Esters
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
general formula of esters
R1COOR2
Which is the correct structural formula for this ester.
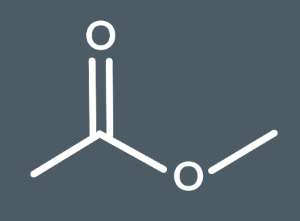
CH3COOCH3
When ethanoic acid and propan-1-ol react, what are the products?


To make esters, we need to add a strong acid. However, the acid is not consumed by the reaction.
This makes the acid a…
catalyst
Which of these molecules is the product of the reaction between propanoic acid and propanol?
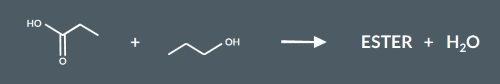
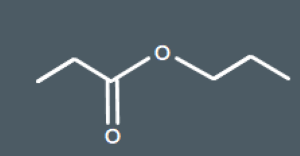
Which of these molecules is the product of the reaction between 3-chlorobutan-1-ol and propanoic acid?

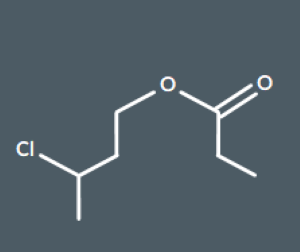
How many carbons are there in the main chain of this molecule?
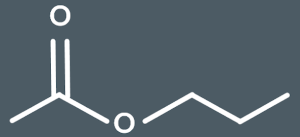
2
What's the IUPAC name for this ester?
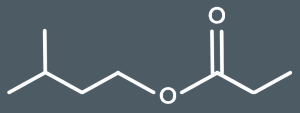
3-methylbutyl propanoate
What's the name of the ester that would form from butanol and ethanoic acid?
butyl ethanoate
What's the name of the ester that would form from hexanoic acid and ethanol?
ethyl hexanoate
What's the IUPAC name for this ester?
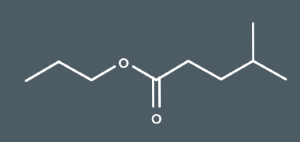
propyl 4-methylpentanoate
What's the IUPAC name for this ester?
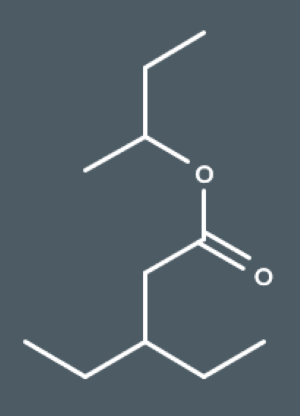
1-methylpropyl 3-ethylpentanoate
What are the products of the hydrolysis of propyl butanoate?
propanol and butanoic acid
Acid catalyses the hydrolysis of esters.
Therefore, does the amount of acid affect the point of equilibrium?…
no
What are the products when propyl methanoate is hydrolysed by sodium hydroxide?
sodium methanoate
What are the products of the hydrolysis of esters in acidic conditions?
acid+alcohol
What are the products of the hydrolysis of esters in alkaline conditions?
salt+alcohol
In an alkaline hydrolysis reaction, the ester will be completely converted into a salt, as long as there is enough
NaOH
The hydrolysis of an ester under acidic conditions…(3)
is reversible.
produces an acid.
produces an alcohol
The hydrolysis of an ester under alkaline conditions…(3)
consumes the alkali (NaOH)
produces a salt.
produces an alcohol.
A chemist wants to produce ethyl 2-methylbutanoate.
State the reagents and 2 conditions required for this reaction.
Reagents:
ethanol and 2-methylbutanoic acid
Conditions:
acid and heat