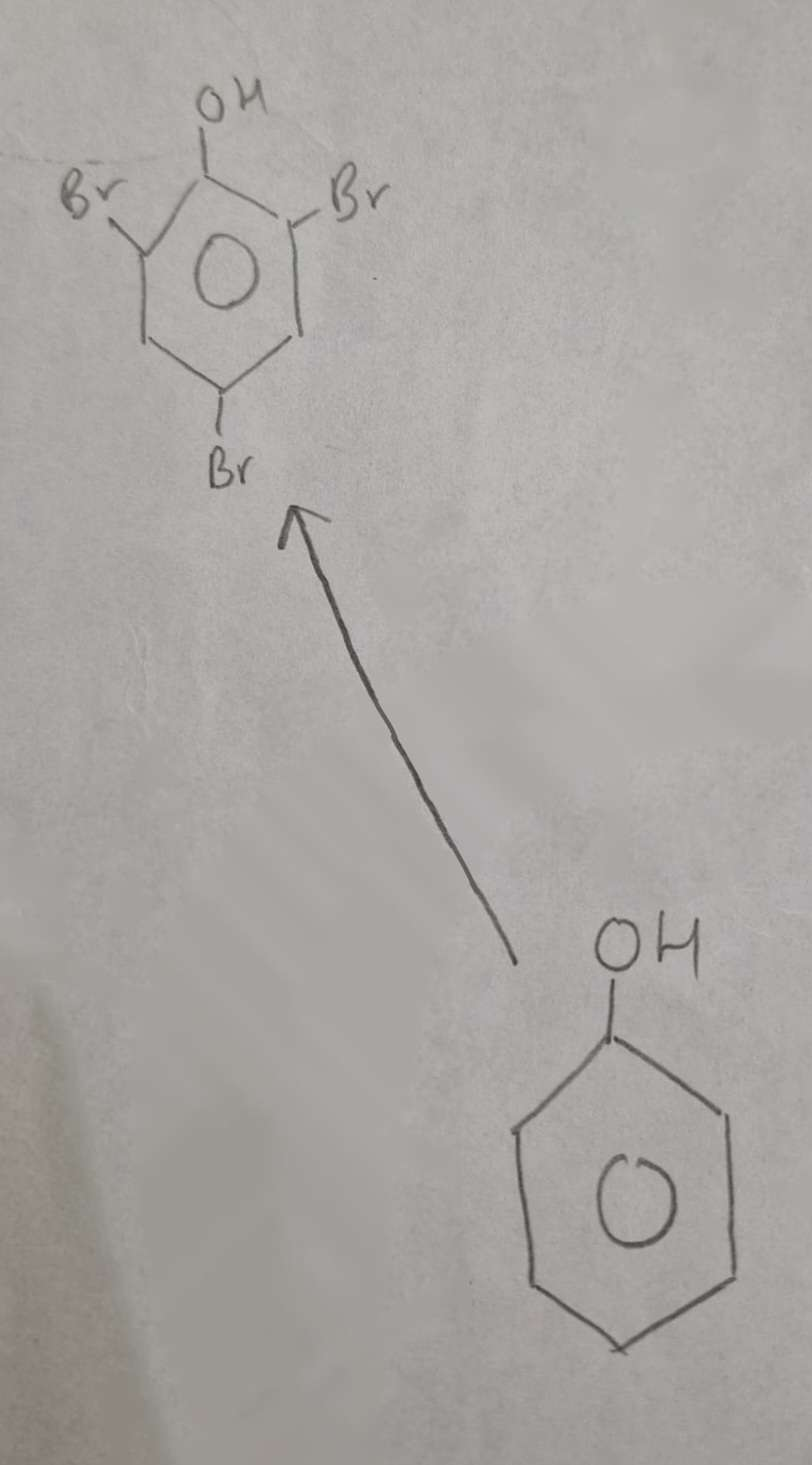
Phenol to Halophenol Conditions:
Warm