combustion of alcohols
1/3
Earn XP
Description and Tags
When fuels are burned, they release heat. However, not all fuels produce the same amount of heat during combustion. In this experiment, you will compare the energy released by ethanol, propanol, butanol, and pentanol by measuring the temperature increase of a known mass of water heated using a spirit burner.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
aim
investigate the temperature rise produced in a known mass of water by the combustion of the alcohols ethanol, propanol, butanol and pentanol
equipment
spirit burner
conical flask
measuring cylinder
draft shield
thermometer
balance
glass rod
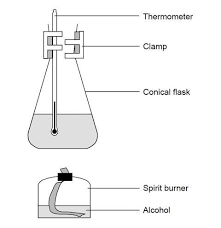
setup
measure and record the mass of the ethanol spirit burner
measure 100cm³ of cold water in measuring cylinder and pour into conical flask
clamp conical flask above spirit burner
use draft shields around setup
place thermometer in water
steps
record initial water temperature
remove burner cap and light it
constantly stir water to prevent hot and cold spots
once temperature has risen 40 degrees C, place the cap back on the burner
record the final mass of the spirit burner
find the change in mass
can repeat for different alcohols