chapter 3 - enzymes
1/104
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
105 Terms
What is a chemical reaction?
A process that involves the rearrangement of the molecular or ionic structure of a substance.
What is a catabolic reaction?
A chemical reaction that breaks a big molecule into smaller molecules.
What is an anabolic reaction?
A reaction that builds a bigger molecule from small molecules.
What is an exergonic reaction?
A reaction that releases energy.
What is an endergonic reaction?
A reaction that absorbs energy.
What kind of reaction is a catabolic reaction?
Exergonic reaction.
What kind of reaction is an anabolic reaction?
Endergonic reaction.
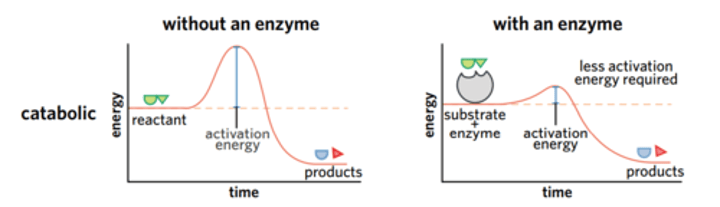
What is activation energy? Why is it required? What are two ways to overcome it?
The minimum quantity of energy which a reacting molecule must possess in order to undergo a reaction.
It is required for a chemical reaction to take place because every reaction (even exergonic) requires an input of energy to get it going.
Because molecules are constantly in motion, sometimes enough energy might be absorbed by a molecule for a chemical reaction to happen, but it won't happen very often if the activation energy is high.
There are two ways to overcome activation energy: 1 -> add more energy (so it can react and overcome activation energy)
or 2 -> lower the activation energy (not going to need as much energy to overcome it).
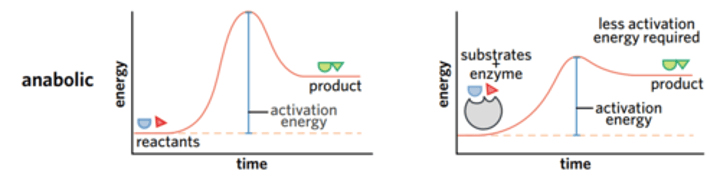
What do anabolic reactions require to take place? How does the cell do this?
A constant source of lots of energy. A lot of activation energy. (In order to build a molecule).
Also, it is an endergonic reaction.
How does the cell do this provide anabolic reactions with a source of energy?
A cell must couple (link or connect in pairs) a catabolic, exergonic reaction with an anabolic, endergonic reaction. (Because of exergonic release energy for endergonic).
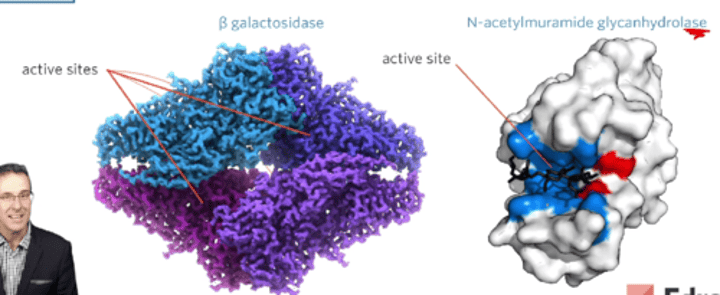
What are enzymes? What is specific about them?
An enzyme is an organic (carbon-based) molecule, typically a protein, that catalyses specific reactions
They are proteins and each enzyme has at least one active site (some have multiple), the site to which its substrate binds (can be different substrates, depending on enzyme).
Enzymes are generally specific to their substrate. The attraction between the active site and the substrate is called an affinity.
what is a substrate?
the reactant of a reaction catalysed by an enzyme
(either broken down or joined together)
How do catabolic enzymes work? What are they?
A catabolic enzyme is an enzyme that takes a substrate and breaks it down into products that are smaller than the substrate.
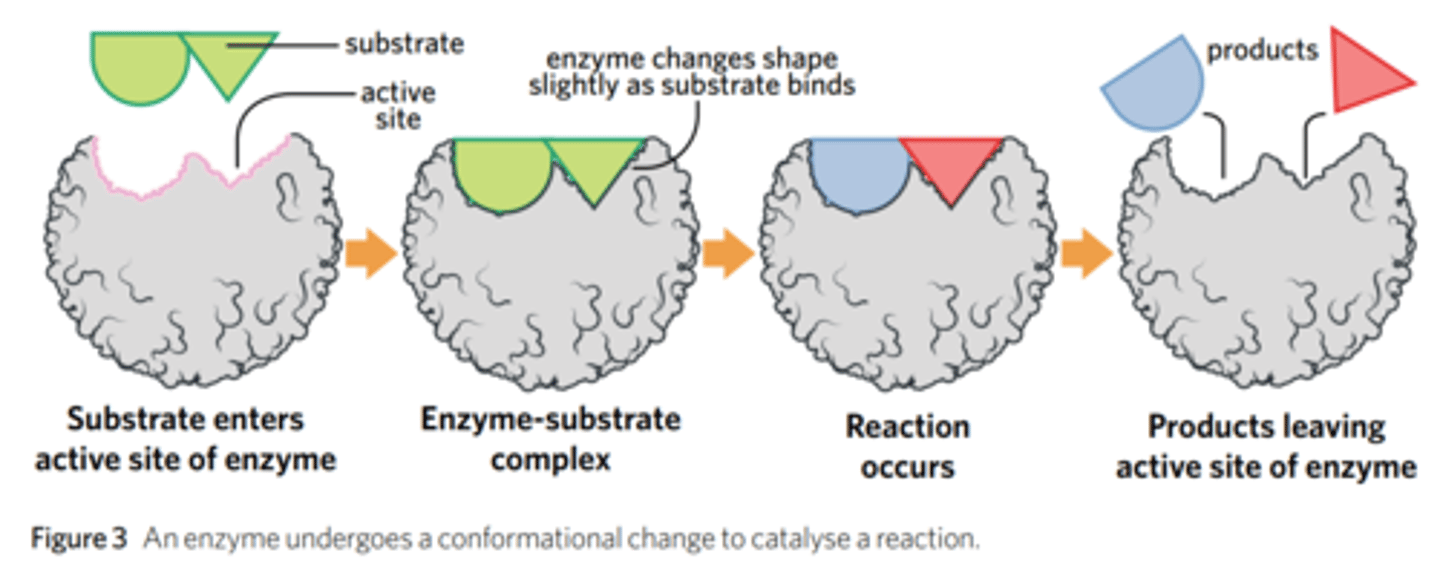
The substrate binds to the active site of the enzyme, because their shapes are almost perfectly complementary.
The enzyme lowers the activation energy by attracting the substrate to its active site.
Upon bonding, the enzyme changes shape, which stresses the bonds in the substrate, helping it to catabolise into two products.
What is an induced fit?
A tweaked version of the original model of the enzyme function which was called 'lock and key'.
The induced fit model explains that an enzyme is flexible and shapes shape a little when the substrate binds to it.
(Conformational change. Mould.)
How do anabolic enzymes work? What are they?
Anabolic enzymes are enzymes that take two substrates and join them together to make one product.
Energy (since it is an endergonic reaction) is used to make the enzyme change shape and align those substrates so that they become a single product.
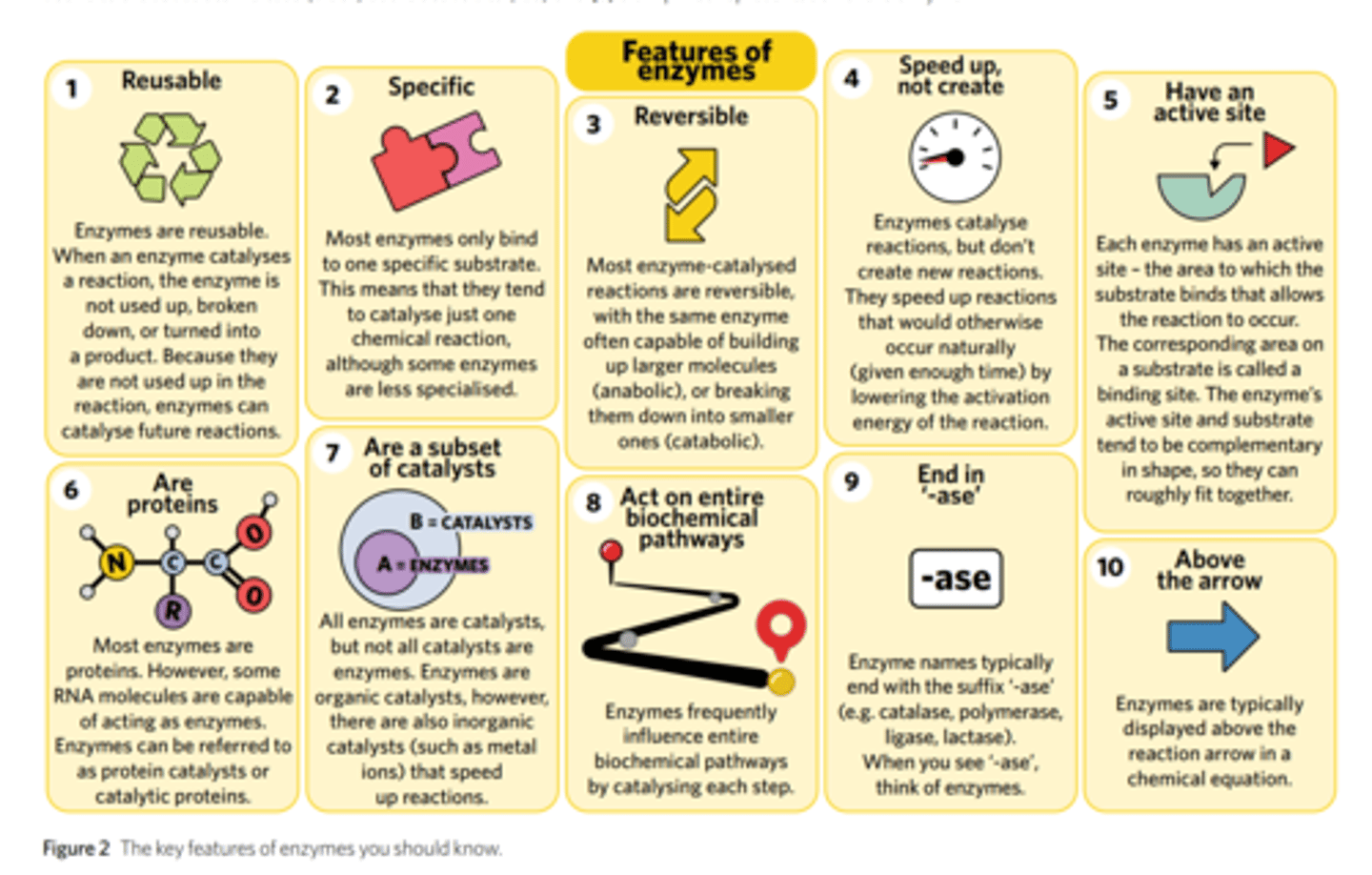
recall the properties of enzymes.
1. They lower the activation energy required for chemical reactions (and therefore increase the rate or time of a reaction).
2. They are not permanently changed or used up in the reaction.
3. They are re-usable.
4. They are (usually) specific to just one substrate.
5. They have an active site.
6. They act on entire biochemical pathways.
7. Situated above the arrow in chemical reactions.
8. Typically end in 'ase.'
9. Mostly proteins
The enzyme RNA polymerase joins RNA nucleotides together to make a molecule of RNA. This process would be expected to:
be exergonic and anabolic.
be catabolic and endergonic.
be anabolic and endergonic.
be catabolic and exergonic.
c - making - requires energy.
Tryptophan synthase is a catalytic enzyme which breaks a phosphate group off Indole-3-glycerol phosphate to create the amino acid tryptophan.
Based on this information, which of the following statements is correct?
Tryptophan synthase requires ATP to do its job.
Tryptophan synthase is made of a single polypeptide chain.
Tryptophan has at least one active site.
Indole-3-glycerol phosphate is the substrate of the reaction.
d - breaks it and acts on
Enzymes are
proteins that raise the activation energy of reactions.
organic catalysts that often influence entire biochemical pathways.
b
what is the product?
the transformed molecule created in a reaction
are all catalysts enzymes?
no but all enzymes are catalysts
do enzymes create new reactions?
no, they do lower the activation energy required though.
what is an active site?
the part of an enzyme where the substrate binds
what is an enzyme-substrate complex?
the structure formed when an enzyme and substrate are bound together
flip
Label the parts of the diagram from the list of terms.
enzyme
products
substrate
active site
enzyme-substrate complex
Which are key features of enzymes? (Select all that apply)
I reusable
II non-specific
III are mostly proteins
IV contain an active site
V shown below the arrow
VI most reactions are reversible
I
III
IV
VI
Enzymes are catalysts that have an ________ where a substrate binds. They function to ________ the activation energy of reactions in order to ________ the reaction rate. Following a reaction, an enzyme ________ go on to catalyse further reactions.
active site
lower
increase
will
The activation energy in a biological reaction is
completely removed in the presence of an enzyme.
the energy required to finish the reaction.
lowered in the presence of an enzyme.
involved in catabolic reactions only.
c
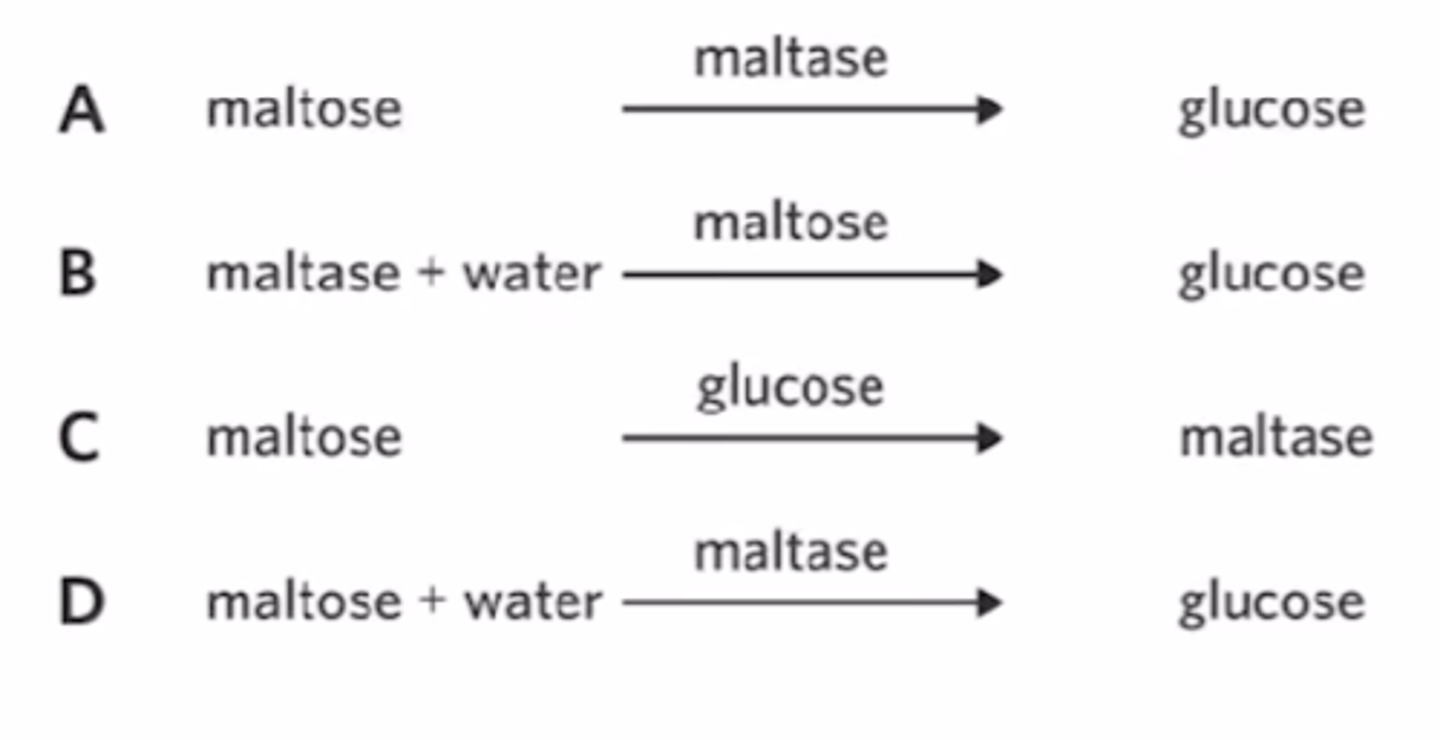
In an experiment, two students added 5 mL of a glucose solution, 5 mL of a maltose solution, 1 mL of a maltase solution, and 100 mL of water to a beaker. After an hour, the amount of glucose in the beaker increased, the amount of maltose decreased, the amount of water also decreased, and the amount of maltase remained unchanged.
The product in this scenario is
maltose.
maltase.
glucose.
water.
The enzyme in this scenario is...
c
maltase
flip
Which of the following displays the equation of the reaction?
what is collision theory?
explanation of chemical reactions that states that in order to react molecules must hit one another
According to collision theory, in order for molecules to react with one another they need to 'collide' with enough kinetic energy (energy possessed by moving objects) to overcome the activation energy of the reaction. Activation energy is like the hurdle that reactants need to get over to start a chemical reaction.
what is a biochemical pathway? what is another name for it?
a series of enzyme-catalysed biochemical reactions in which the product of one reaction becomes the substrate of the next reaction. Also known as a metabolic pathway
Recall that enzymes are specific to their substrate, so enzymes must function in pathways to reach the desired outcome.
Examples of an exergonic (catabolic) reaction include the formation of
protein from amino acids.
fatty acids and glycerol from lipids.
ATP and water from ADP and inorganic phosphate.
glucose and oxygen from carbon dioxide and water.
b
Enzymes are
A carbohydrates that have the ability to catalyse reactions.
B proteins that speed up chemical reactions.
C carbohydrates that are denatured at very high temperatures.
D proteins that are used up in chemical reactions.
b
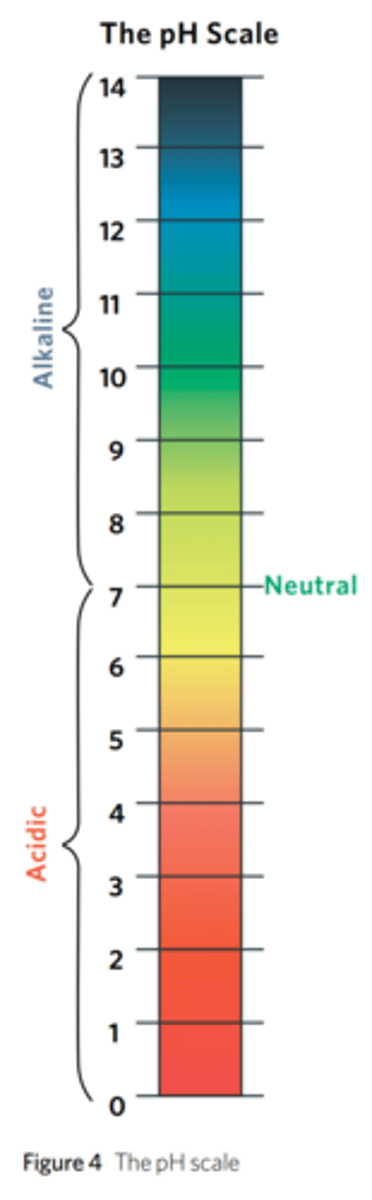
What is pH?
Acidity or alkalinity of a substance. (7 is neutral, 1 is acidic and 14 is basic).
what are the factors that affect enzyme-catalysed reactions?
Temperature
pH
Enzyme concentration
Substrate concentration
Competitive inhibitors
Non-competitive inhibitors
Reversible inhibitors
Irreversible inhibitors
Cofactors
Coenzymes
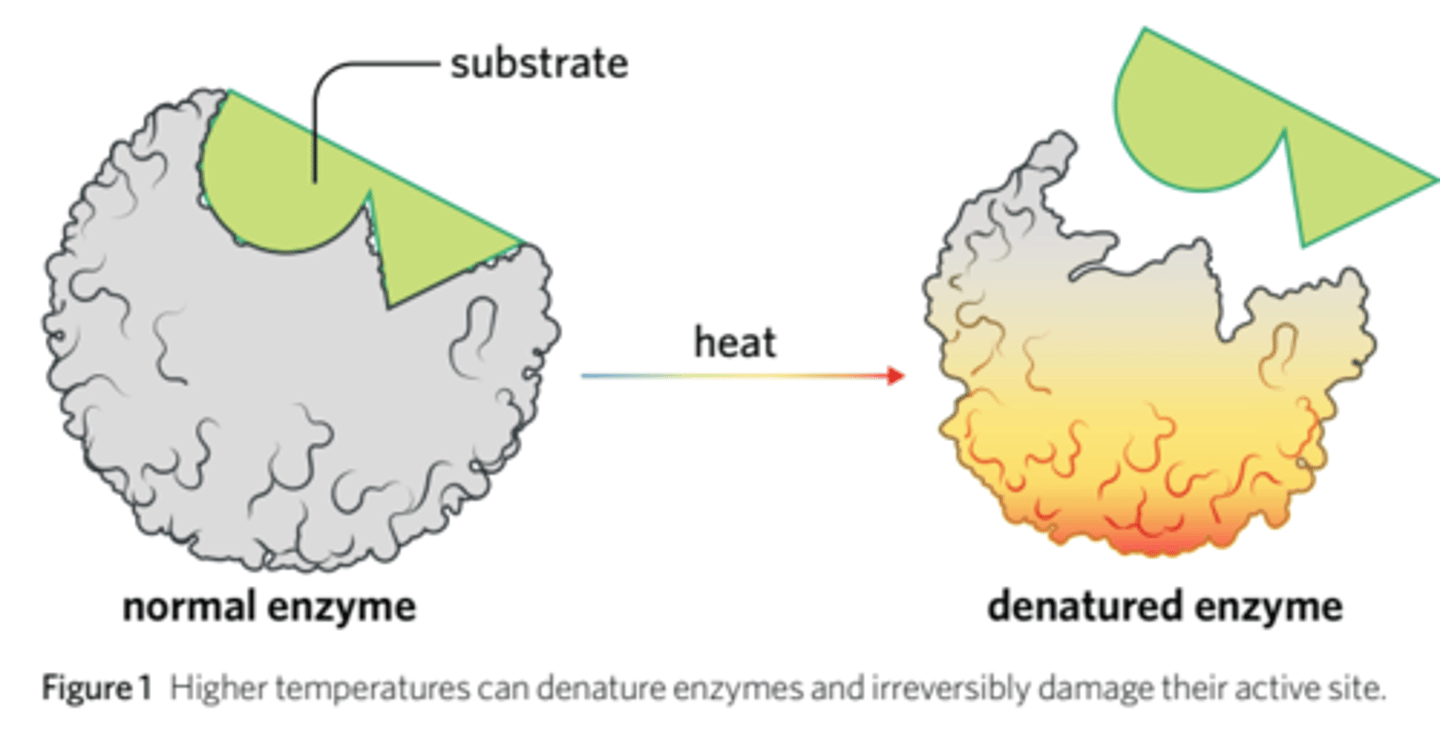
How does temperature affect enzyme-catalysed reactions?
Each enzyme has an optimal temperature; the temperature at which it works fastest. (Molecules move faster - enzymes and substrates move more frequently).
At temperatures lower than the optimal temperature, the reaction is slower because molecules obtain less kenetic energy. (This is reversible as enzyme can be reheated - enzyme is not denatured).
At temperatures higher than the optimal, enzymes can be denatured. Thus, the substrate cannot bind.
(Can only be denatured at high temperatures and such change is not reversible. IRREVERSIBLE.)
What is kinetic energy?
energy of movement
What does it mean when an enzyme is denatured?
A permanent change in the shape of the enzyme due to the breaking of hydrogen bonds between non-adjacent amino acid residue side chains.
The disruption of a molecule's structure by an external factor.
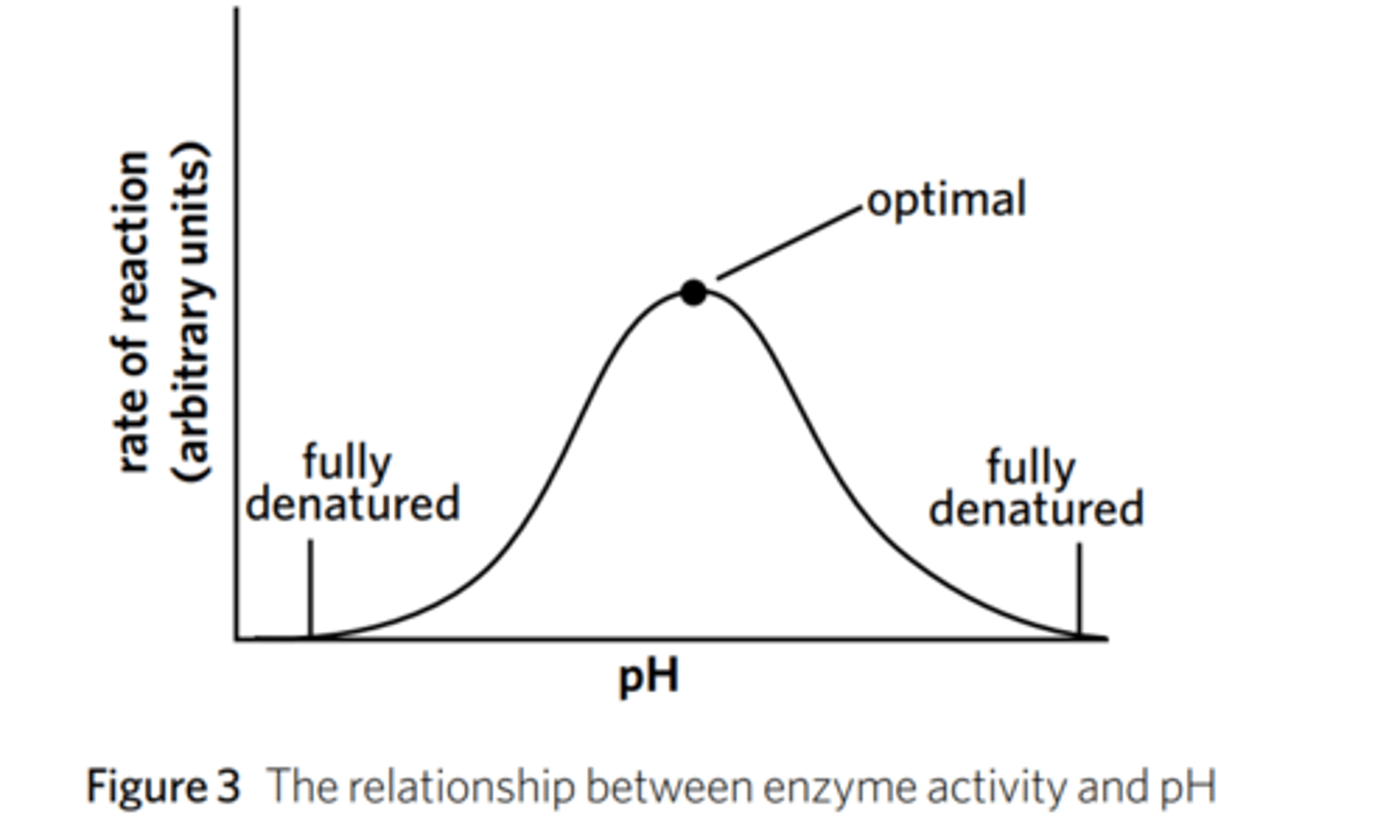
How does pH affect enzyme-catalysed reactions?
Each enzyme has an optimal pH (that is distinct).
If the pH is either higher of lower than the optimal, the rate of the reaction will slow down because pH can change the shape of the enzyme and the shape of the substrate. Enzymes may be denatured by extreme pH (either high or low).
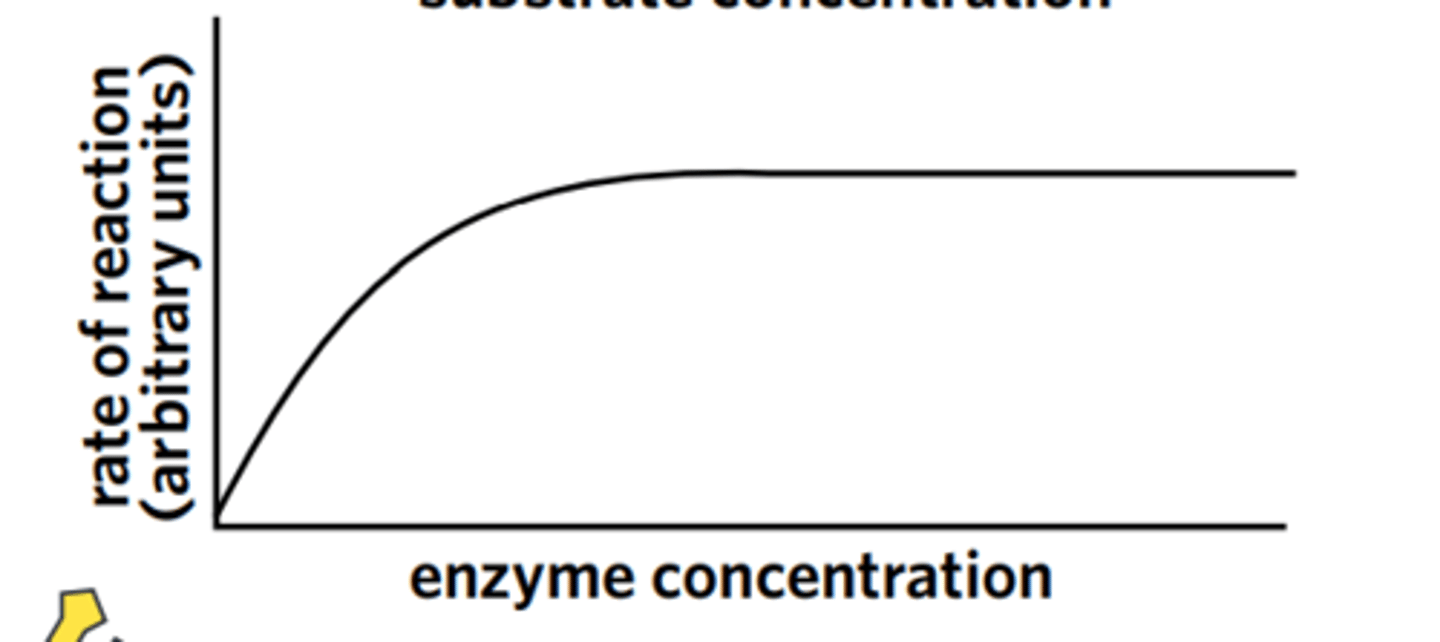
How does enzyme concentration affect reaction rate?
At higher concentrations, the rate of a reaction will be faster.
More interactions between enzymes and substrates - more active sites available for substrates.
This is true until enzymes are in excess, at which point the reaction rate will plateau regardless of any continued increase in enzyme concentration. (Constant)
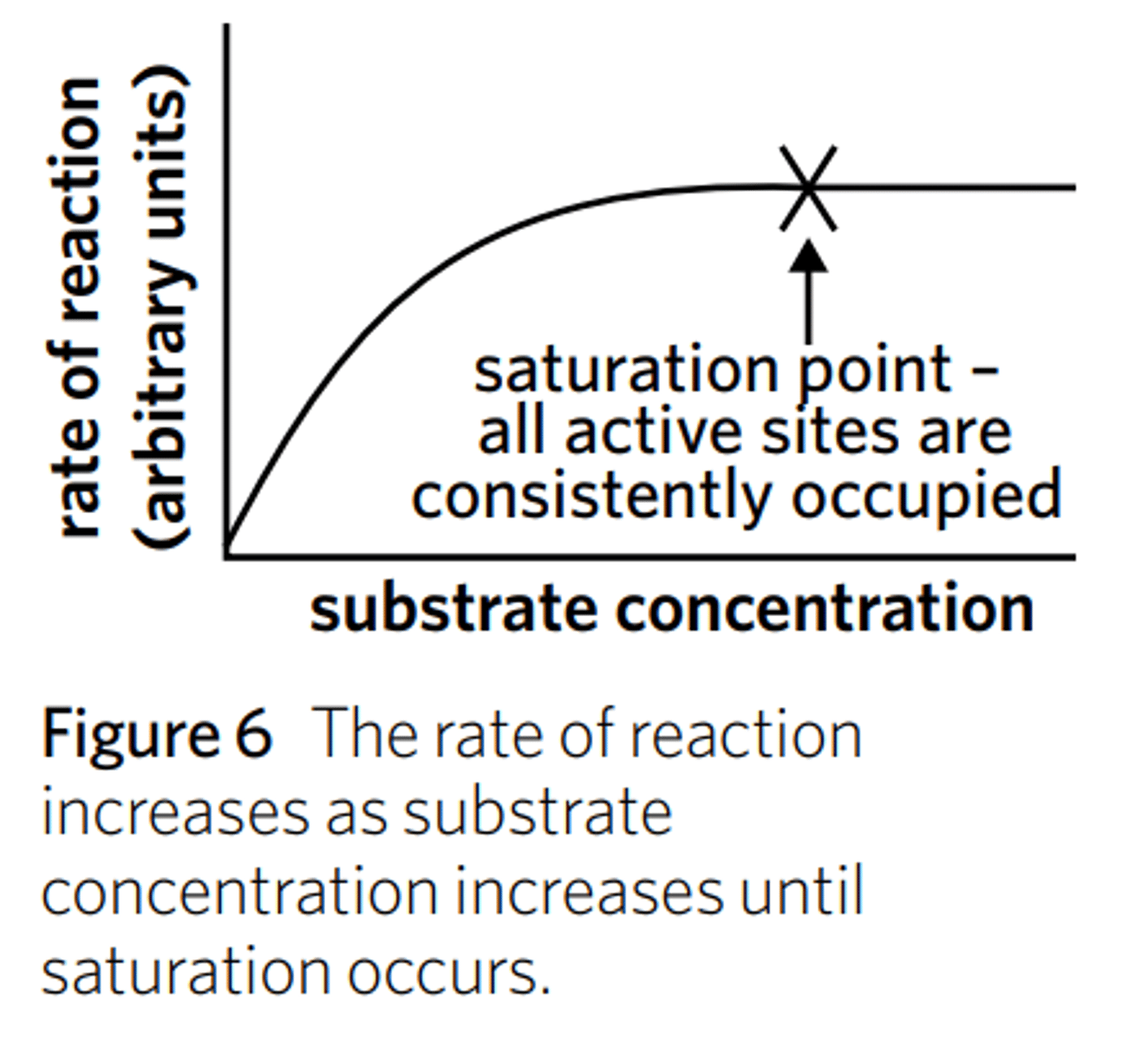
How does substrate concentration affect reaction rate?
If the concentration of substrate is higher, the rate of reaction will be higher too - up to a point where the enzyme is saturated with substrate.
Increasing the substrate concentration beyond the point of saturation (the point at which the active sites of all enzymes in a solution are occupied by a substrate molecule) will not increase the rate of reaction further.
After the saturation point is reached, the rate of reaction remains constant, resulting in a plateau. However, at the saturation point and beyond, the reaction is still occurring very quickly – the rate of reaction, however, is no longer increasing.
Before the graph plateaus, we can say that the substrate concentration is a limiting factor in the reaction, or, more specifically a limiting reagent.
When the graph’s plateau starts, however, we can say that the substrate concentration is no longer the limiting factor/reagent in the reaction. This means that another factor such as temperature, pH, or enzyme concentration is the limiting factor preventing the reaction rate from increasing.
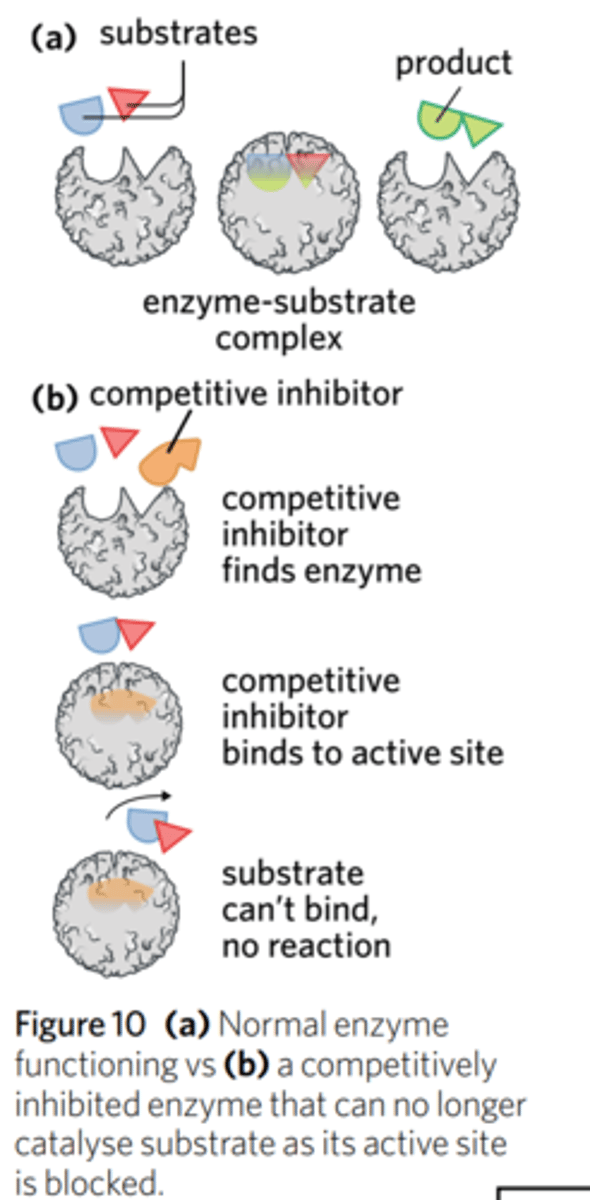
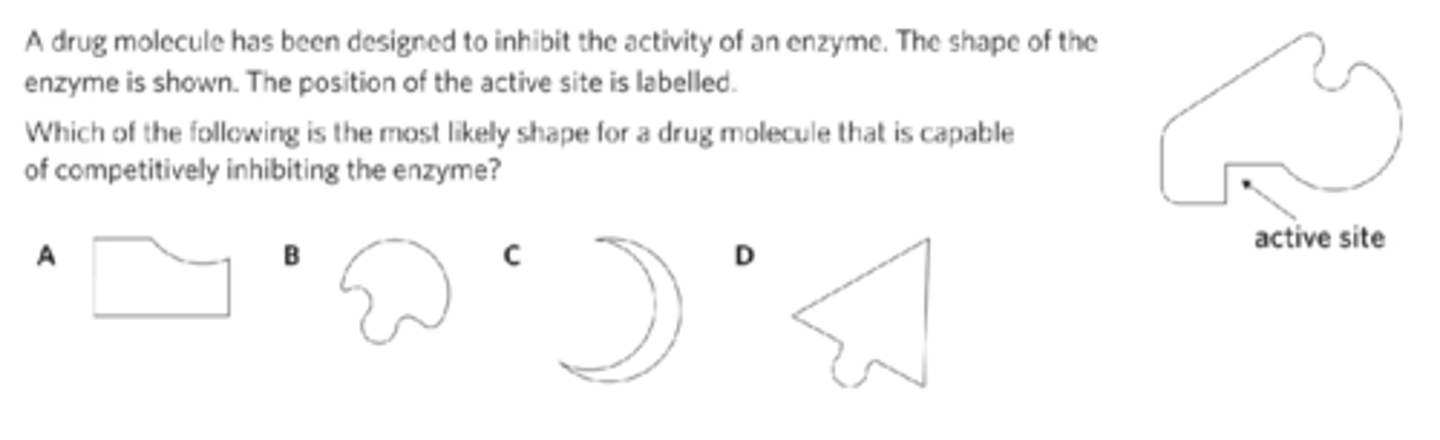
what is competitive inhibition?
the hindrance of an enzyme by blocking the active site and preventing the substrate from binding
what are enzyme inhibitors?
a molecule that binds to and prevents an enzyme from functioning
how do competitive inhibitors affect enzyme-catalysed reactions?
Competitive inhibitors bind to the active site of the enzyme and prevent the substrate from binding to the same active site.
To block an active site, a competitive inhibitor has to have a shape that is complementary to the active site in some way, and therefore must share similarities in shape to the substrate. Unlike the substrate, however, when an inhibitor binds to an active site, it does not trigger a reaction. This form of inhibition is said to be ‘competitive’ because both the substrate and inhibitor are attempting to bind to the active site – they are competing for the active site.
But, adding more substrate can remove a competitive inhibitor from the active site. They can displace the inhibitor because it is not stationary. So, if an inhibitor 'jumps' out then a substrate will 'jump' in.
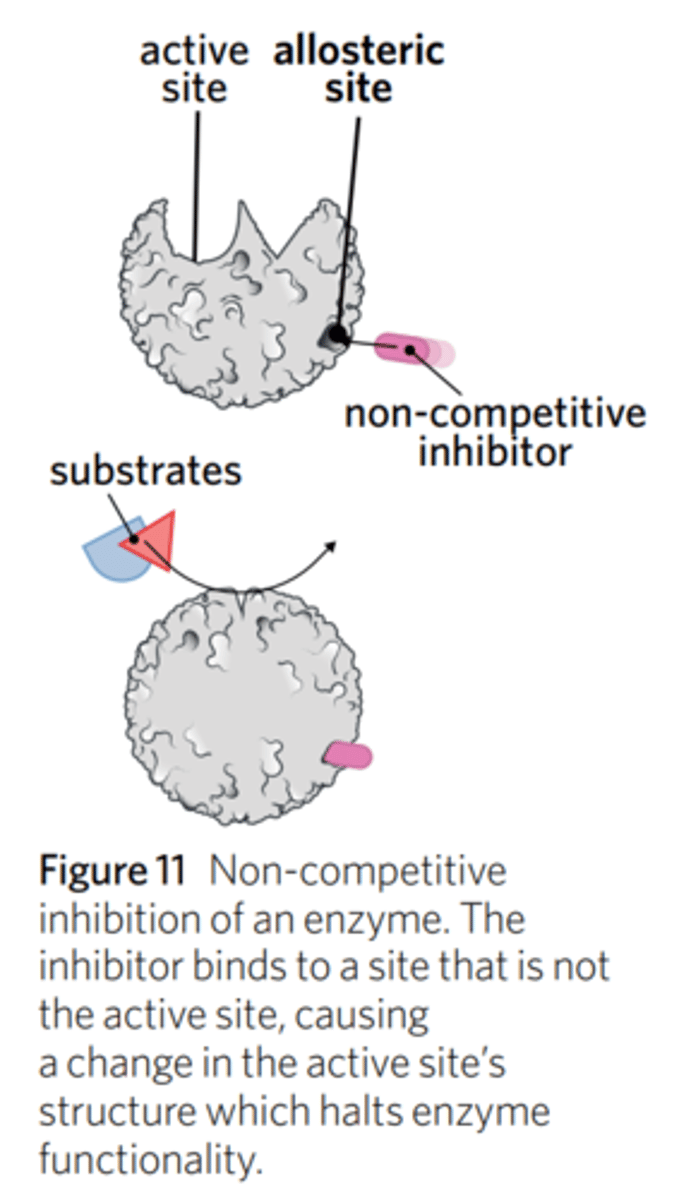
what is non-competitive inhibition? what is another name for it?
Also known as allosteric inhibition.
It is the hindrance of an enzyme by binding to an allosteric site and changing the shape of the active site to prevent the substrate from binding
how do non-competitive inhibitors affect enzyme-catalysed reactions?
what is an allosteric site?
Non-competitive inhibitors bind to an allosteric site (any part of the enzyme other than the active site) on the enzyme, and in doing so, change the shape (conformational change) of the enzyme, such that the substrate does not bind to the active site.
Because the protein changes shape (undergoes conformational change) due to this non-competitive inhibitor, the substrate cannot fit into the active site.
Adding more substance does NOT displace the non-competitive inhibitor from the allosteric site.
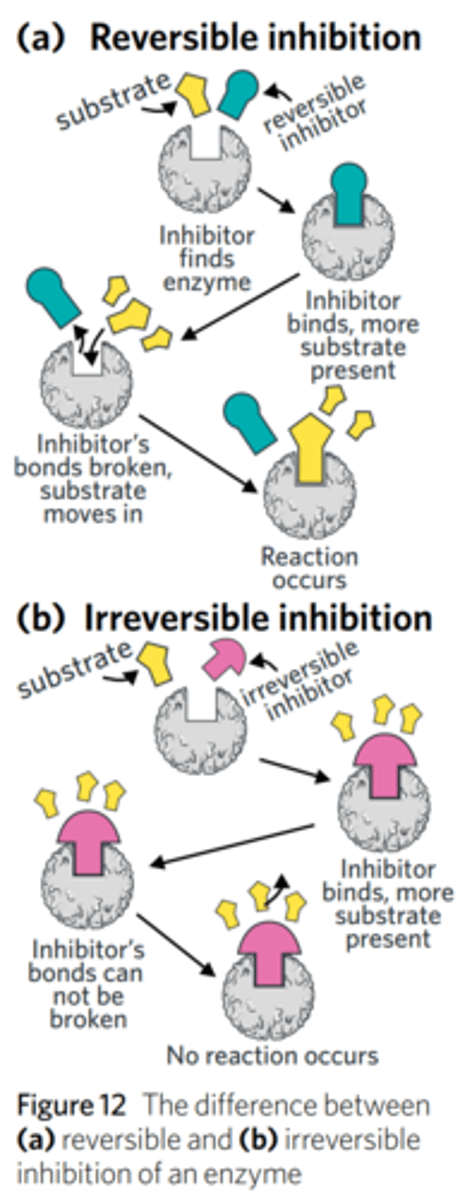
how do reversible and irreversible inhibitors affect enzyme-catalysed reactions?
Irreversible inhibitors form a covalent bond (a strong bond between atoms which they share electrons) with part of the enzyme - causing a permanent change to the enzyme. They become a part of the enzyme. Can be competitive or non-competitive. This means that if an irreversible inhibitor binds to an enzyme, the enzyme is unable to bind with any substrate or catalyse any reactions indefinitely. This means that regardless of how much extra substrate is present, the reaction can never occur. Most irreversible inhibitors occupy the active site of an enzyme, and so are usually classified as competitive.
Reversible inhibitors bind to an enzyme non-covalently (a weak attraction between molecules based on electrostatic forces).
This means reversible inhibitors typically slow the rate of a given enzyme-catalysed reaction, but do not stop it indefinitely. Can be competitive or non-competitive.
how do enzyme inhibitors play an important role in regulating a range of biochemical pathways in the body?
what is a self-regulating biochemical pathway?
They regulate biochemical pathways in the body by regulating how much of a certain product is created, depending on the body's needs.
Thus, the products of a biochemical pathway will not be overproduced.
A self-regulating pathway is one that the product created is regulated by ONE enzyme in the pathway.
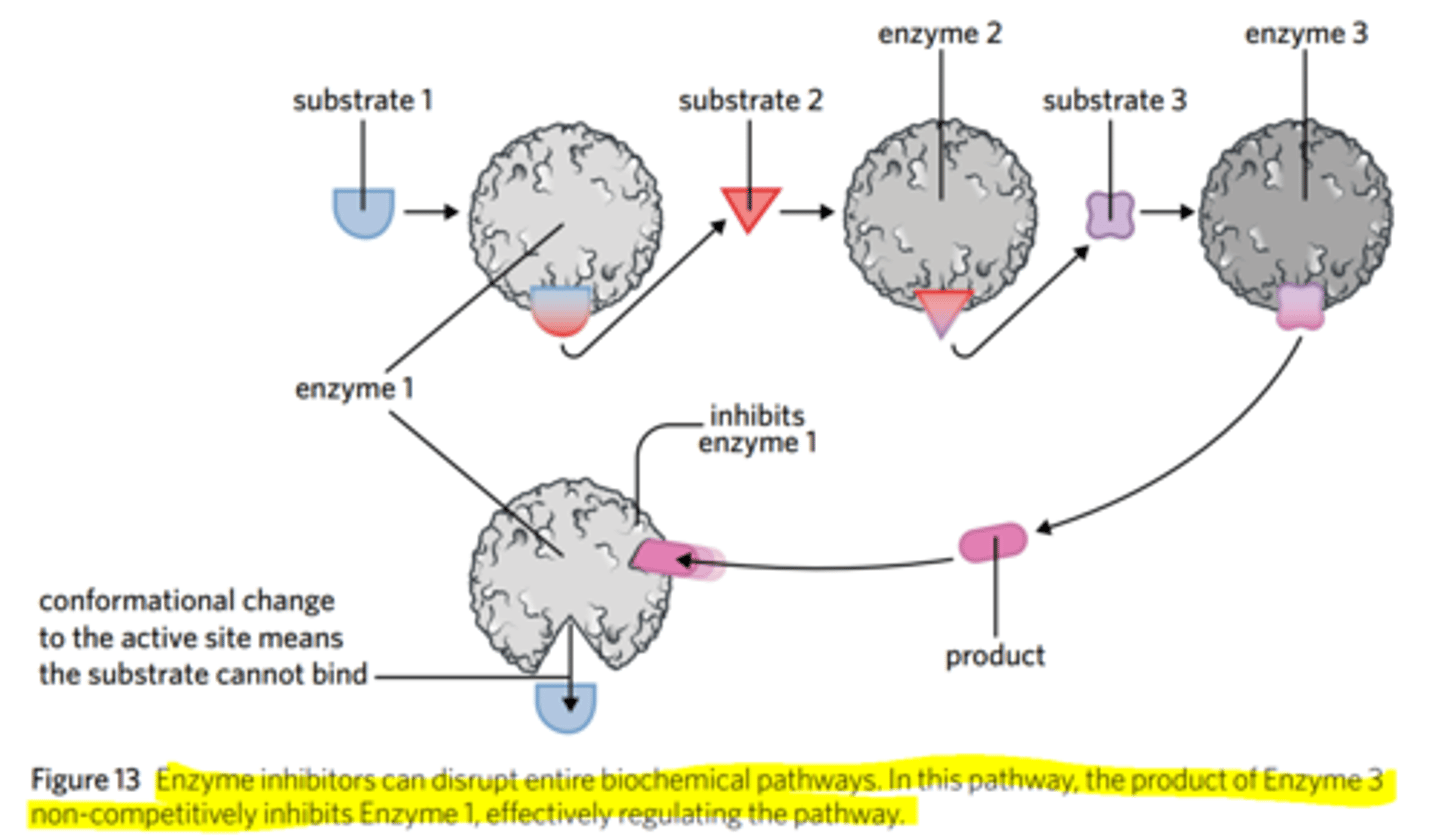
how do cofactors and coenzymes affect enzyme-catalysed reactions? what are they?
In order to function efficiently, many enzymes require 'helper' molecules. These molecules are collectively known as cofactors. Some cofactors are inorganic (often metal ions) and are often known as enzyme activators.
COFACTOR -> any molecule an enzyme needs to function efficiently.
Other cofactors are organic (carbon bonded to hydrogen). Organic cofactors are either prosthetic groups or coenzymes. Coenzymes bind only loosely with an enzyme and are charged in the reaction. Prosthetic groups are bound tightly to the enzyme.
COENZYMES -> an organic non-protein factor that binds loosely to the enzyme and is changed in the reaction.
In coenzyme-assisted enzymatic reactions, the enzyme remains unchanged as always. The structure of the coenzyme, however, is changed. During the reaction, the coenzyme binds to the active site, donates energy or molecules, and then cannot be immediately reused. After the reaction, the coenzyme leaves the enzyme and is recycled by accepting more energy, so it can then go on to assist in more reactions. This is referred to as the cycling of coenzymes and is integral to certain biochemical processes.
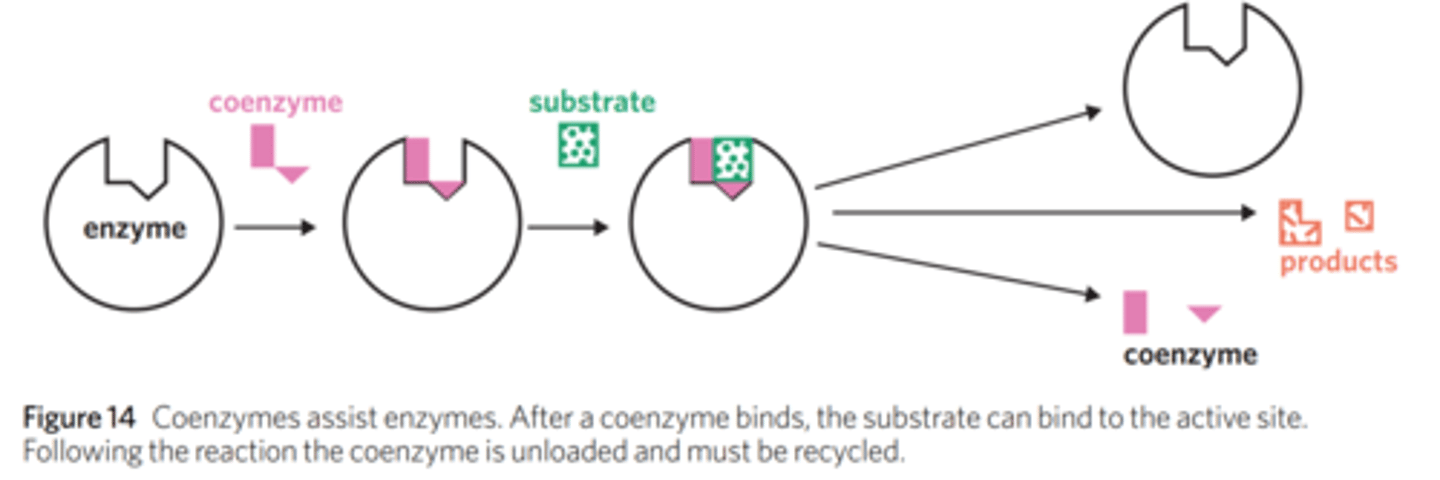
What are some examples of coenzymes? How are they represented in chemical reactions?
DNA, vitamins, NADH, NADPH, FADH2, ATP.
The function of a coenzyme can be represented using a curved arrow below the arrow.
what is an example of a cofactor?
Magnesium Chloride (MgCl2). Cofactor for Taq polymerase - an enzyme used in PCR.
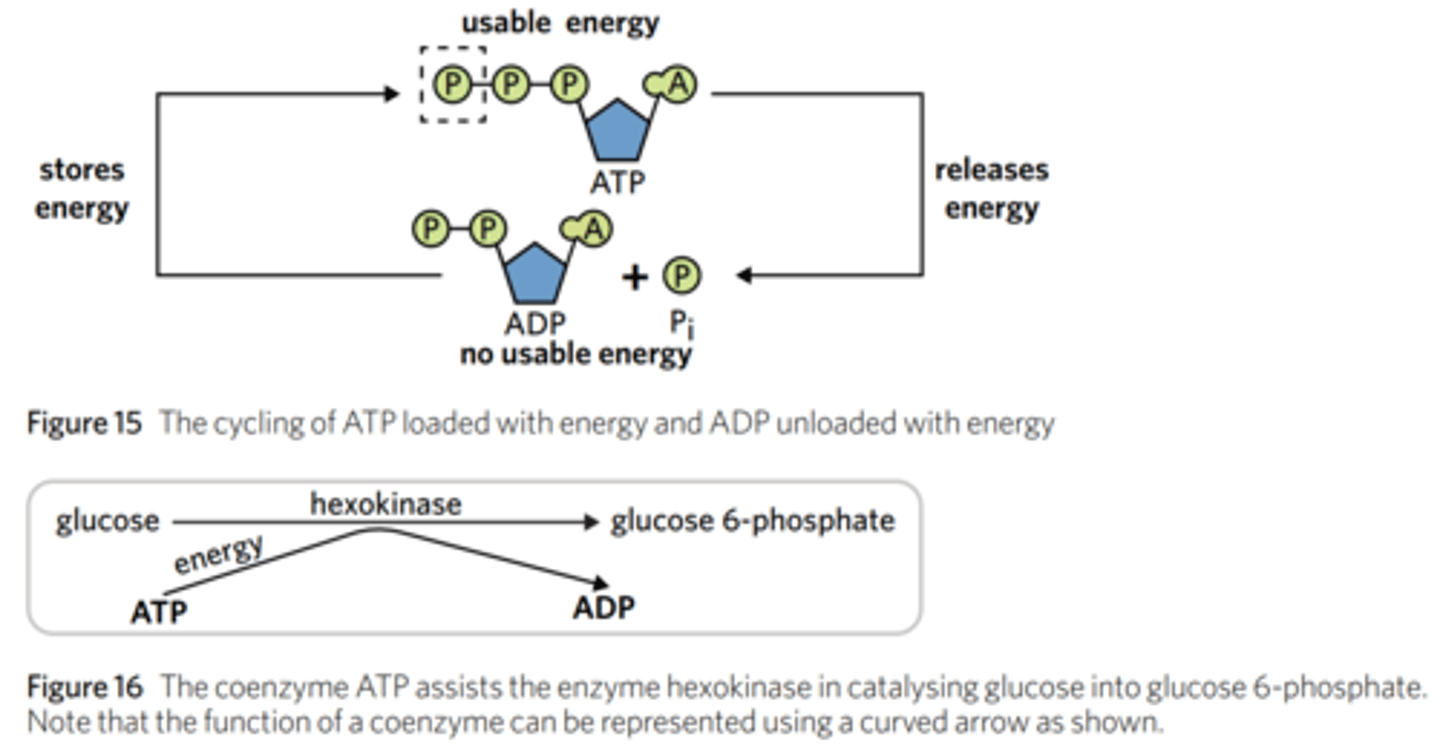
how does ATP act as a coenzyme? how does it convert into ADP? provide an example.
ATP (Adenosine Triphosphate) is the main energy transfer unit of the cell, as cells frequently rely on this coenzyme to donate energy to catalyse reactions.
Upon releasing energy for a reaction, ATP loses a phosphate group and becomes ADP (triphosphate = three phosphates, diphosphate = two phosphates).
The ADP molecule then goes on to have a phosphate group re-added, so it can become ATP and go on to catalyse more ATP-assisted reactions.
The conversion of ADP to ATP is a phosphorylation (adding phosphate) reaction, whilst ATP turns into ADP via a dephosphorylation (removing phosphate) reaction.
This back-and-forth process is coenzyme cycling.
For instance, the coenzyme ATP assists the enzyme hexokinase in catalysing glucose into glucose 6-phosphate
Which of the following accurately describes the relationship between temperature and the rate of enzyme activity?
Enzymes work fastest when the temperature is above the “critical temperature”.
36.7°C is the optimal temperature for enzymes.
When a solution is cooled down, enzymes work faster because their substrates move more slowly.
Enzymes work fastest at a temperature that is just below that at which they start to denature.
d
β-amylase is an enzyme that digests starch to sugars in plants. The optimal pH of β-amylaseis 5.4. If some β-amylase were added to a solution of starch with a pH of 6.5,
the enzyme would be denatured, because the difference between 5.4 and 6.5 is greater than 1.
the enzyme would work slowly, because the pH is more acidic than the optimal pH.
the enzyme would work slowly, because the pH is more basic than the optimal pH.
the enzyme would work more quickly, because 6.5 is greater than 5.4.
c
A biologist set up two test tubes, labelled 𝐴 and 𝐵. Each test tube contained the same concentration of starch. Into each test tube he placed β-amylase, which digests starch to simple sugars. The amount of β-amylase added to test tube 𝐴 was double the amount added to test tube 𝐵. After 5 seconds, the concentration of sugar was measured in each tube.
It is reasonable to predict that
the concentration of sugar would be equal in both tubes.
the concentration of sugar in test tube 𝐵 would be higher.
no sugar would be detected.
the concentration of sugar in test tube 𝐴 would be higher
d
If the concentration of substrate is doubled, we can be sure that
the rate of the reaction will stay the same.
the total amount of product produced will increase.
the amount of substrate used per second will increase.
the concentration of enzyme will also increase.
b
Relenza® is a rationally-designed therapeutic drug, developed by the CSIRO in Australia to treat influenza. The drug is a ‘release phase inhibitor’. It binds to to the active site of neuraminidase; an enzyme on the surface of influenza virus particles, preventing newly formed virus particles from binding to molecules on the inner surface of the host cell. This is necessary in order for it to leave the host cell to infect other cells. In this way, the reproductive cycle of the virus is impeded, giving the immune system more time to overcome the infection.
In terms of the enzyme neuraminidase, Relenza® is
a competitive inhibitor.
a non-competitive inhibitor.
a cofactor.
a coenzyme.
a
NADPH (Nicotinamide adenine dinucleotide phosphate) is a coenzyme for many enzymes in plants and animals. In animals, it is a coenzyme for the enzyme adrenodoxin reductase, which is involved in the synthesis of steroid hormones.
Based on this information and your knowledge of enzymes, it is fair to conclude that
NADPH is made of protein.
NADPH is organic.
NADPH binds tightly to the active site of adrenodoxin reductase.
NADPH is not changed or used up in the reaction catalysed by adrenodoxin reductase.
b
Enzymes can be denatured by
high and low temperatures and pH values.
high and low pH values but only high temperatures.
b
Which of the following are true of non-competitive inhibition? (Select all that apply)
I A reaction is triggered by the inhibitor.
II The active site of an enzyme is blocked.
III The enzyme’s function is reduced or eliminated.
IV The active site undergoes a change in structure.
V The substrate can no longer bind to the enzyme.
VI Binding occurs at a site other than the active site.
VII Non-competitive inhibition is always a form of irreversible competition.
III
IV
V
VI
define optimal/optimum in regards to enzymes
the point at which for a given condition, the maximum function of an enzyme occurs. Also known as optimum
generally, what are enzyme's optimal temperature in the human body?
36 to 38 degrees celsius. however, some enzymes in bacteria, for example, have an optimal temperature of above 70 degrees.
define conformational change
a change in the three-dimensional shape of macromolecules such as proteins
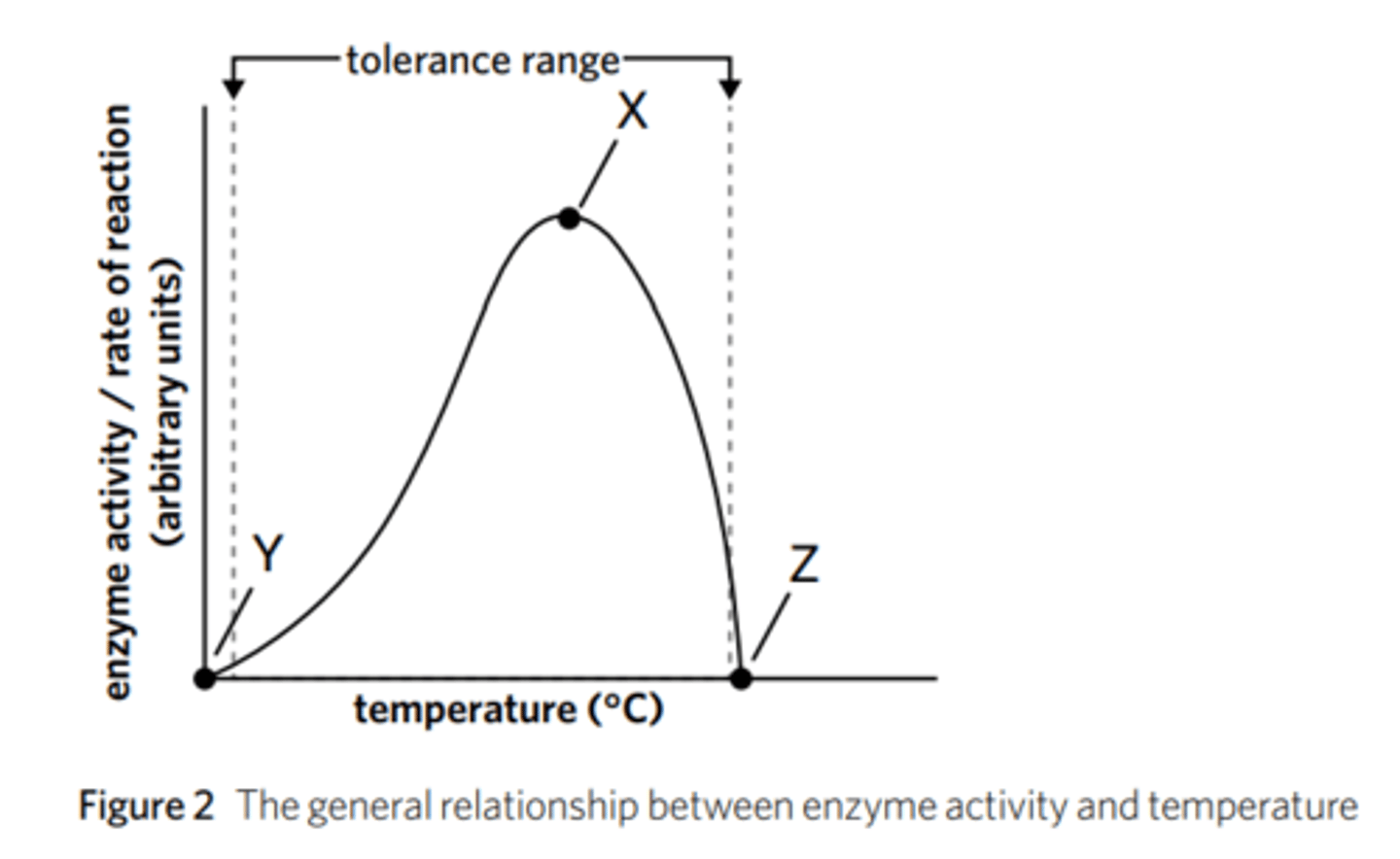
what is tolerance range? provide an example in regards to temperature.
Optimal temperature, which is the narrow range at which enzyme function is best.
However, tolerance range is used to describe the wider range of a given condition (like temperature) that an enzyme can function under. Outside of its tolerance range, an enzyme is inactive.
For example, an enzyme might have an optimal temperature of 58-60 °C but could have a tolerance range of 30-70 °C. In this case, the enzyme will function variably within this range but might freeze if the temperature drops below 30 °C, or denature if the temperature goes above 70 °C.
flip
describe the trend in this graph and what each point illustrates.
flip
It is reasonable to conclude that
A. Celluclast® is inactive at 61 °C.
B. Celluclast® is denatured at 35 °C.
C. the optimum pH for Celluclast® is pH 5.
D. the optimum temperature for Celluclast® is around 57 °C.
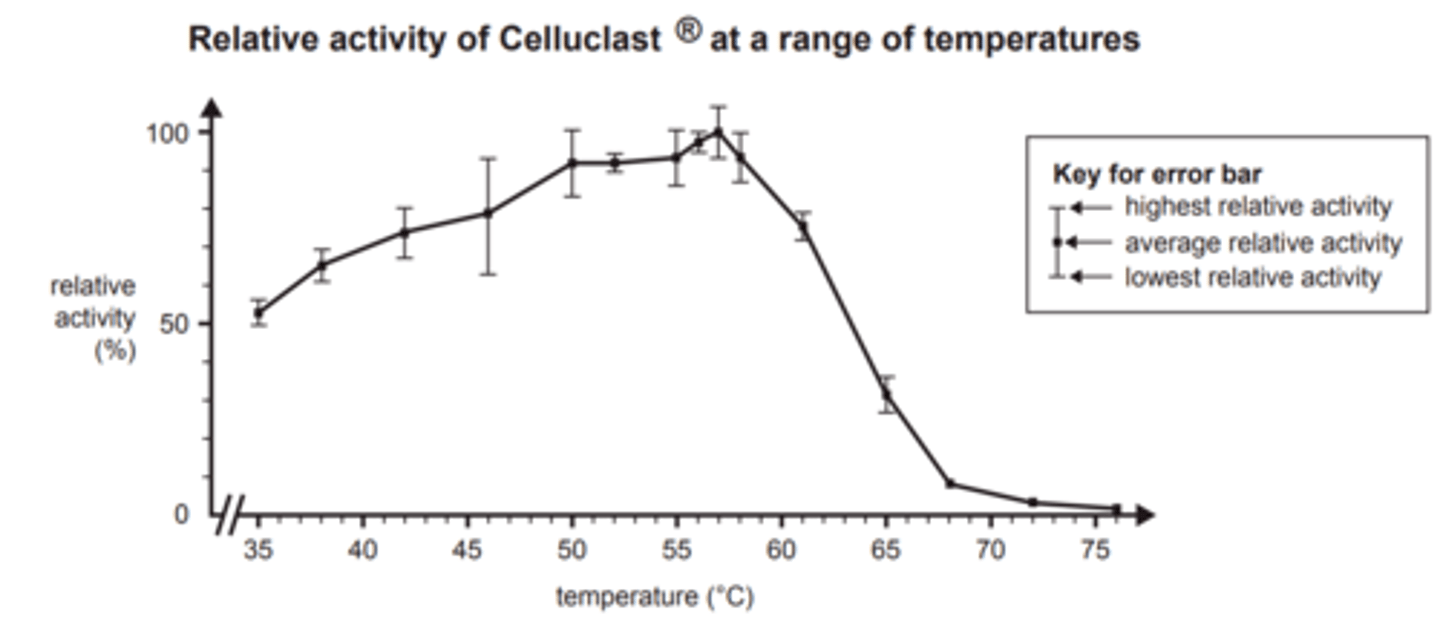
define saturation point in regards to enzymes
the point at which a substance (e.g. an enzyme) cannot receive more of another substance (e.g. a substrate)
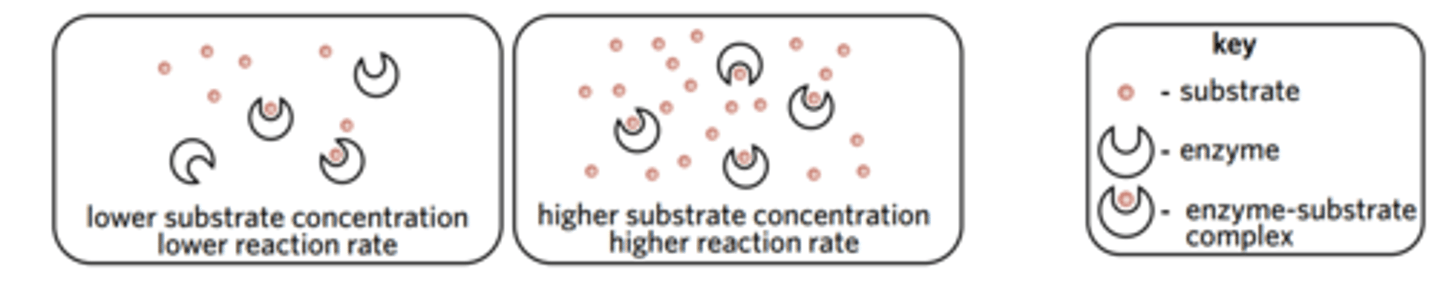
what is a limiting factor?
a factor that prevents the rate of reaction from increasing
what is a limiting reagent? provide an example.
a reactant that prevents the rate of reaction from increasing
for example, substrate before reaction rate reaches a constant.
flip
examine how the (graph a) concentration of limiting reagent and (graph b) total quantity of limiting reagent differ in the total product formed.
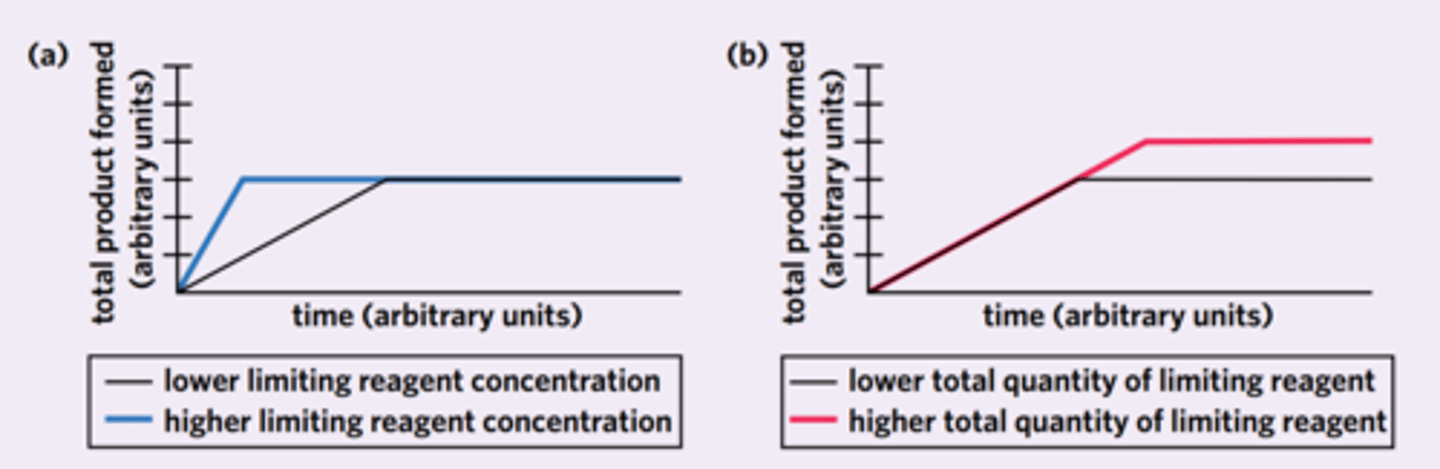
flip
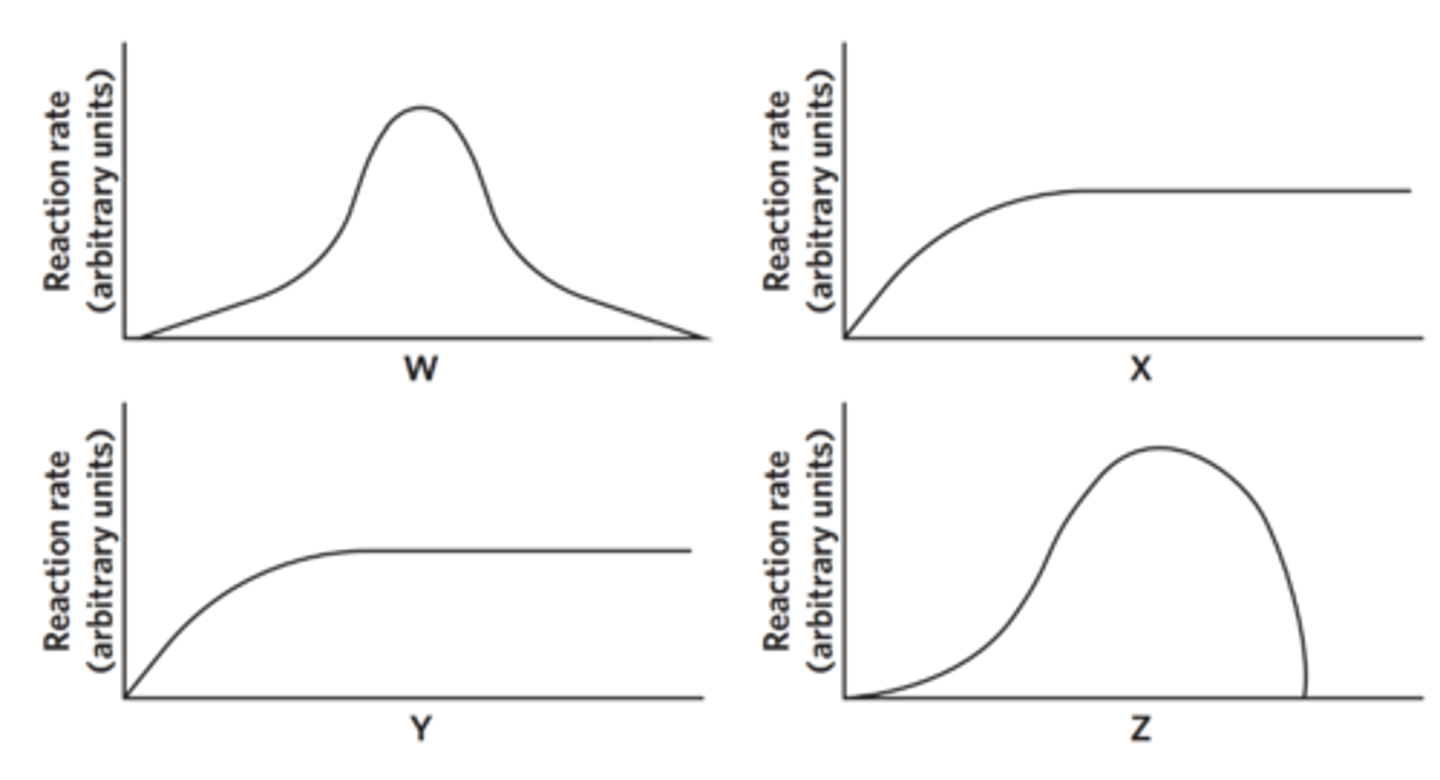
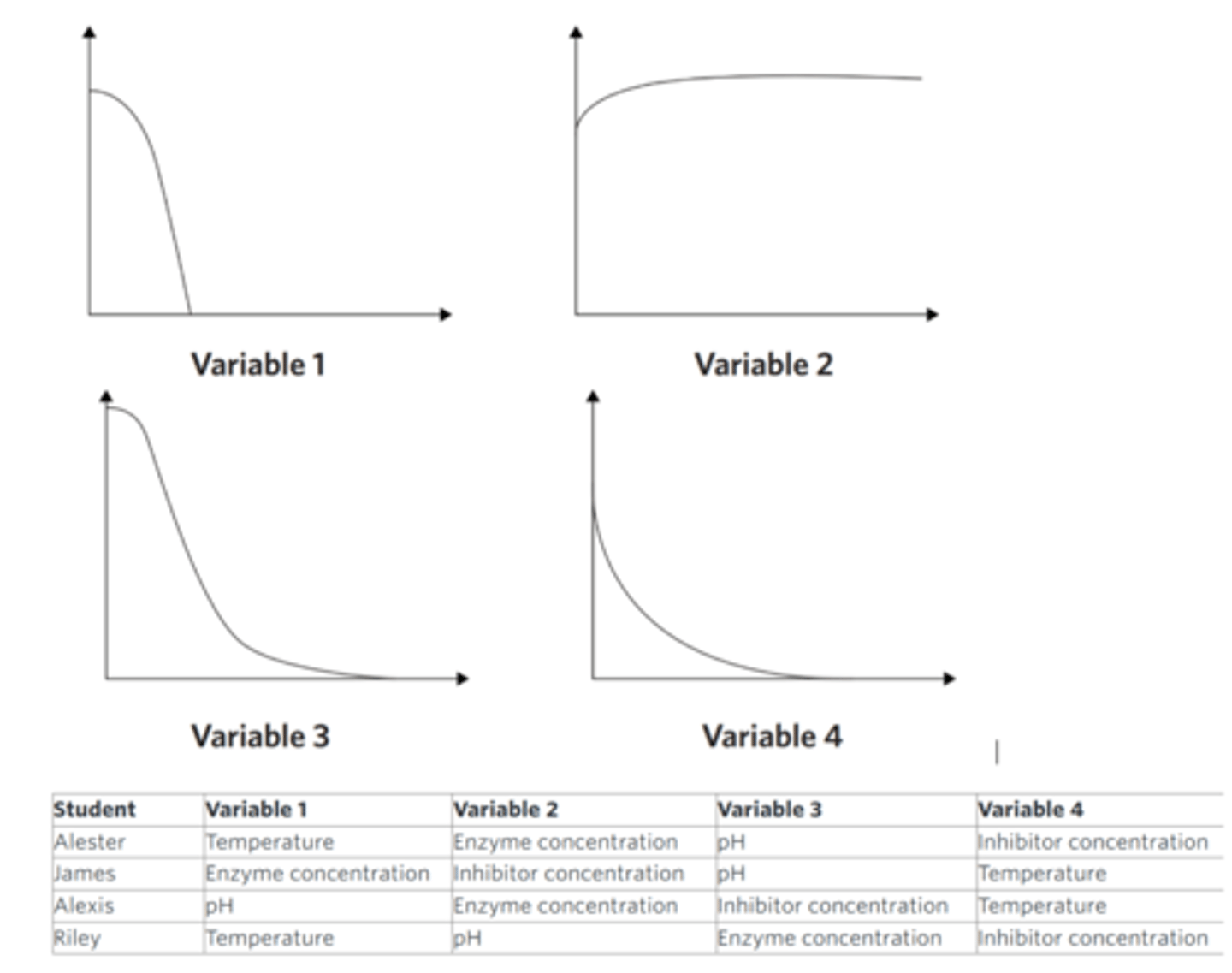
Four students performed a series of experiments to investigate the effect of four different variables on the rate of an enzyme-catalysed reaction. In each experiment the students changed one of the following variables: substrate concentration, pH, temperature and enzyme concentration. After recording their data, the students displayed their results in a series of graphs, as shown below. Each graph is a line of best fit for their data.
The students did not label the horizontal axis on any of their graphs. The next day, the students could not agree on which variable should be labelled on the horizontal axis of each graph. The students made the following suggestions as to what each variable could be.
Identify the correct student based on their results.
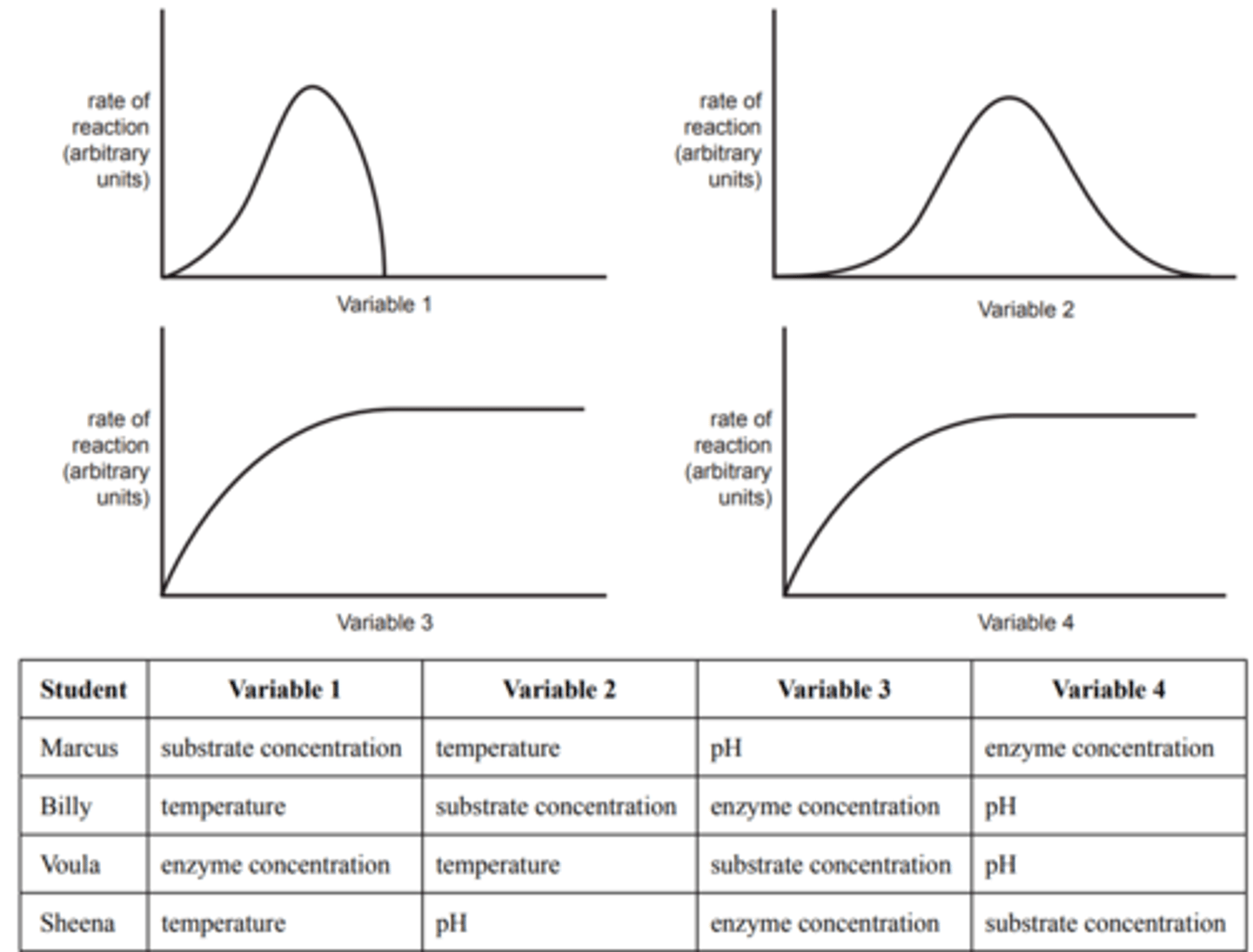
flip
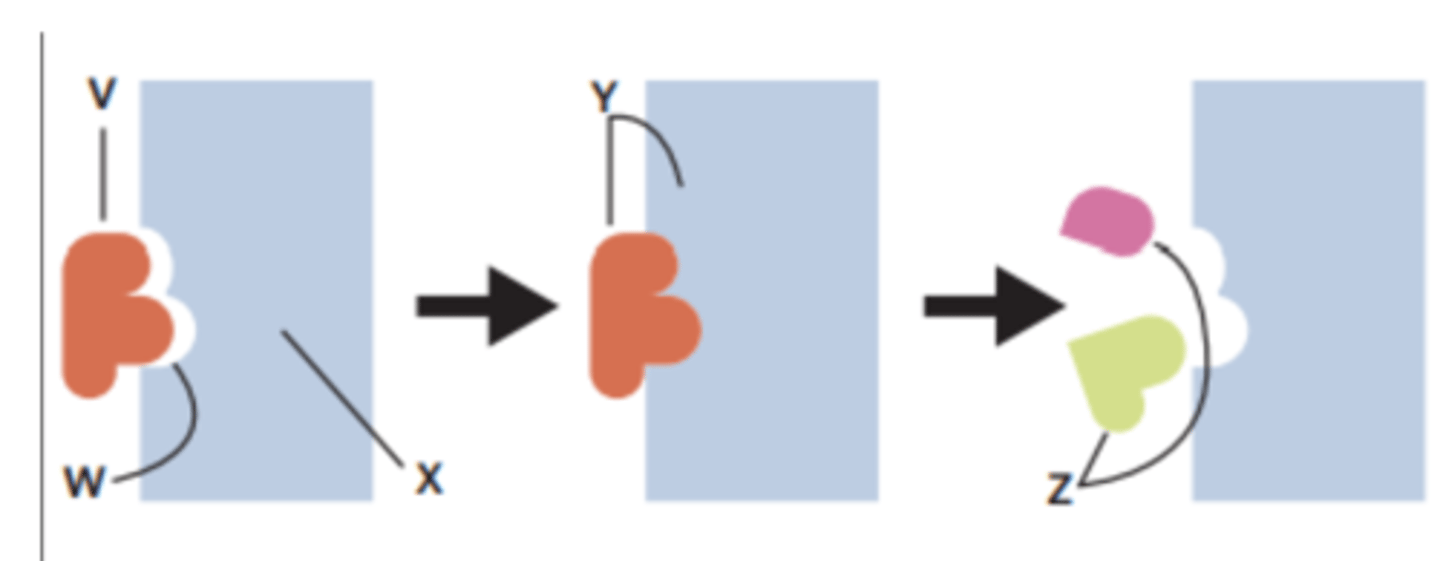
Label the parts of the enzymatic reaction from the list of terms.
coenzyme
active site
substrate
product
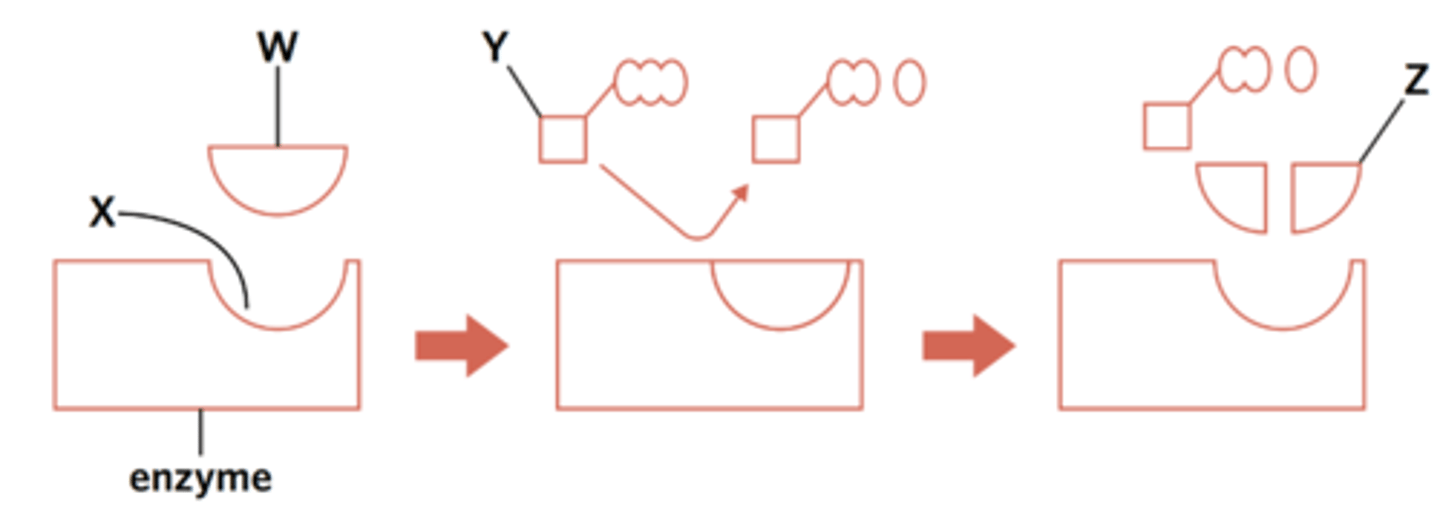
flip
Label the parts of the graph axes from the list of terms.
pH
temperature
enzyme concentration
substrate concentration
Order the steps to correctly describe a coenzyme-assisted enzymatic reaction.
I The reaction occurs.
II An enzyme is present.
III The substrate can now bind to the active site.
IV The enzyme is now free to catalyse further reactions.
V Substrate arrives at the enzyme but cannot be catalysed yet.
VI A loaded coenzyme arrives at the enzyme to bind and donate energy.
VII The products are released from the active site and the unloaded coenzyme leaves to be recycled.
II; V; VI; III; I; VII; IV
flip
In this series of experiments, the amount of enzyme, the pH, and the temperature remain constant.
At point X
all active sites are consistently occupied.
the substrate is the limiting reactant.
the rate of reaction is decreasing.
no reactions are occurring.
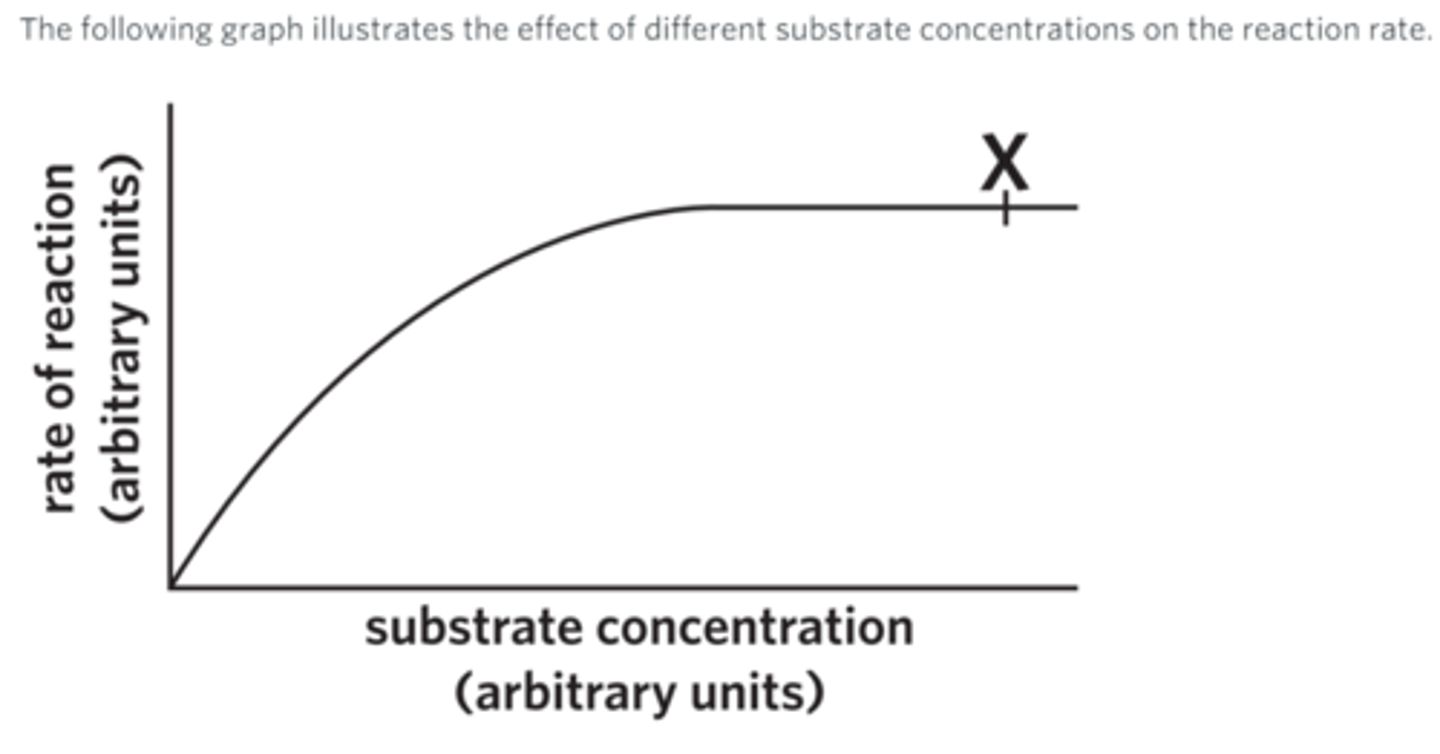
Bacteria such as Thermus aquaticus live in hot springs where temperatures are around 90 °C. What can be said about the temperature tolerance range of enzymes found in T. aquaticus?
The enzymes’ tolerance range likely centres around 90 °C.
The enzymes’ tolerance range is limited to a narrow range.
90 °C is outside the enzymes' tolerance range, however, they can still operate.
The enzymes must be capable of operating over a wide range of temperatures.
a
flip
Which of the following is false when considering this reaction?
This reaction is reversible.
The product of this reaction stores energy.
The product of this reaction contains three phosphate subunits.
This reaction is catalysed by a coenzyme to produce adenosine triphosphate.
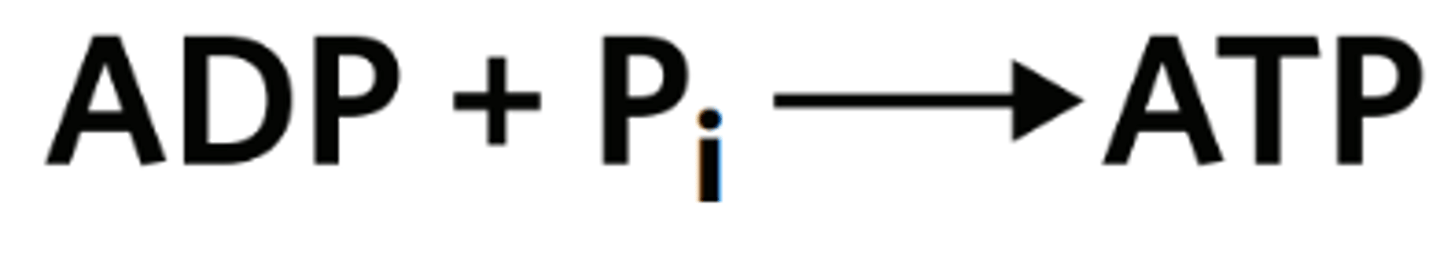
flip
CTP is a substance used by cells to make RNA. The cell initially synthesises CTP using a metabolic pathway starting with the amino acid aspartate (A) and another complex molecule (B).
The pathway for making CTP is represented. The enzyme involved in the first step of the pathway is called ATCase.
Inhibiting the action of Enzyme X would
result in a buildup of molecule D.
cause Enzyme Z to function faster.
decrease the concentration of ATCase.
increase the concentration of CTP produced.

An inhibitor that competitively inhibits Enzyme X would be
complementary to ATCase.
similar in structure to Enzyme X.
similar in structure to molecule D.
complementary to the substrate of Enzyme Z.
c
flip
The enzyme lactate dehydrogenase is found in a wide variety of organisms. It catalyses the conversion of both pyruvate to lactate, and lactate to pyruvate. The bacterium Thermoanaerobacter ethanolicus lives in geothermal (hot) springs. The river buffalo (Bubalus bubalis) is a domestic animal common in Pakistan. Scientists studying the enzyme lactate dehydrogenase from these two organisms produced the following graphs (adapted from Nadeem et al. (2011) (left) and Zhou and Shao (2010) (right)).
From the graphs, which of the following conclusions is false?
The optimal pH of the bacterial lactate dehydrogenase is 7.0.
Above 60 °C the buffalo form of the enzyme would likely denature.
Below 40 °C the bacterial form of the enzyme would likely denature.
The form of enzyme found in the buffalo operates over a narrower pH range than the bacterial form.
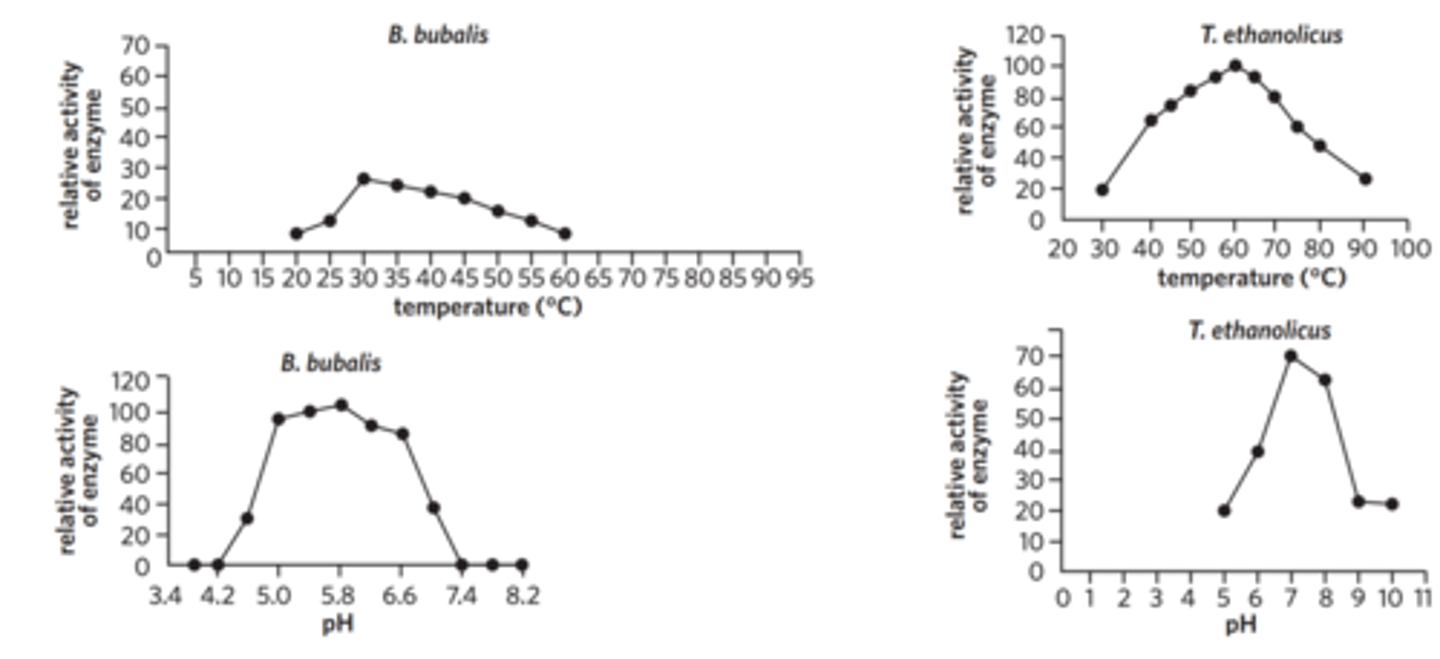
flip
Laundry powder is sometimes advertised as containing powerful enzymes that break down dirt. These enzymes are called extremozymes. They come from some species of bacteria and archaea. The following table gives the optimal functioning of enzymes from some of these species.
Given this information and your knowledge of enzyme function, which of the following conclusions could be made?
Enzyme K would likely denature in extremely acidic environments.
Enzyme L has the widest optimal temperature range.
Enzyme M functions well in a basic environment.
Enzyme J is likely found in the human body.
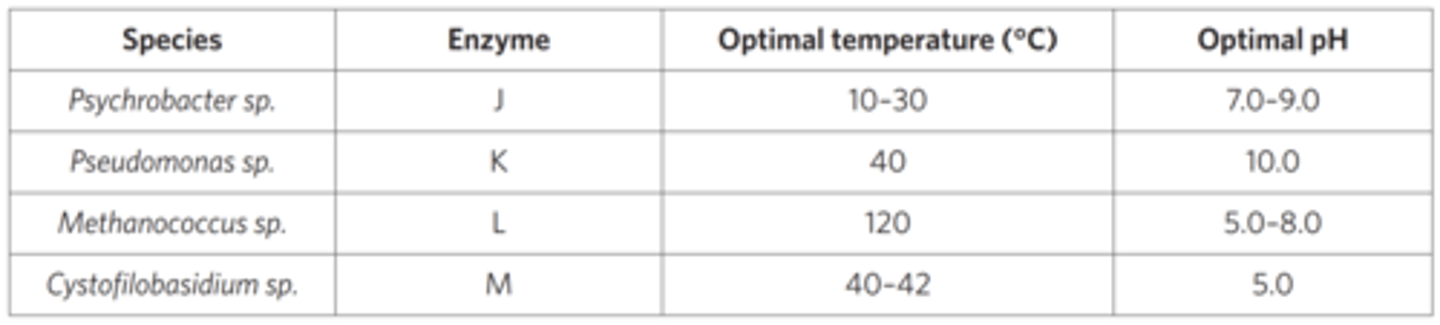
flip
In the production of isoleucine from threonine in bacteria (Biochemical Pathway 1 [BP 1]), the end product acts as a competitive inhibitor of the first enzyme in the pathway. In the production of arginine (Biochemical Pathway 2 [BP 2]), the end product has no influence on other enzymes in the pathway.
It is reasonable to conclude that in
BP 1, an increase in isoleucine results in an increase in enzyme 2.
BP 2, an increase in arginine results in an increase in substrate.
BP 1, isoleucine regulates the production of compound X.
BP 2, arginine regulates the production of compound M.
![<p><strong>In the production of isoleucine from threonine in bacteria (Biochemical Pathway 1 [BP 1]), the end product acts as a competitive inhibitor of the first enzyme in the pathway. In the production of arginine (Biochemical Pathway 2 [BP 2]), the end product has no influence on other enzymes in the pathway.</strong></p><p></p><p><strong>It is reasonable to conclude that in</strong></p><p></p><p>BP 1, an increase in isoleucine results in an increase in enzyme 2.</p><p></p><p>BP 2, an increase in arginine results in an increase in substrate.</p><p></p><p>BP 1, isoleucine regulates the production of compound X.</p><p></p><p>BP 2, arginine regulates the production of compound M.</p>](https://knowt-user-attachments.s3.amazonaws.com/701a0fa2-90c3-4ba8-9ce6-16cce617442e.image/png)
flip
The enzyme maltase catalyses the breakdown of maltose into glucose. Maltase was added to a tube containing a solution of maltose in water and incubated at 37 °C. The amount of glucose produced was monitored over a period of time. Some maltose remained at the end.
The graph showing the change in glucose concentration in the tube is
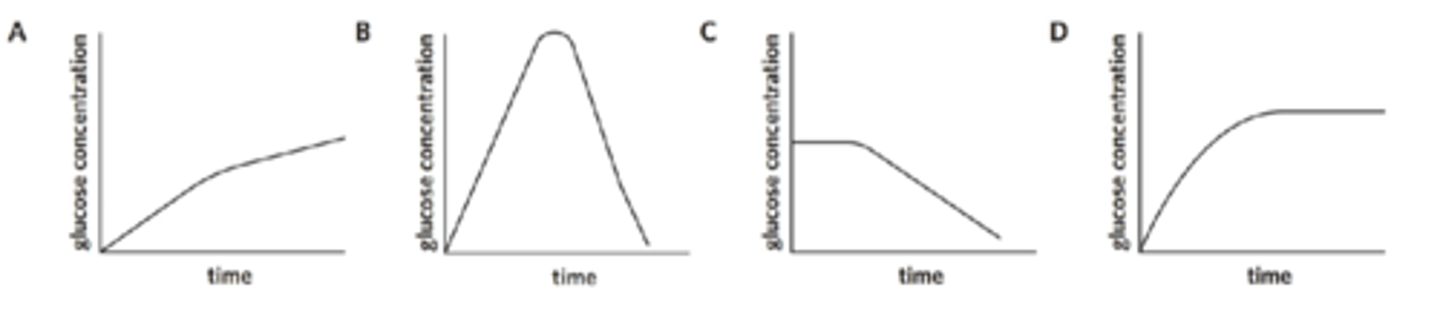
flip
Sucrose (cane sugar) is a disaccharide used by plants as a transport molecule. Sucrose is formed in the reaction shown.
With reference to this process, which of the following statements is false?
Fructose acts as a coenzyme to produce sucrose from glucose.
The enzyme lowers the activation energy of the reaction.
The production of sucrose is an energy-storing reaction.
An enzyme can be used to form sucrose.
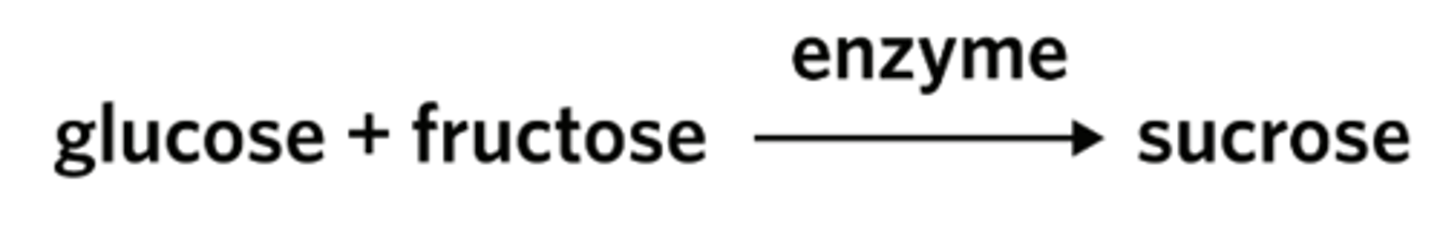
Yeast cells divide rapidly provided they are mixed with warm water to form a suspension, and sucrose is added.
Sucrose is unable to cross the yeast cell membrane, and is digested into glucose and fructose by the enzyme sucrase. Sucrase is synthesised within the yeast cell, but acts in the water surrounding the yeast cells.
Warming the yeast suspension increases the rate at which sucrose is broken down to glucose and fructose, because warming
A ensures the pH is optimal for formation of enzyme-substrate complex.
B improves the ‘fit’ between active site of the enzyme and substrate.
C increases the frequency of collisions between molecules.
D increases the concentration of the products.
c
Bacteria such as Thermus aquaticus live in hot springs where temperatures are around 90°C. The most likely reason that the bacteria are able to carry out their metabolic functions in this environment is that the bacteria
A
have enzymes with a high optimal temperature.
B
can lower the temperature of the cellular environment.
C
use compounds other than enzymes to catalyse reactions.
D
have enzymes other than proteins that do not respond to changes in temperature.
A
Acetylcholinesterase is an enzyme that catalyses the breakdown of the neurotransmitter acetylcholine into acetate and choline.
Some chemicals used to kill insects contain aldicarb.
Aldicarb is a reversible inhibitor of acetylcholinesterase that
A
permanently blocks the active site of acetylcholinesterase.
B
acts by strongly binding to the active site of acetylcholinesterase.
C
increases the rate at which acetylcholine is broken down into acetate and choline.
D
reduces acetylcholinesterase activity by reducing the number of active sites for acetylcholine to bind to.
D
Explain why enzymes are necessary in living organisms.
Describe the 'active site' of an enzyme and explain its role.
Enzymes are necessary amidst living organisms because they lower the activation energy required to initiate a given reaction and thus they increase the rate of chemical reactions that would normally take much longer to occur given that an enzyme was not present.
An active site is part of an enzyme that allows for substrates to bind to an enzyme.
flip
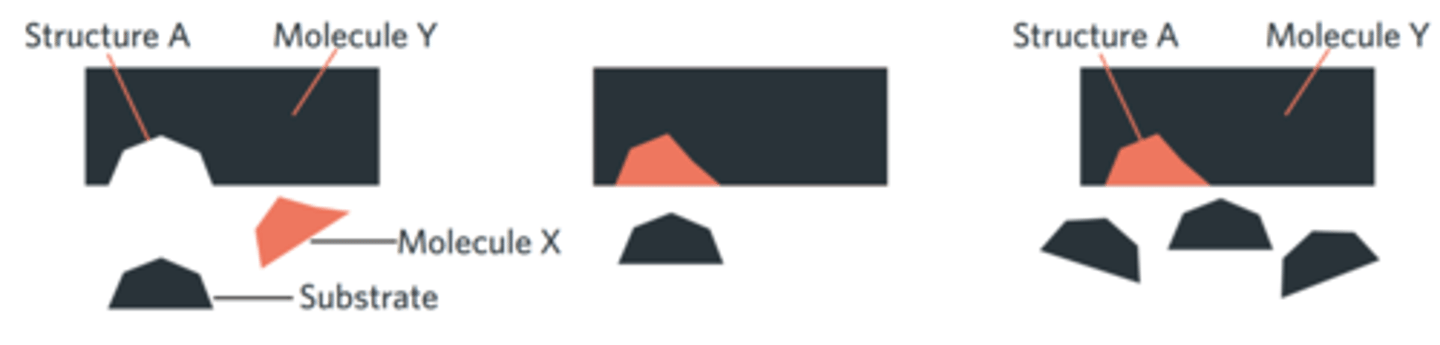
Which one of the following statements is correct?
Molecule X is an enzyme.
Structure A is a coenzyme.
This is an example of competitive inhibition.
Molecule X binds allosterically to the active site.
flip
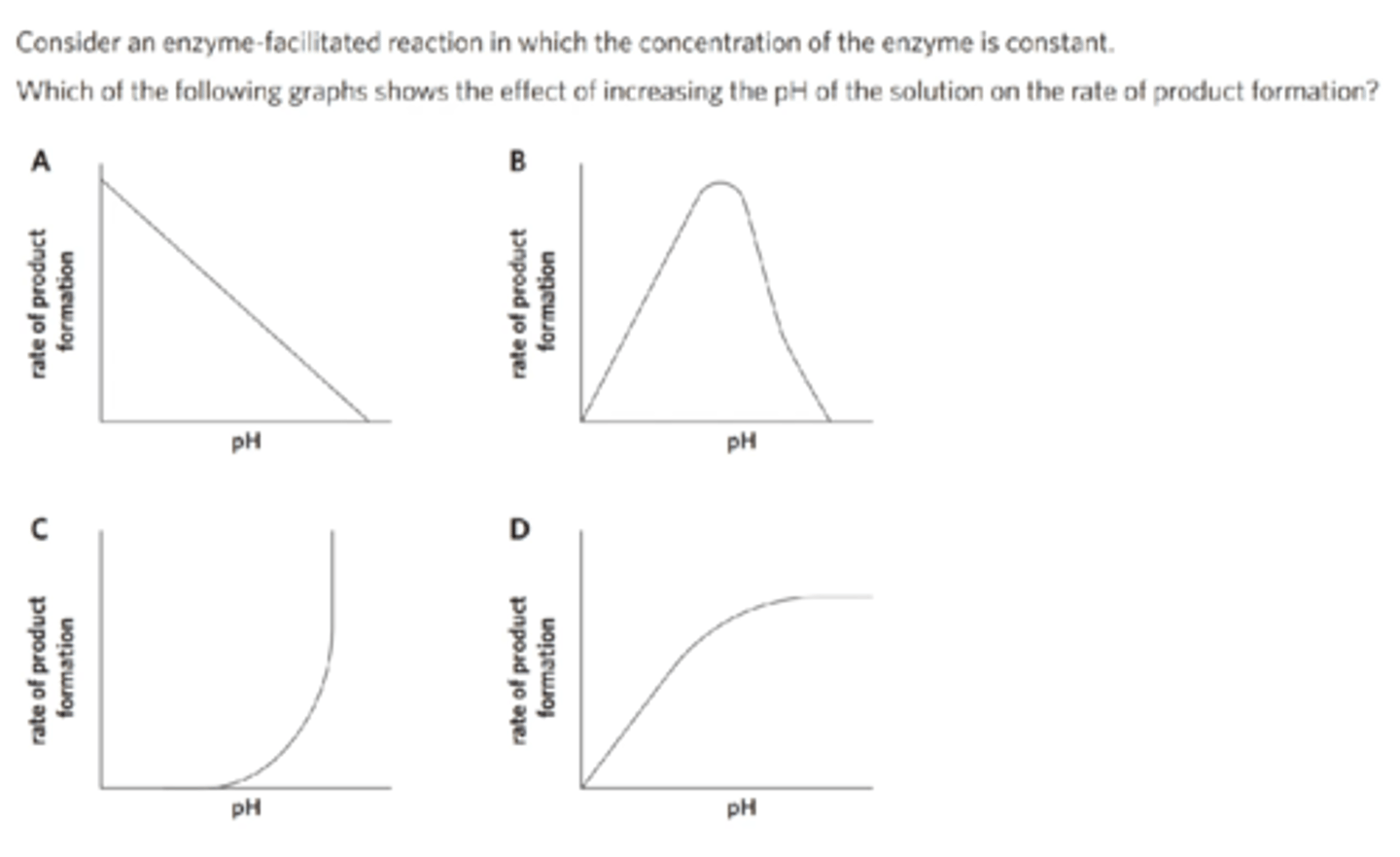
The activity of an enzyme does not
increase with temperature indefinitely, even though more molecular collisions typically occur as temperature increases.
decrease with pH values outside an enzyme’s optimal range.
increase when temperature is within its optimum range.
decrease in the presence of an inhibitor.
a
flip
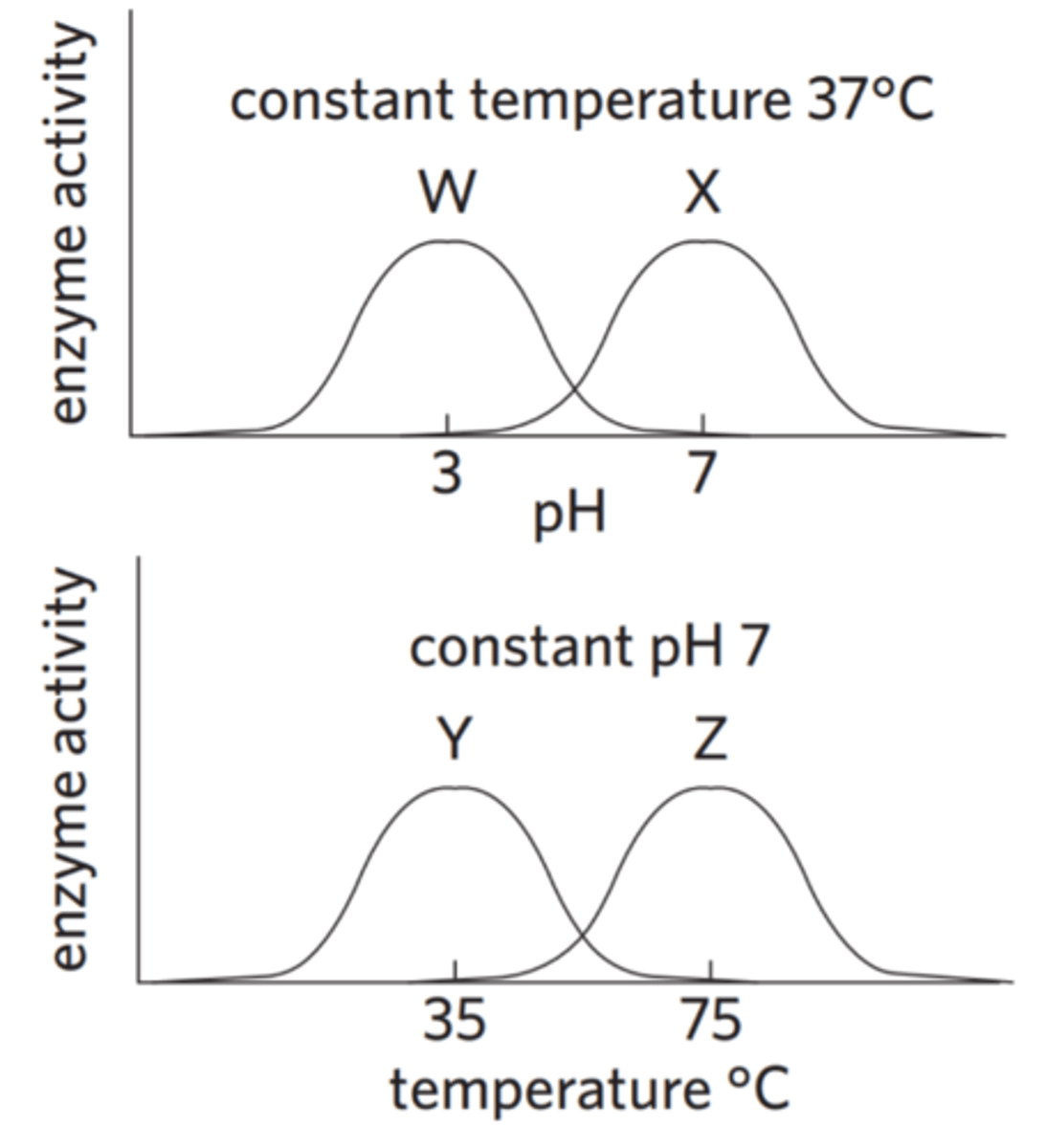
The following graphs show the way four enzymes, W, X, Y, and Z, change their activity under different pH or temperature situations.
Which of the following statements about the activity of the four enzymes is correct?
The optimal pH of enzyme Z is 3.
Enzyme W functions well in an alkaline environment.
At pH 5, enzyme X has greater activity than enzyme W.
Enzyme Y is more likely to be found in the human body than enzyme Z.
flip
flip
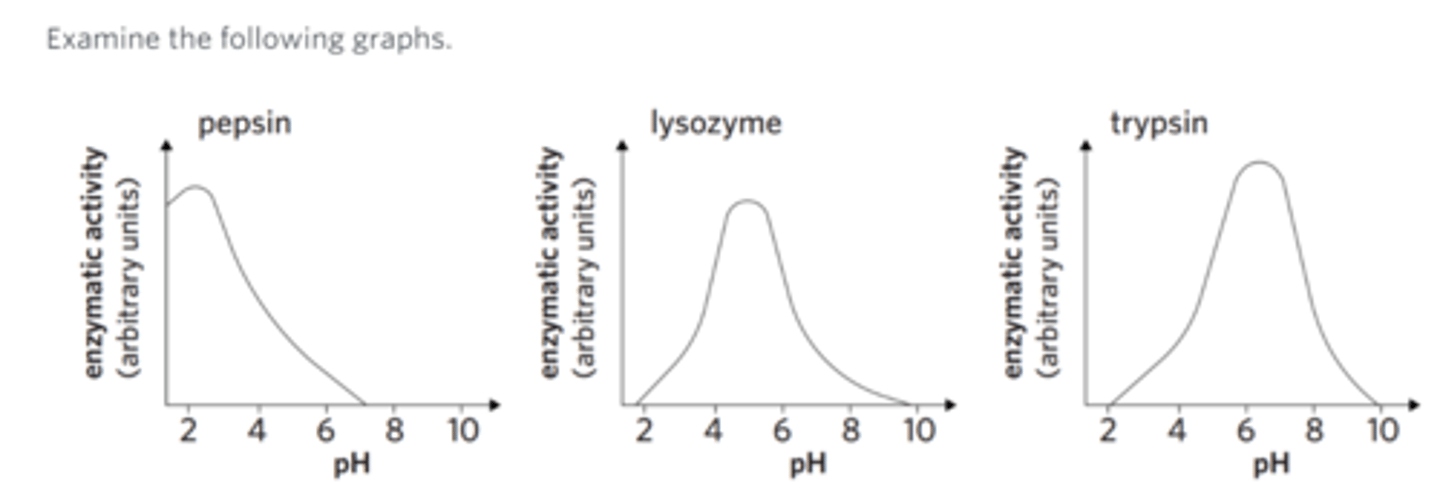
Examine the following graphs.
From these graphs, it is reasonable to infer that at a pH of 6
all the lysozyme would be denatured.
trypsin converts a large amount of substrate.
all three enzymes would lack a functional active site.
the active site of pepsin would bind well to the substrate.
flip
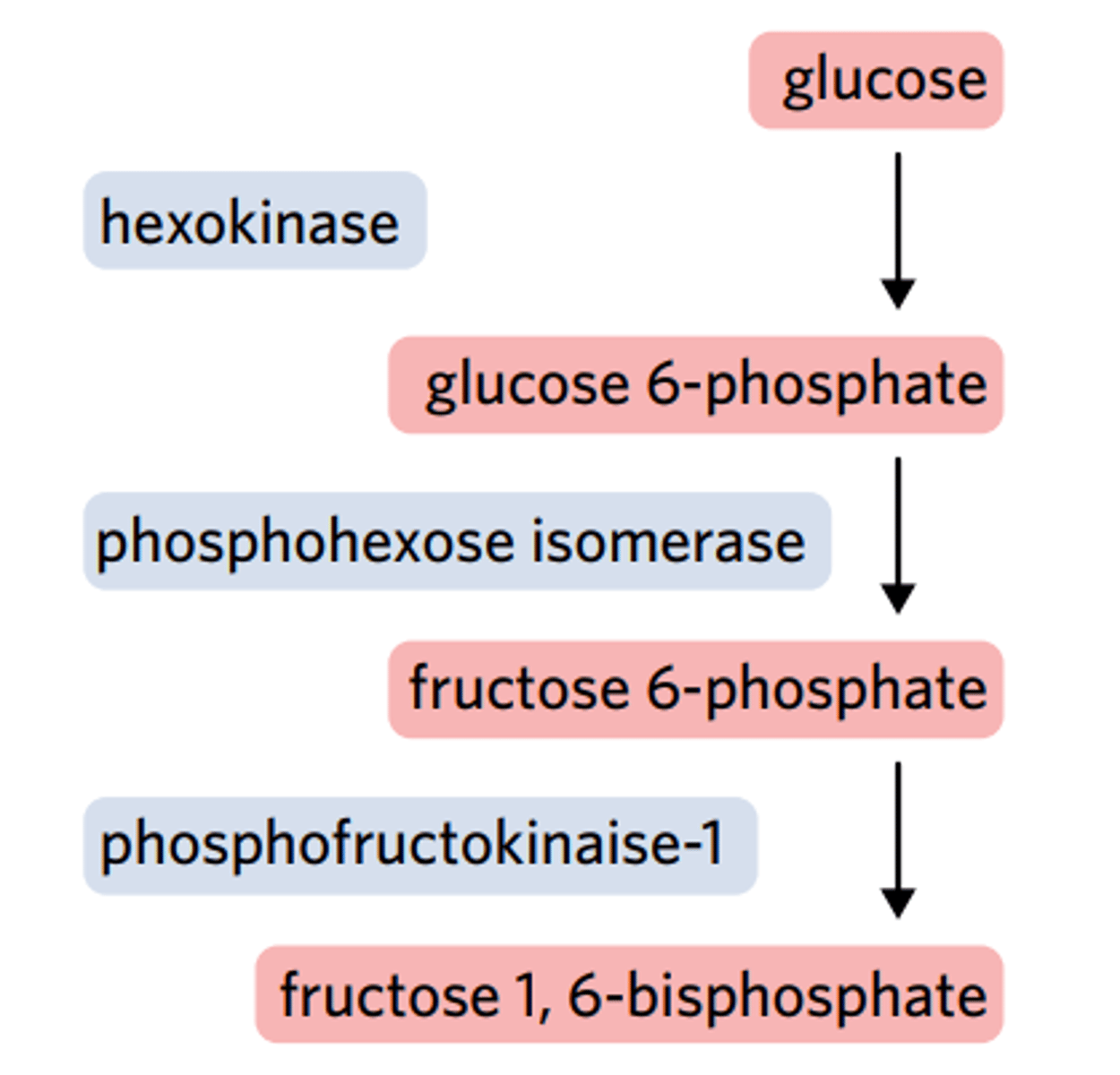
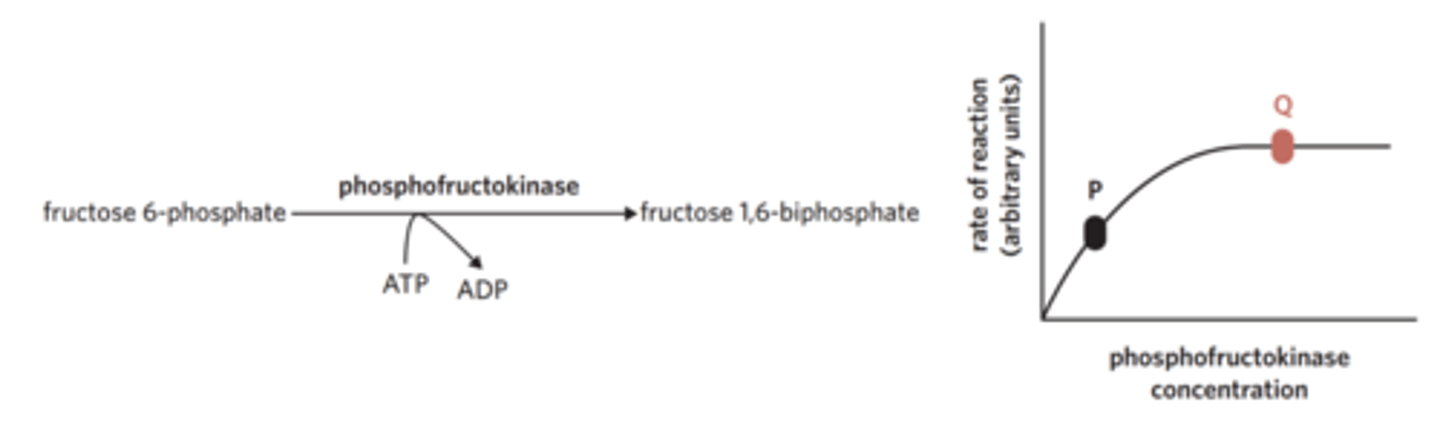
The biochemical pathway of glycolysis involves nine intermediate reaction steps. One of these steps is represented in the diagram, as well as a graph displaying the rate of the reaction. In this reaction, the environmental temperature and pH are optimal.
Based on the information in the graph, what can be concluded about point Q?
Fructose 6-phosphate is the limiting reactant.
The enzyme phosphofructokinase has denatured.
Fructose 1,6-biphosphate is the limiting reactant.
ADP has become the limiting reactant of the reaction.
flip
Four students performed a series of experiments to investigate the effects of four different variables on the rate of an enzyme-catalysed reaction. In each experiment, the students increased one of the following variables: temperature, pH, enzyme concentration, and the concentration of a known enzyme inhibitor. Each experiment started at a pH and temperature value known to be within the optimal range of the enzyme. When starting each experiment, one student made the mistake of not recording the data for the first several minutes. The students still displayed their results in a series of graphs, as shown, but the reaction rate was high when recording started. Each graph is a line of best fit.
The students did not label the horizontal axis on any of their four graphs. The next day, the students could not agree on which variable should be labelled on the horizontal axis of each graph. The students made the following suggestions as to what each variable could be.
What student is correct?
What type of error was made by not recording data at the beginning of the experiment?
flip
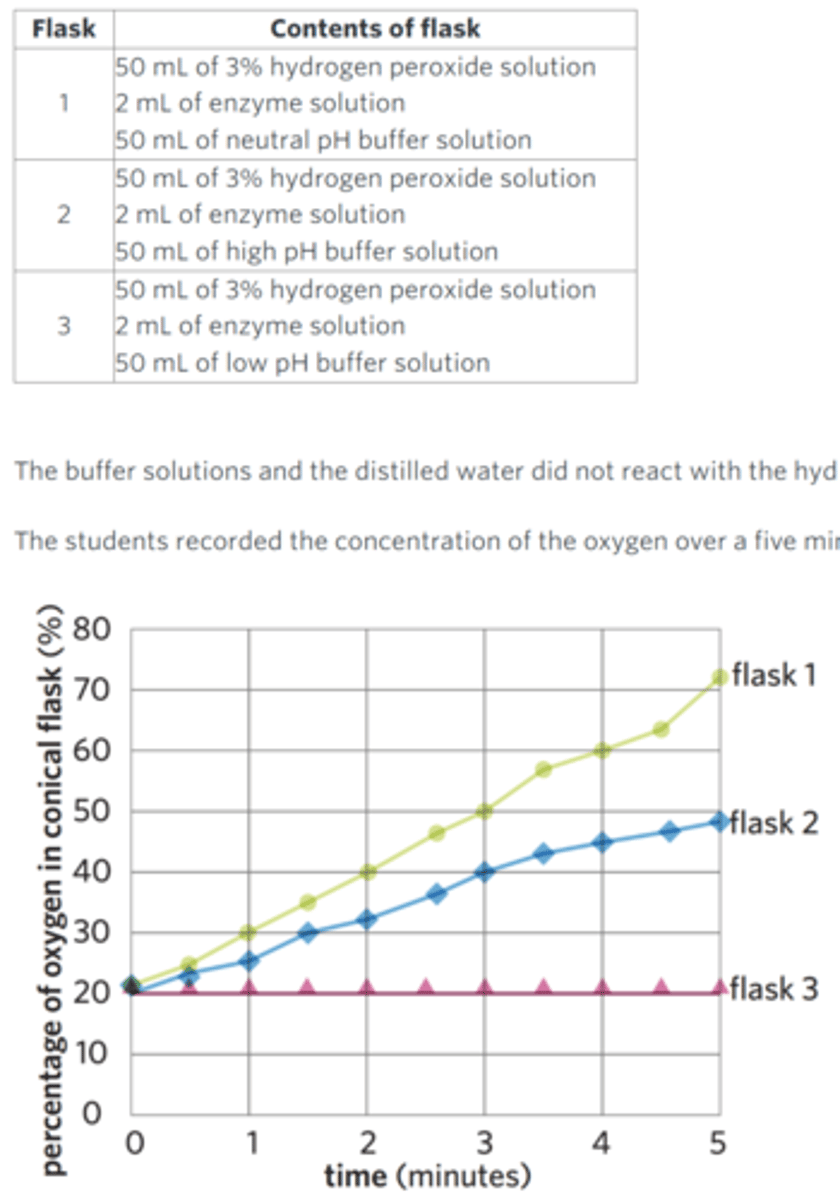
A group of students wanted to investigate the activity of an enzyme that catalyses the breakdown of hydrogen peroxide into water and oxygen.
The students measured oxygen concentration using an oxygen sensor. The oxygen sensor fits into the top of a conical flask. The students set up three conical flasks with the contents listed in the table.
The buffer solutions and the distilled water did not react with the hydrogen peroxide, and all conical flasks were at room temperature.
The students recorded the concentration of the oxygen over a five minute period. The results of the experiment are shown in the graph.
Describe how a control could be implemented and outline its purpose.
Differentiate between a reactant and a substrate.
Substrate is the name given to the reactant in an enzyme-catalysed reaction.
what is high pH?
alkaline - basic