Nuclear Chemistry MCQ
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms

What are isotopes?
atoms of the same element that have the same # of protons but different # of neutrons or mass.
Elements 43, 84 and above are _____ because they decay.
radioactive
When the phrase “no stable isotopes” is used in the Chemistry Regents, what does it mean?
Radioactive
If an element is naturally radioactive, every other version is ____.
Radioactive
Atomic mass:
The ones with parentheses are _____.
Ex: atomic mass - (238.0289u)
radioactive
The element is radioactive when the ratio of neutrons to protons is _____ (for light nuclei)
Examples:
Carbon-14, Lithium-7
greater than 1:1
When does natural radioactivity occur?
When nuclei are unstable
What happens when a nucleus decays?
A nucleus decays to attain more stable atomic configuration.
It emits particles
What is the mass of an alpha particle? (in amu)
4 amu
What is the mass of a beta particle? (in amu)
0 amu
What is the mass of a positron particle? (in amu)
0 amu
What is the mass of a gamma particle? (in amu)
0 amu
What is the charge of an alpha particle?
2+
What is the charge of a beta particle?
1-
What is the charge of a positron particle?
1+
What is the charge of a gamma particle?
none
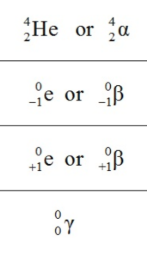
What is the symbol of an alpha particle?
α, 4/2He
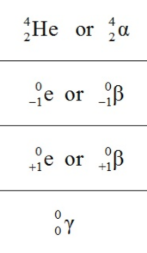
What is the symbol of a beta particle?
-β, 0/-1e
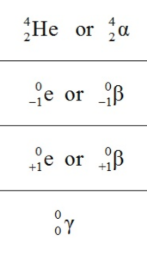
What is the symbol of a positron particle?
+β, 1/0e
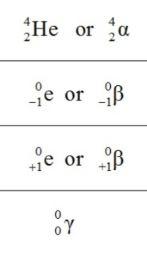
What is the symbol of a gamma particle?
γ, 0/0 γ
What is the penetrating power of an alpha particle?
(Hint: it has maximum mass)
Low
What is the penetrating power of a beta particle?
Moderate
What is the penetrating power of a positron particle?
Moderate
What is the penetrating power of a gamma particle?
(Hint: it it only energy - no charge or mass)
High
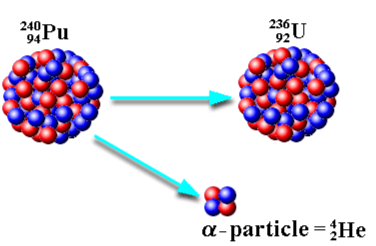
What is alpha decay?
Keep in mind:
Symbol | Change in Mass # | Change in Atomic # |
α | -4 | -2 |
When an unstable nucleus emits an alpha particle
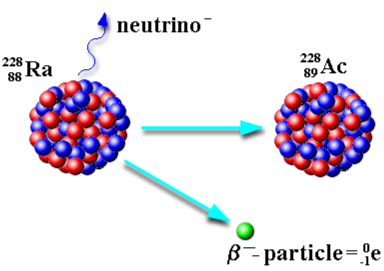
What is beta decay? (beta minus decay)
Keep in mind:
Symbol | Change in Mass # | Change in Atomic # |
β | 0 | +1 |
When an unstable nucleus emits a beta particle
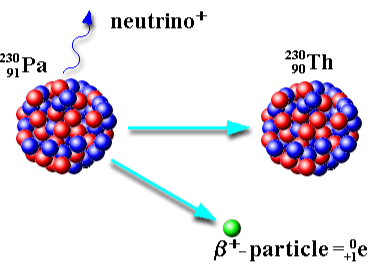
What is positron emission? (beta plus decay)
Is caused by a proton converting to a neutron.
Keep in mind:
Symbol | Change in Mass # | Change in Atomic # |
β+ | 0 | -1 |
When an unstable nucleus emits a positron
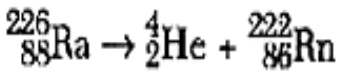
What is natural transmutation? (only has ONE REACTANT)
Begins with one nucleus that spontaneously decays
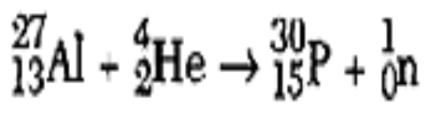
What is artificial transmutation? (always has TWO REACTANTS)
Bombardment of the nucleus with high energy particles
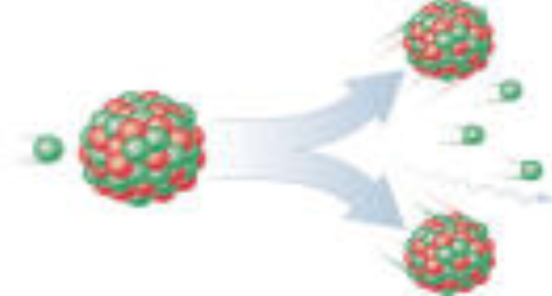
What is a fission reaction?
Fission reactions produce/capture neutrons. They can become involved in another fission reaction.
Involves breaking/splitting an atom into other atoms.
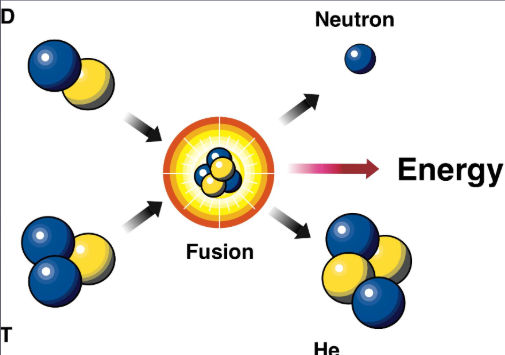
What is a fusion reaction?
Two atoms of hydrogen fusing together to form an atom of helium.
What does fission and fusion create? (Hint: They break the law of conservation of matter.)
A tremendous amount of heat and energy.
The energy of nuclear reactions is _____ the energy associated with chemical reactions.
greater than
The energy of fission is _____ the energy of fusion.
less than
How come it is very hard to make nuclear fusion happen? (What is so impossible about the fusion?)
Hydrogen is positive- hydrogen atoms repel each other
What is half life?
(See table N for half-lives and modes - will never hit 0!)
the time it takes for half of a radioactive sample of an element to decay.
Radioactive substances ______ at a rate that is _____ on temperature,
pressure, or concentration.
decay, not dependent
What radioactive elements are used for dating?
U-238, K-42, C-14
What radioactive elements are used in the medical field?
I-131, Tc-99, Co-60, gamma
What is radioactive element I-131 used for?
Medical field - thyroids
What is radioactive element Tc-99 used for?
Medical field - cancer
What is radioactive element Co-60 used for?
Medical field - cancer
What is radioactive element U-238 used for?
Dating - rocks
What is radioactive element C-14 used for?
Dating - Living material
What is radioactive element K-42 used for?
Dating - dinosaurs
What must radioactive elements used in the medical field must have?
Short half life and be quickly eliminated from the body
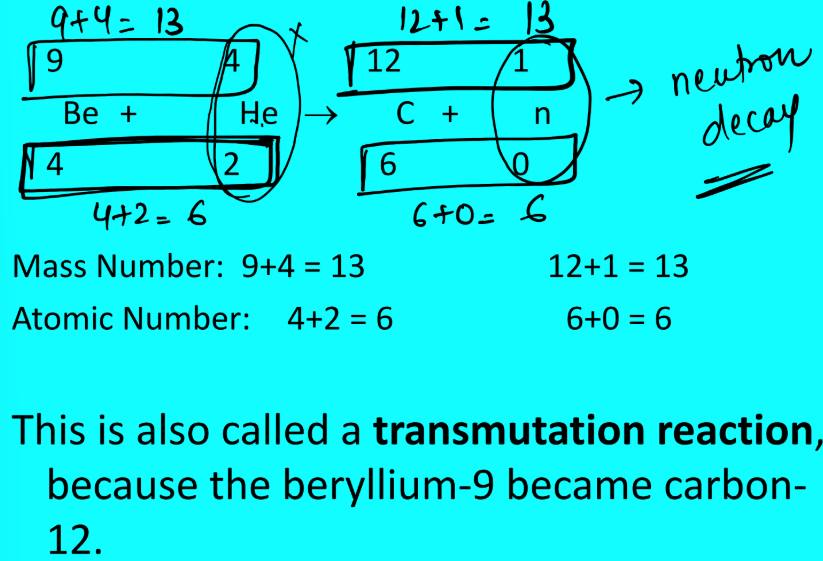
In nuclear equations, the total of the _____ and the total of the _____ must be equal on both sides of the equation.
atomic number, mass number