Phenol
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Describe the Weak acidity of phenol
phenoxide - is a weak conjugate base because of the delocalization of electrons by the +M effect
more delocalization - harder for h+ to find electrons
solubility increasing with pH
as pH increases, solubility of phenol increases
less h+ in solution, hence dissociation is favored
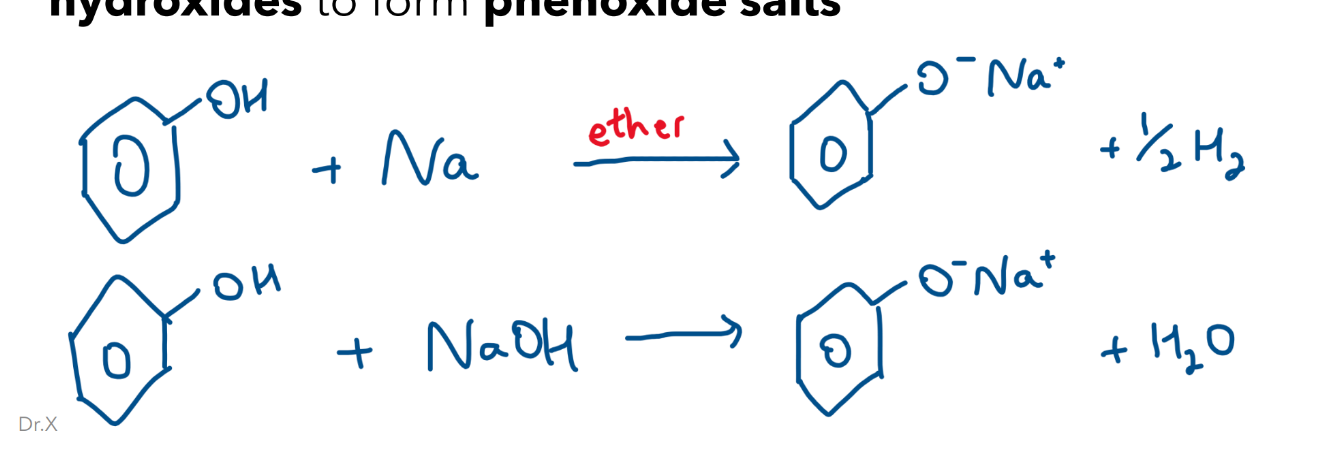
Reaction with Na and NaH and NaOH
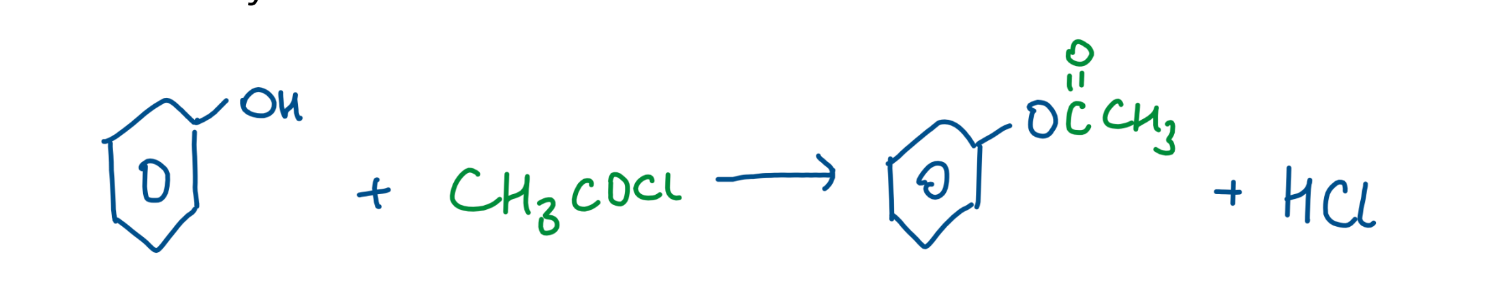
Reaction with acid chloride
lone pair availability of O is low - due to it being delocalized most of the time
acid chlorides are v electronegative so will pull e-
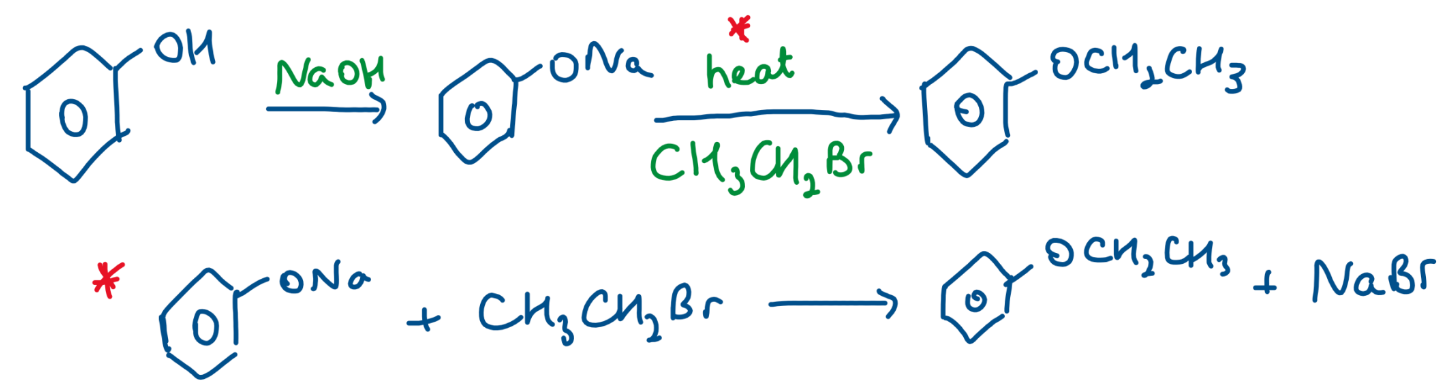
Reaction with Halogenoalkane
produce aromatic ether
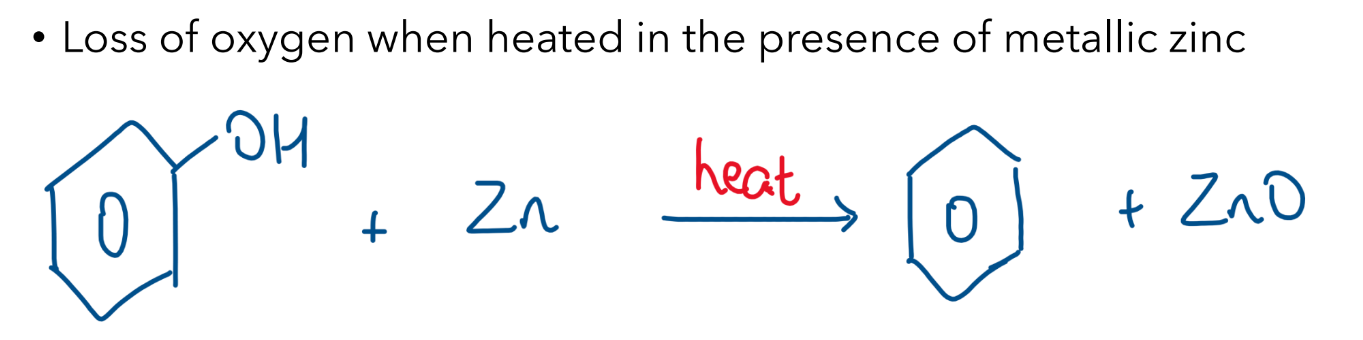
Reduction
Neutral Iron (III) Chloride
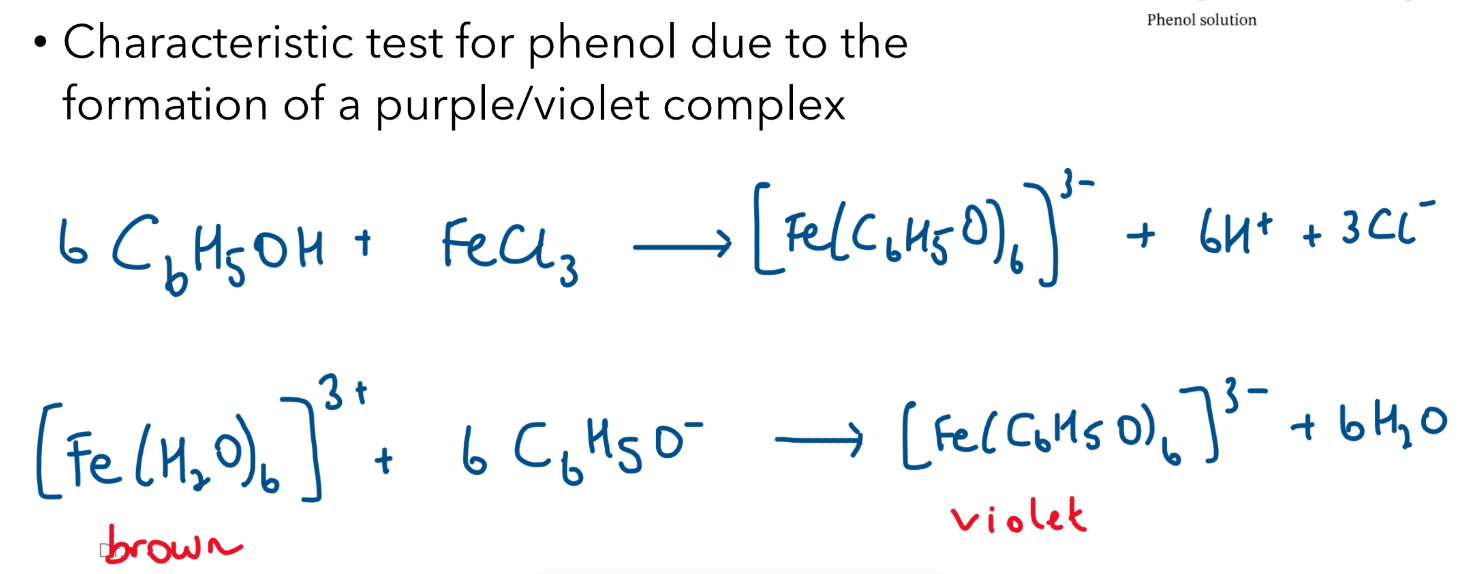
Why cant reactions with pcl5 and lucas reagent take place
most of the structure is a double bond which is much harder to break
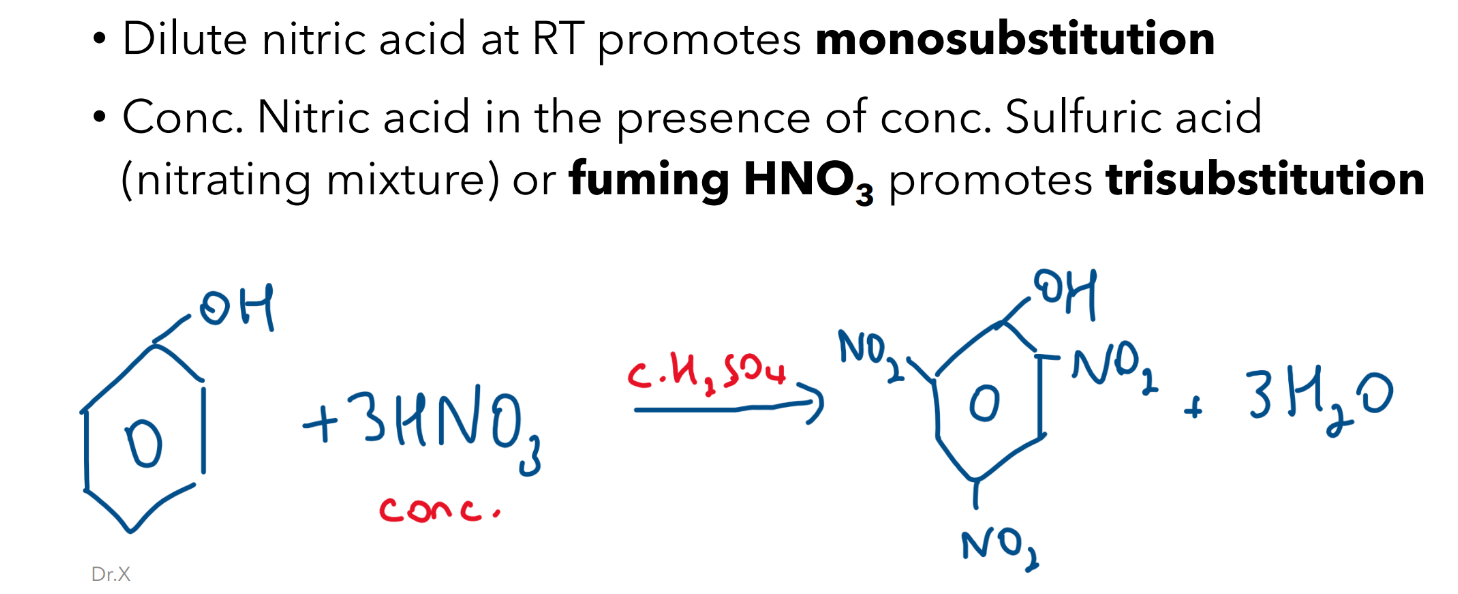
Nitration
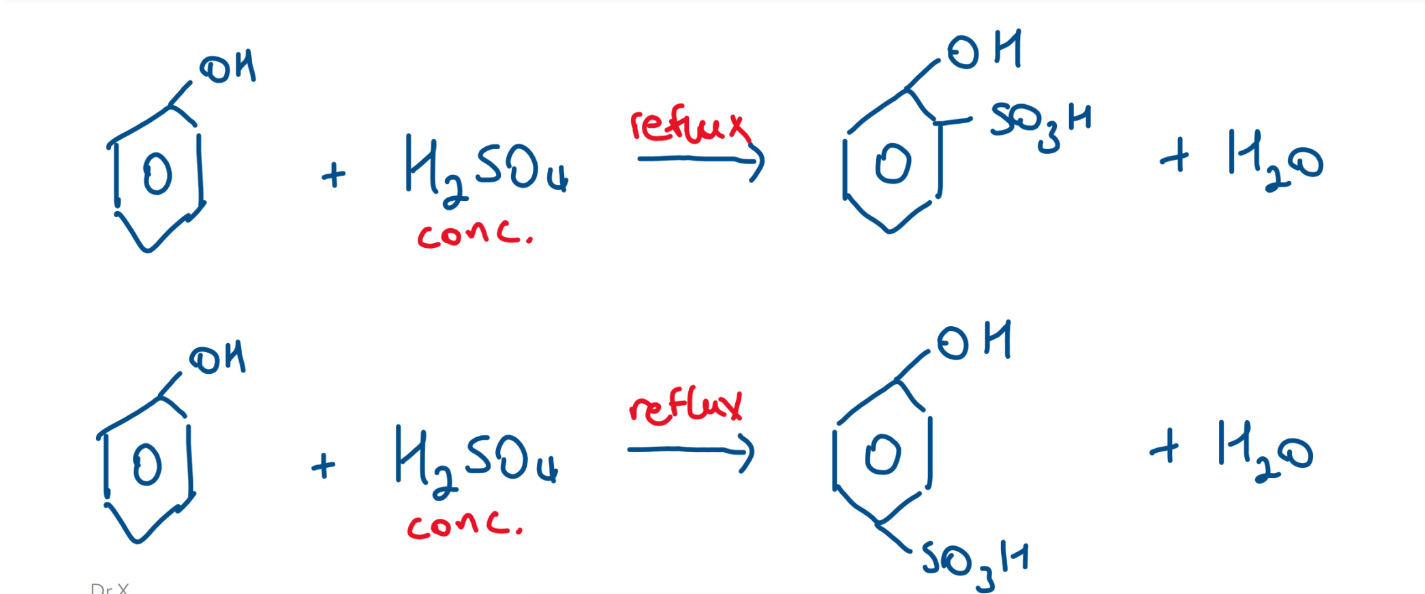
Sulfonation
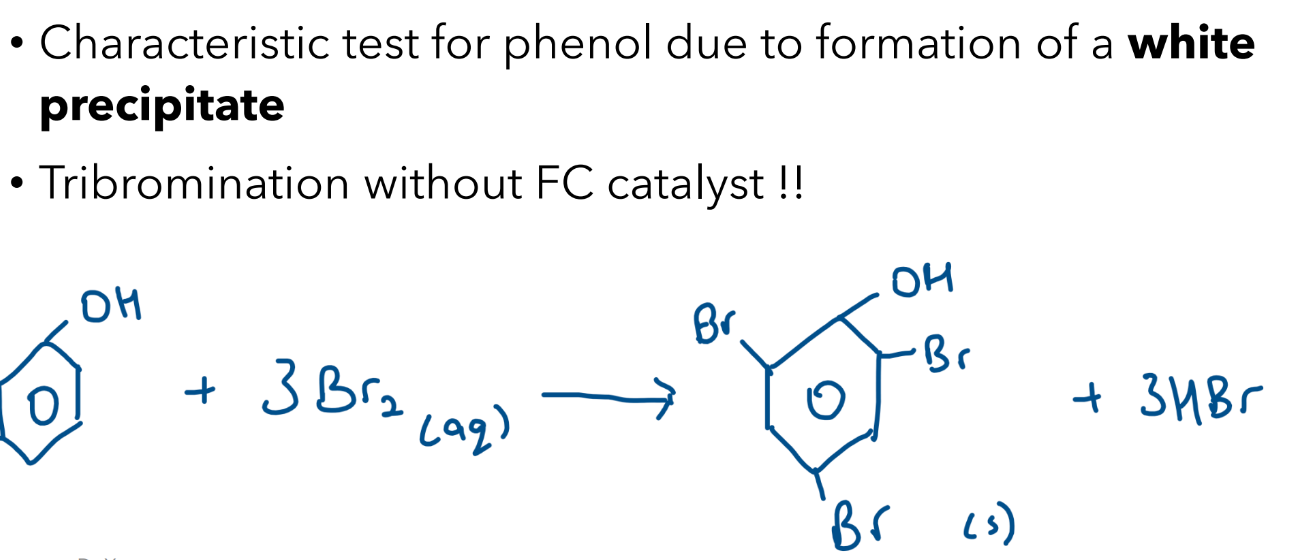
Halogenation with Bromine Water
Alkylation
reaction is same as benzene
requires FC catalyst and anhydrous conditions
Acylation
AlCl3 and anhydrous
Hydrogenation
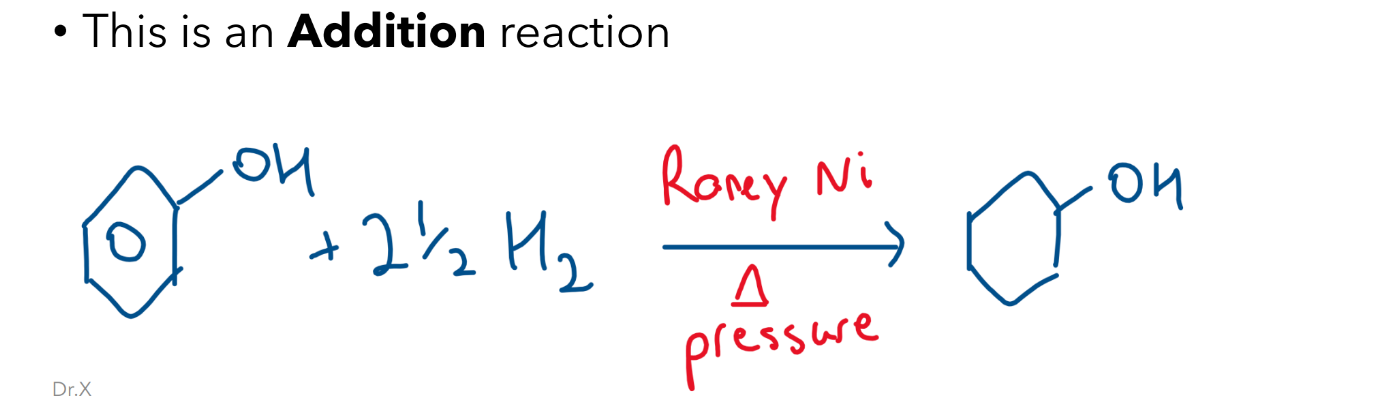
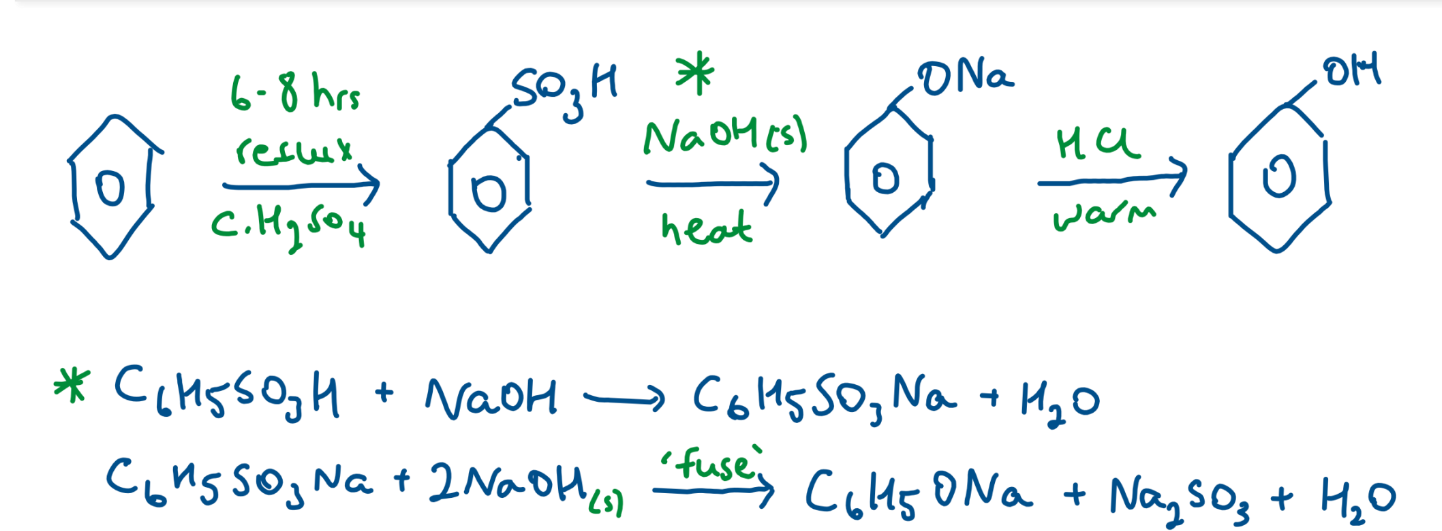
Preparation of Phenol