Quiz 2 & 4
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Dimensional Analysis Formula:
Measurement x (Top (The Element You want))
----------------------------————————
(Bottom (Element You want to get rid of))
How to measure precision?
Go as many decimal places as the instrument allows. Add another decimal point if you’re estimating between the lines.
What should you do
Always write your units
Moles —> Grams (Formula)
mol (of element) x molar mass (of element)
Grams —> Moles (Formula)
mass (of element) (grams)
———————————-
molar mass
1 mol = ?
22.4 L
What is Molar mass
1 mol of an element
Mol —> Mol (Formula)
(x mol a = y mol b) (x and y = coefficients of a balanced chemical reaction
Given Mol x Desired Mol (mol ratio)
——————————-- = Desired Mol
Given Mol (mol ratio)

Mol —> Particles
Mol x 6.02 × 10^23 (Avogadro number)
Mass to Mass calculations
Starting Weight → Convert to Mol → Multiply by Molar Ratio → Convert back to grams
\text{g A} \times \frac{1 \text{ mole A}}{\text{molar mass } (\text{g}) \text{ A}} \times \frac{\text{Y mol B}}{\text{X mol A}} \times \frac{\text{molar mass } (\text{g}) \text{ B}}{1 \text{ mol B}}
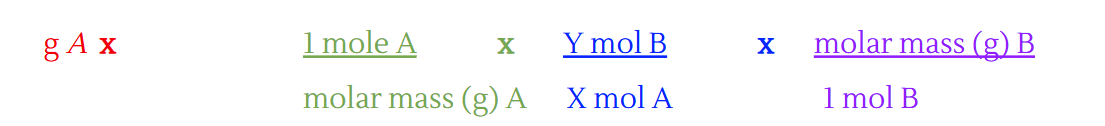
What is the molar ratio?
Coefficients from balanced reaction of reactants and products in a chemical reaction.
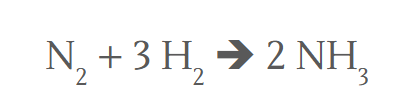
Molar Ratio here
1 : 3 : 2
First step of mass to mass calculation
Convert given mass to moles
Second step of mass to mass calculation
Convert mol of given mass to mol of the mass that needs to be figured out from the balanced reaction.
Third step of mass to mass calculations
Convert the moles of the substance from step 2 to grams
What should you do with mass to mass calculations
Read the problem closely so you know what elements you are trying to find
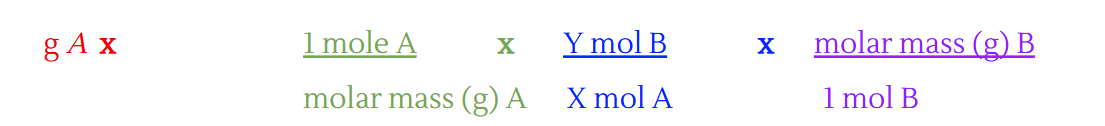
When molarity is given in a question, what do you change to mass to mass formula?
Change the first step to the molarity formula:
(there is no molar ratio here usually)
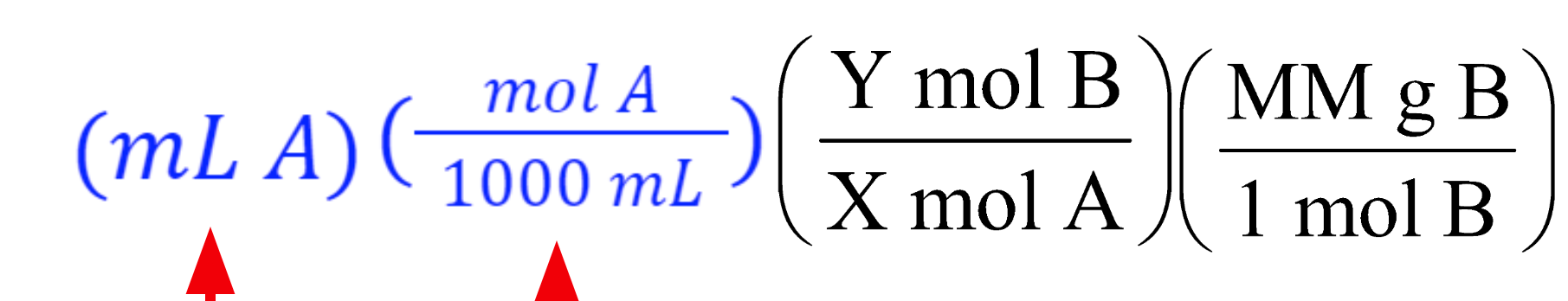
What is the molarity formula:
Mol (of solution)
———————-
1000ml Soln (or 1 L)

What is % Yield Formula
% Yield = | experimental value | / accepted value (100)

What is % Error Formula
% Error = | accepted value - experimental value | / accepted value (100)

When explaining errors in labs you should NOT:
Explain the error with human error (I measured wrong, balance off, calculate something wrong)
Only say what you can directly support
When explaining labs you should:
Make observations during experiment and offer an explanation. For example the oxygen reacted with the aluminum overnight.
How to answer:
Mol of x element —> Mol of y element
How many moles of X element will form if moles of X element are used according to the reaction.
Multiply the given number by the molar ratio.
How to answer:
Mol of x element —> Grams of y element
Multiply by molar ratio then convert to grams
How to answer:
Grams of x element —> Grams of y element
Convert grams of x to moles
Convert moles of x to moles of y (molar ratio)
Convert moles of y to grams (molar mass *)
How to answer:
Volume of x element would x moles of element produce.
Multiply element by liters (22.4L)
How do you solve for a specific variable in a formula?
Rearrange the formula algebraically to isolate the variable you want, keeping units consistent.