Core Practical 13a : Follow the rate of the iodine-propanone reaction using a titrimetric method
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
Overview:

Procedure:
Note burette readings should be given to 0.05cm3

A graph of the titre against time ( where the titre is proportional to the concentration of iodine):
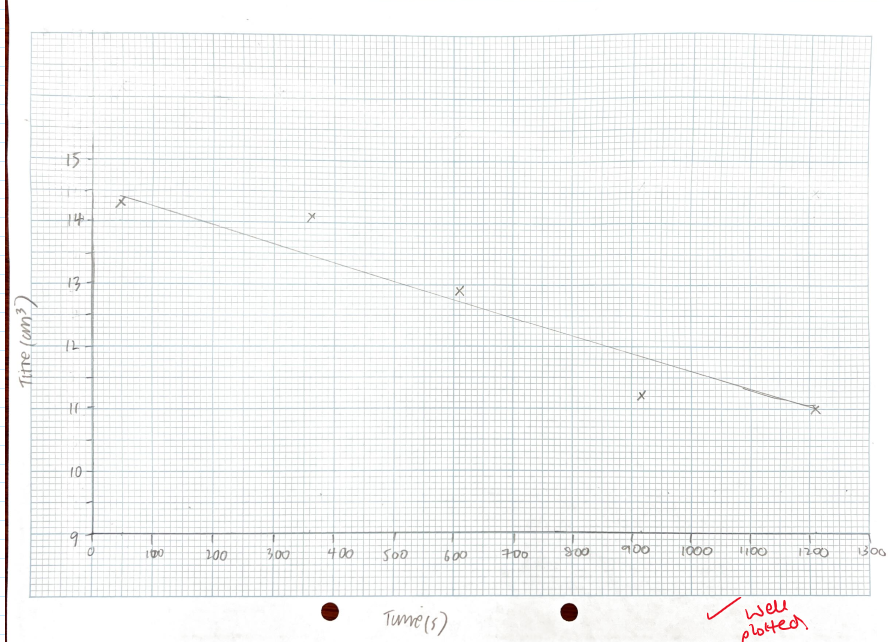
Deduce the order of reaction with respect to iodine from the graph
0 order with respect to iodine.
This is because the graph shows a straight line, therefore the rate of reaction does not change (as the gradient reflects the rate).
This shows that the volume of iodine has no effect on the rate of reaction.
Learning tips:
the reaction between propanone and iodine in aqueous solution can be catalysed by an acid
the influence of iodine on the reaction rate can be studied if the concentrations of propanone and hydrogen ions effectively remain constant during the reaction
this is achieved by using a large excess of both propanone and sulfuric acid in the starting reaction mixture
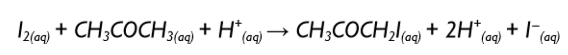
Similar experiments show that the reaction is first order with respect to both propanone and to hydrogen ions. Use this information to answer the following questions.
What is the effect on the rate if the concentration of hydrogen ions doubles?
The rate doubles.
Similar experiments show that the reaction is first order with respect to both propanone and to hydrogen ions. Use this information to answer the following questions.
What is the effect on the rate if the concentration of the propanone is doubled?
The rate doubles.
What is the effect on the rate if the concentration of the iodine is doubled?
There is no effect on the rate.
Write the overall rate expression for this reaction.
Rate = k[H+][CH3COCH3]
Two students monitored the concentration of propanone as the reaction proceeded and plotted a concentration-time graph from their results.
What shape would you expect the graph to be? How would you use this graph to prove that the reaction is first order with respect to propanone?
You would expect the graph to be a curve.
to determine whether it’s a 1st or 2nd order with respect to propanone, you would need to calculate the 1st and 2nd half lives from the graph.
if the 1st and 2nd half lives are the same, this would prove that the reaction is first order with respect to propanone