BIS104: Final Material
1/210
Earn XP
Description and Tags
UC Davis Lectures 5/14-6/4 Dinesh-Kumar Cell
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
211 Terms
What type of vesicle transports proteins from the ER to the cis-Golgi?
COPII-coated vesicles
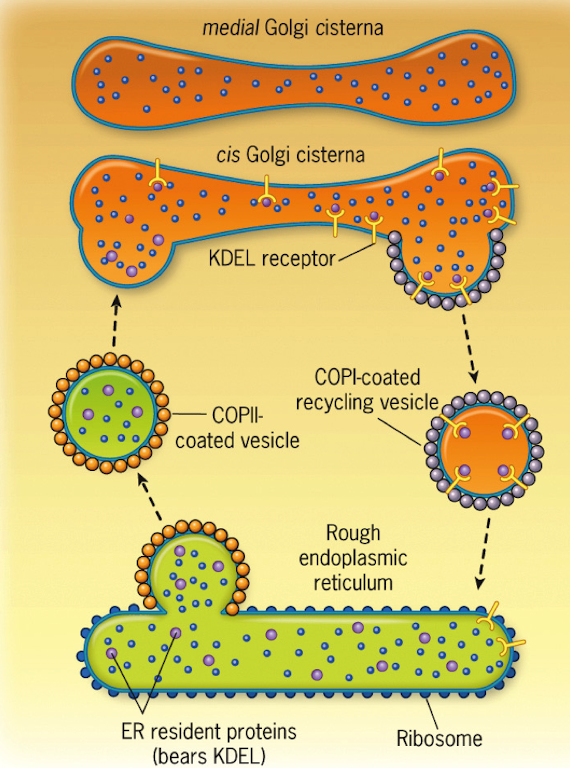
How are proteins selected for export in COPII-coated vesicles from the ER?
Non-specifically — all available proteins are packaged, including some ER-resident proteins
What is the default fate of ER proteins if no retrieval signals are present?
Soluble proteins are secreted
Membrane proteins are inserted into the plasma membrane
What two signals are required for ER lumen-resident proteins?
Signal sequence (to enter the ER)
KDEL sequence at the C-terminus (Lys-Asp-Glu-Leu)
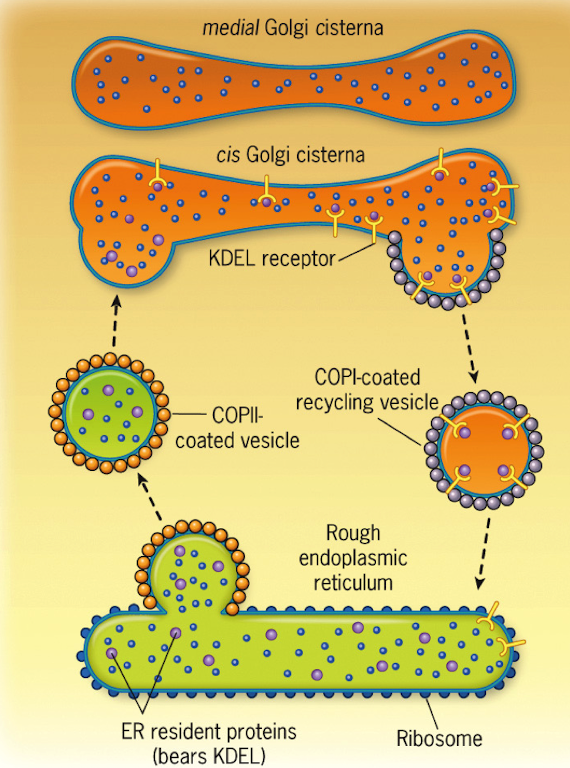
What receptor binds the KDEL sequence, and where is it located?
The KDEL receptor, located in the cis-Golgi
What vesicle type returns KDEL-containing proteins to the ER?
COPI-coated vesicles
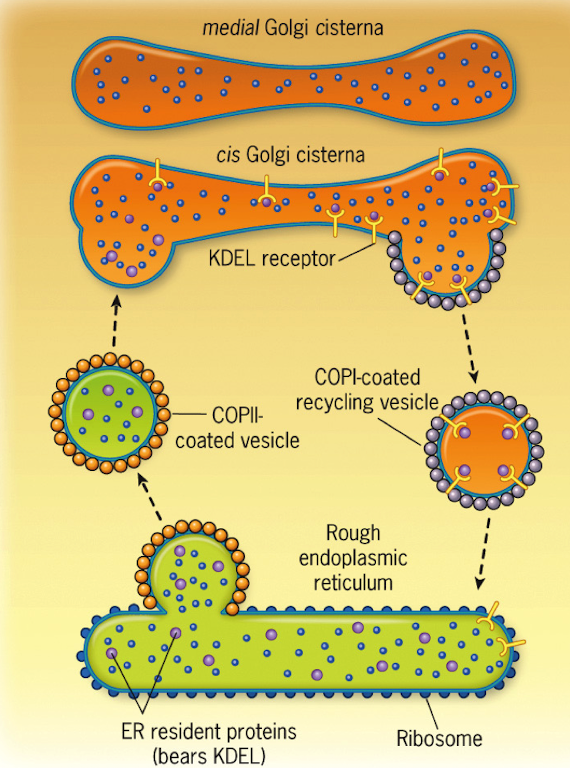
What machinery is involved in the retrograde fusion of vesicles to the ER?
ARF1 (coating)
GAP (uncoating)
Rab, Rab effector
v-SNARE and t-SNARE (fusion)
What happens if the KDEL sequence is not at the C-terminus?
The protein will not be recognized fro retrieval and will be secreted
What signal do ER membrane-resident proteins use for retrieval?
KKXX (Lys-Lys-X-X) at the C-terminus
How are KKXX-tagged proteins recognized?
Directly by a COPI subunit in the cis-Golgi
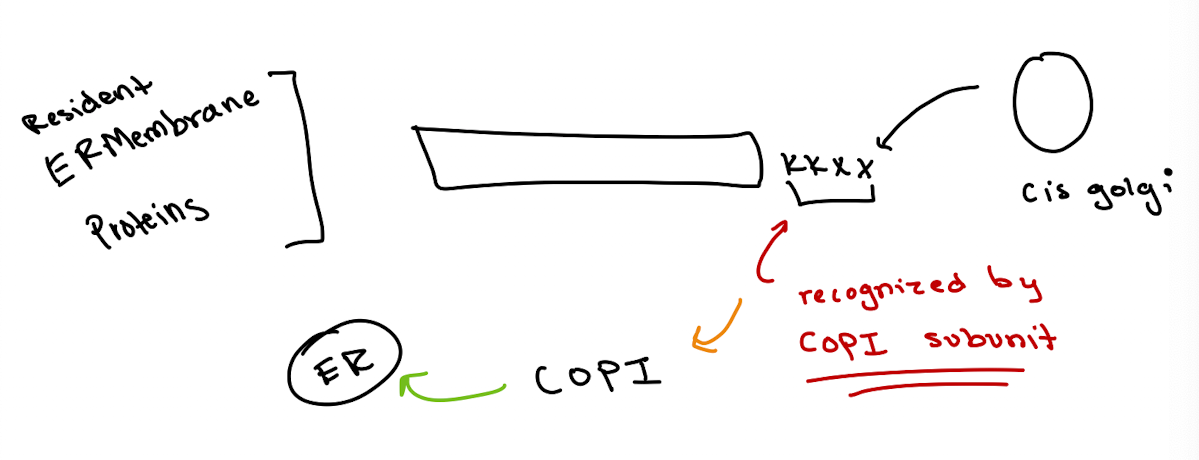
Does the type of membrane protein (I-IV) affect KKXX function?
No, KKXX works for all membrane types as long as it’s at the C-terminus
What is the pathway of cargo through the Golgi?
ER → COPII vesicle → cis-Golgi → medial Golgi → trans-Golgi
Golgi Complex
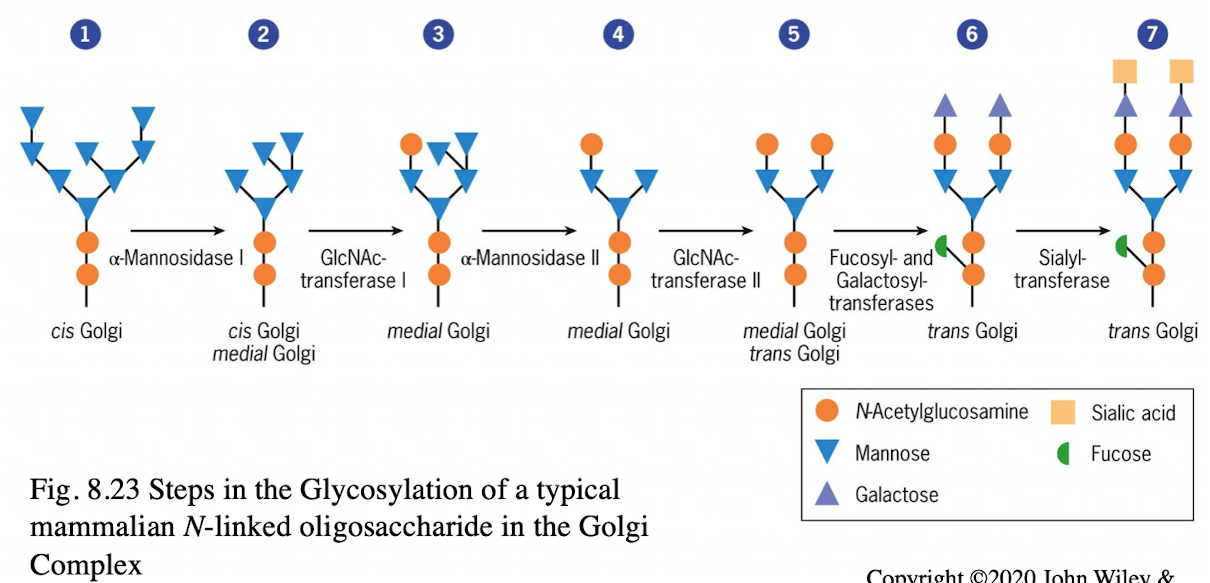
Assembly of carbohydrates found in glycolipids & glycoproteins
Sequence of incorporation of sugars into oligosaccharides is determined by glycosyltransferases
What are two historical models of Golgi Trafficking?
Cisternal Maturation Model (currently accepted)
Vesicular Transport Model
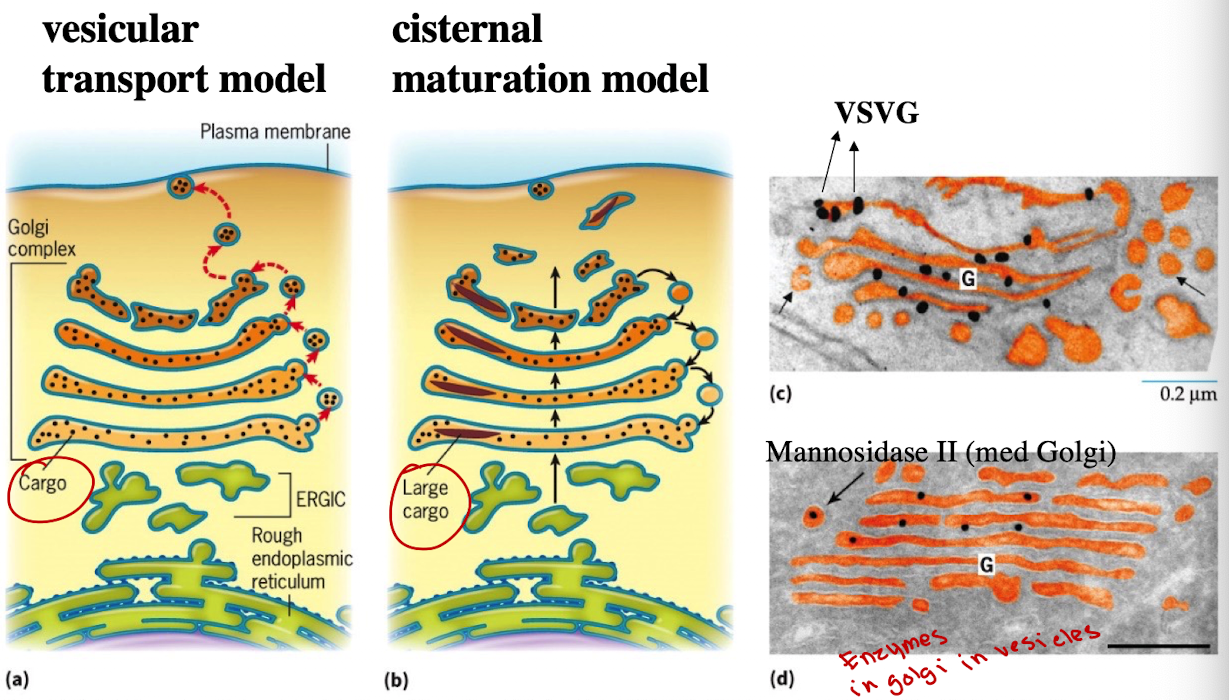
How does cisternal maturation model work?
Golgi compartments are dynamic; cis-Golgi matures into medial → trans. Vesicles carry enzymes backward
Steps:
New cisternae form at the cis face from vesicles arriving from the ER.
These cisternae physically move forward through the Golgi stack toward the trans face.
As they move, they change their enzymes and contents to become more like the next compartment.
Enzymes from later Golgi compartments are sent backward in vesicles (retrograde transport) to earlier cisternae to help with this transformation.
Eventually, the matured trans cisterna breaks apart into vesicles that carry proteins to their final destinations (e.g., lysosomes, plasma membrane).
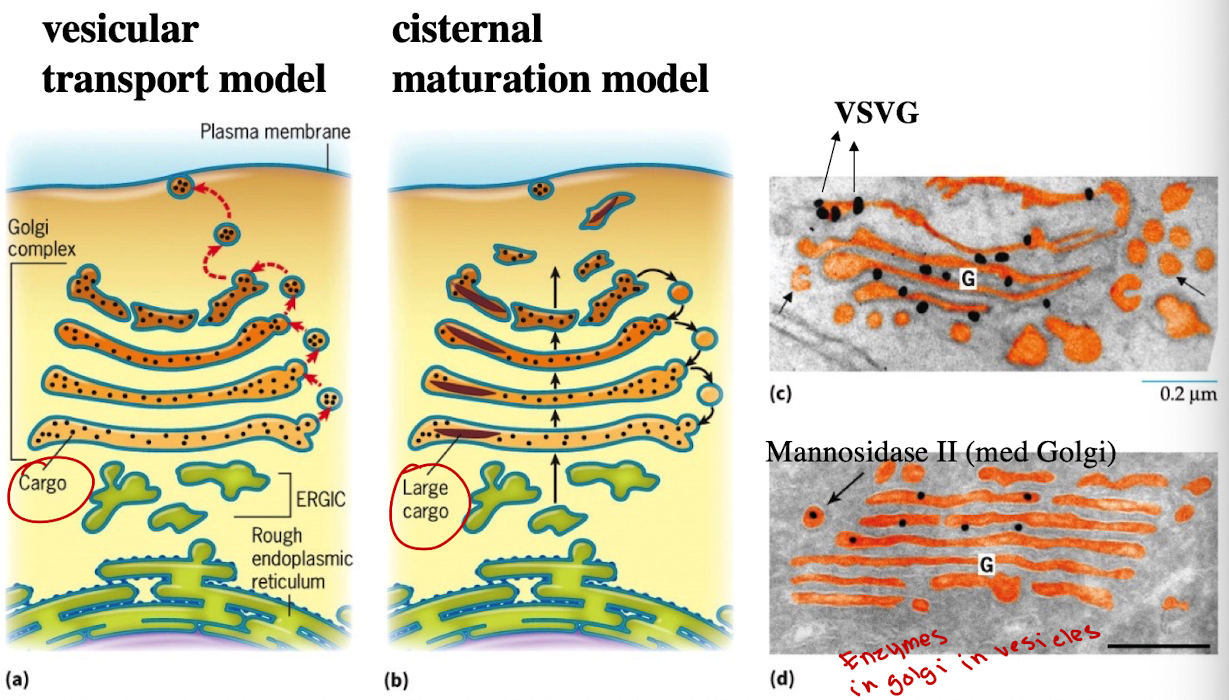
How does the Vesicular Transport Model differ from the Cisternal Maturation Model?
Golgi compartments are static; vesicles move cargo from cis→medial→trans golgi
In cis-Golgi, cargo proteins are packaged into new vesicles.
These vesicles fuse with the next cisterna (e.g., medial-Golgi).
This process continues until proteins reach the trans-Golgi, where they’re sorted for delivery to the plasma membrane, lysosomes, or other location
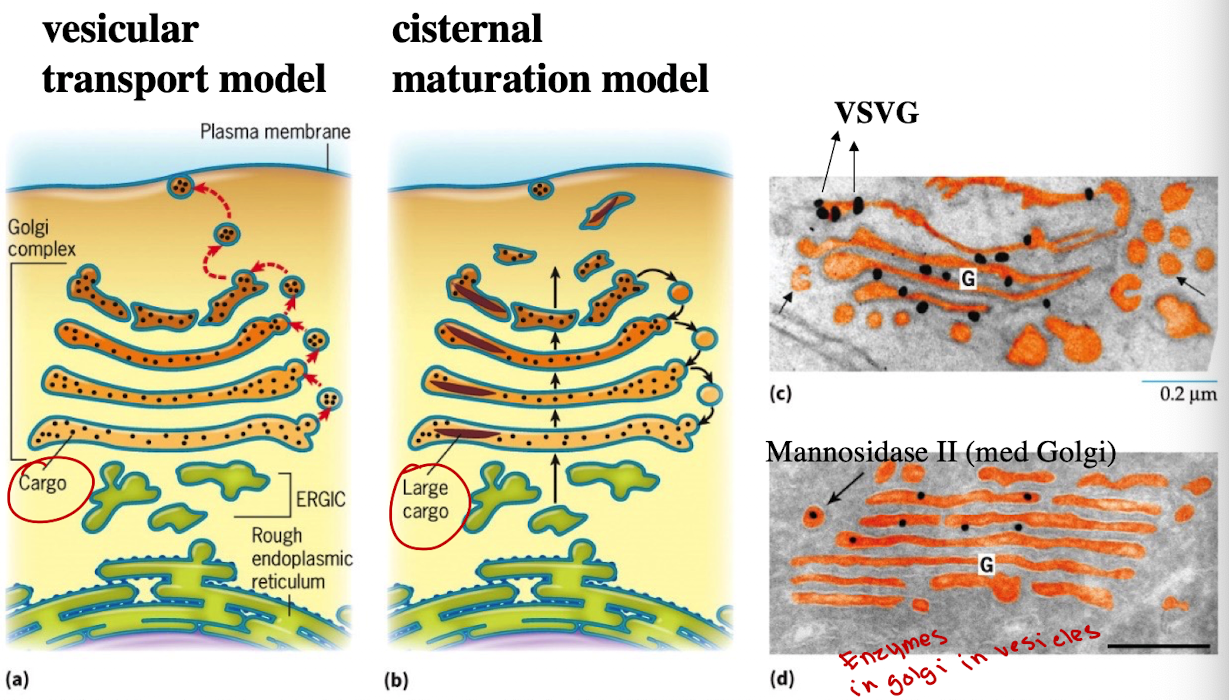
What model is accepted today and why?
Hybrid model:
Cargo moves via cisternal maturation
Golgi enemies recycle via vesicles (retrograde transport)
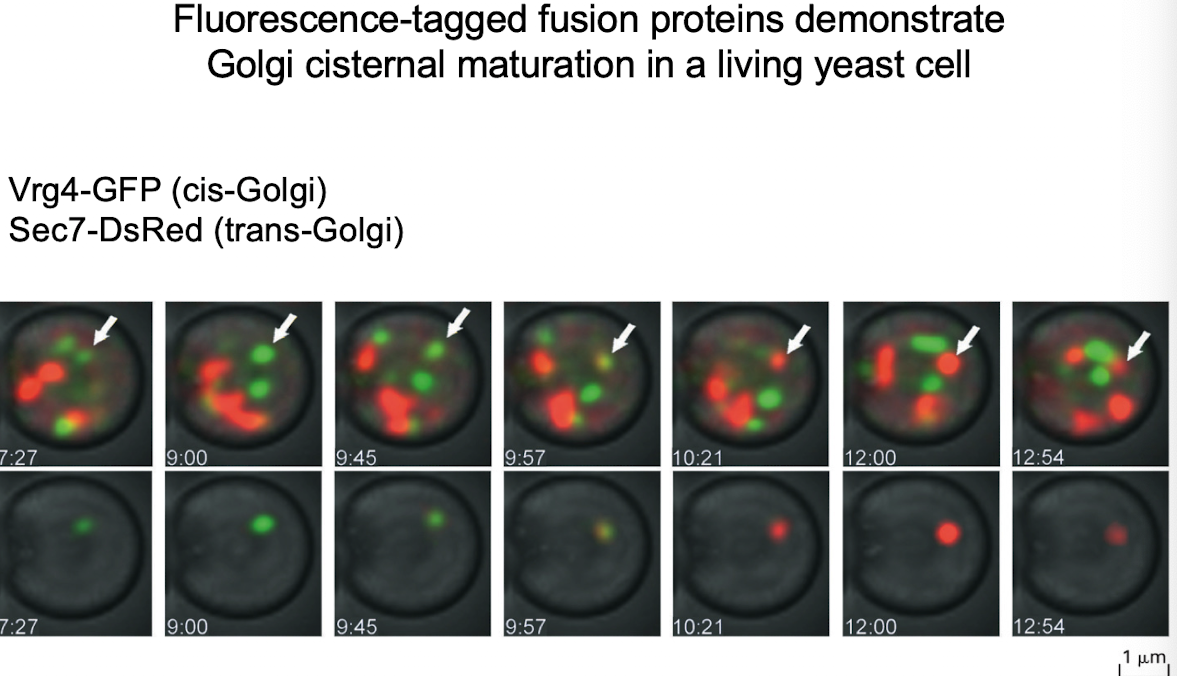
What experiment showed support for cisternal maturation?
GFP-tagging of cis-Golgi protein (VRG4) and trans-Golgi protein (Sec7) showed transition of the same Golgi compartment from green to red.
G-protein was observed only in Golgi stacks, not vesicles — supports cisternal maturation.
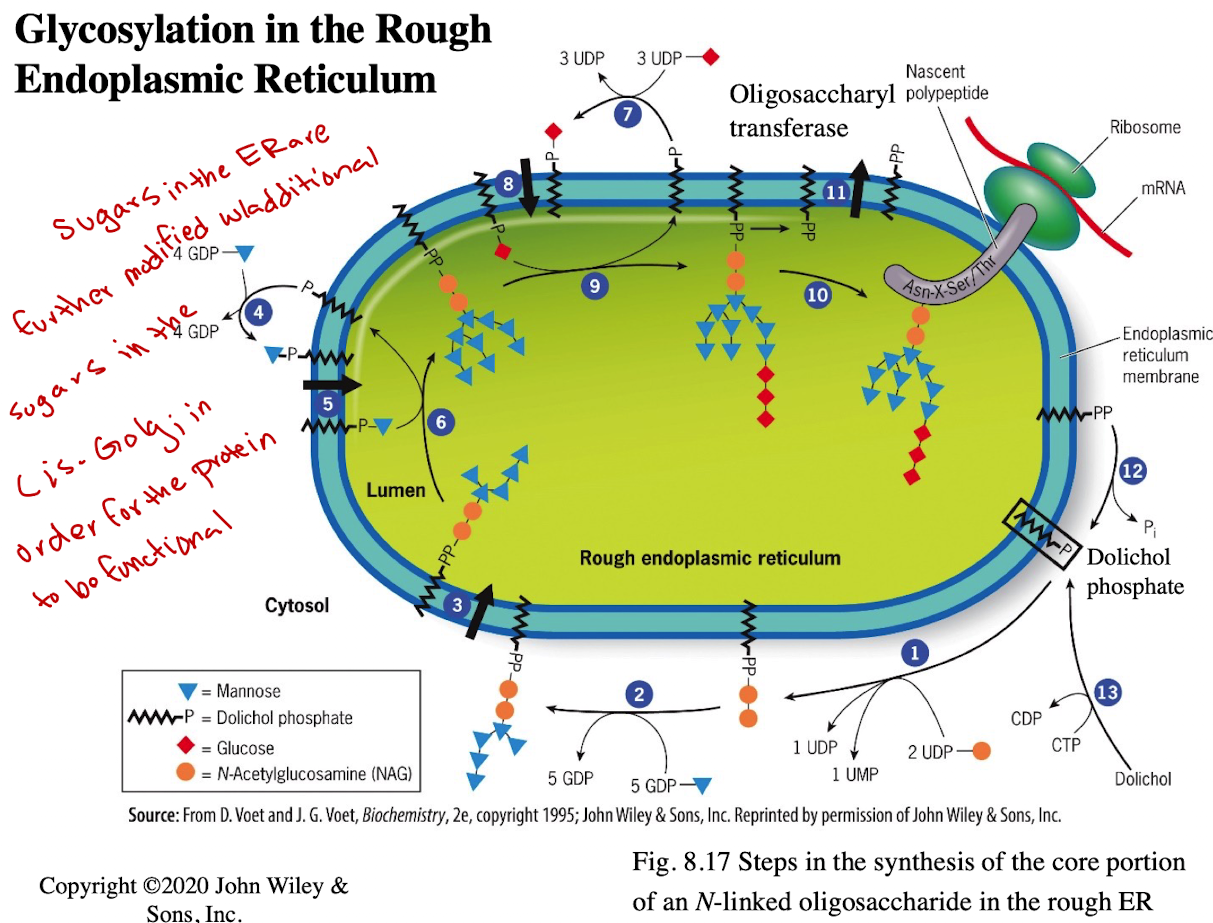
What happens to N-linked sugars in the Golgi?
They are further modified in the cis-Golgi for full functionality
Is KDEL alone enough to send a protein to the ER?
No, it must also have a signal sequence to enter the ER
What happens if a protein has a KDEL but no signal sequence?
It may localize to the nucleus or elsewhere, not the ER
What if a secreted protein is engineered to contain KDEL?
It will be secreted, but retrieved back to the ER via COPI, assuming it entered ER initially
iClicker
If you add KDEL sequence to C-terminus of a normally secreted protein - where would you expect to find this protein?
A. Nowhere, it will never be made
B. In the cytoplasm
C. In the ER
D. In the Golgi
E. Outside the cell
C
iClicker
If you add KDEL sequence to C-terminus of a nuclear localized protein - where would you expect to find this protein?
A. In the cytoplasm
B. Nucleus
C. In the ER
D. In the Golgi
E. Outside the cell
B
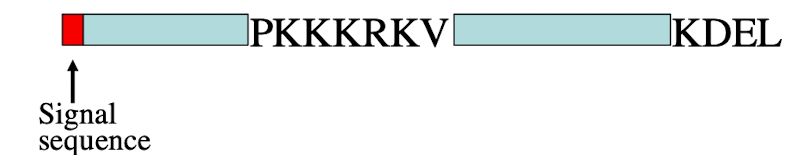
iClicker
What will be the destination of the protein shown?
A. In the cytoplasm
B. Nucleus
C. In the ER
D. In the Golgi
E. Outside the cell
C
Lecture 5/16
What is the default pathway for proteins that reach the trans-Golgi network (TGN)?
Lumenal proteins are secreted, and membrane proteins are delivered to the plasma membrane.
Exception: Proteins targeted to lysosomes
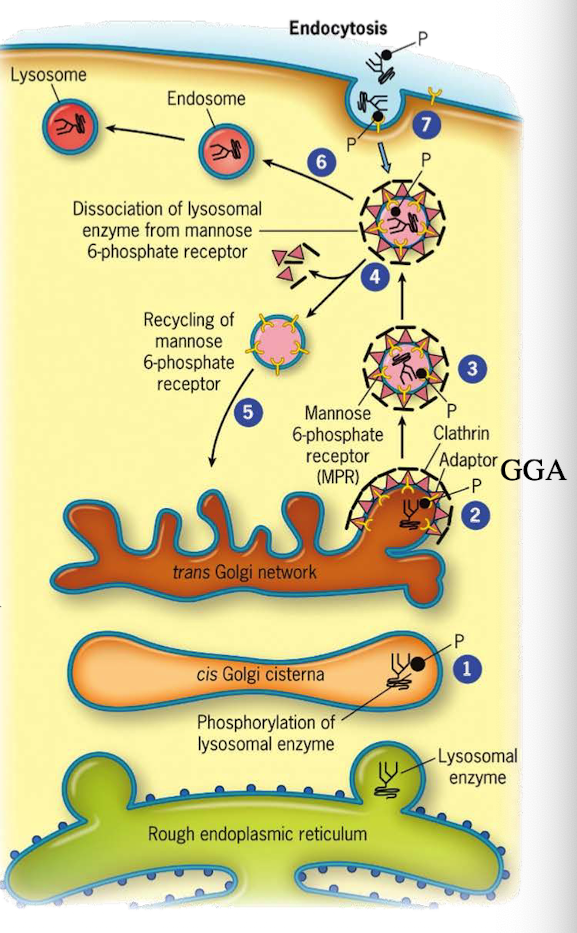
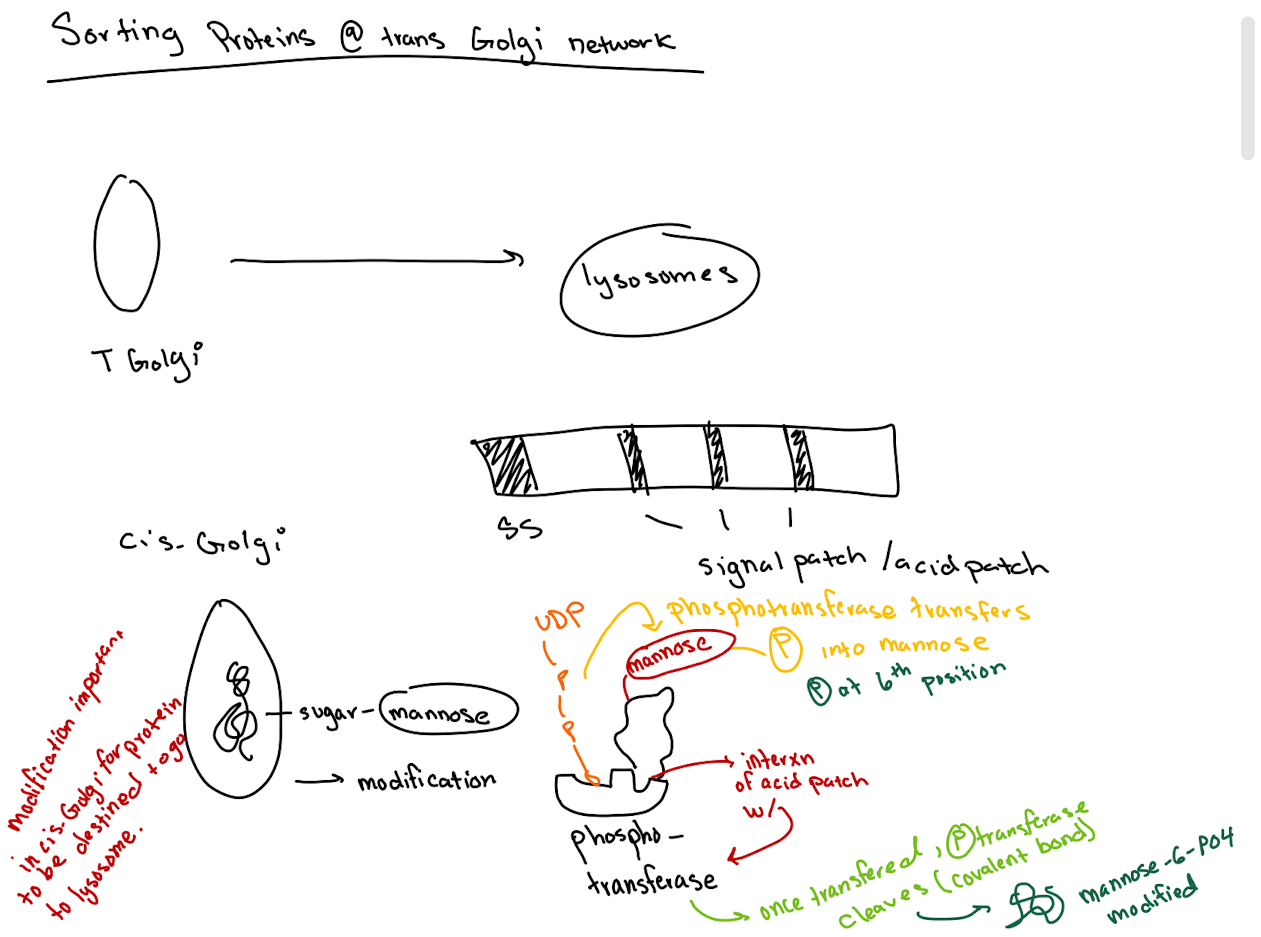
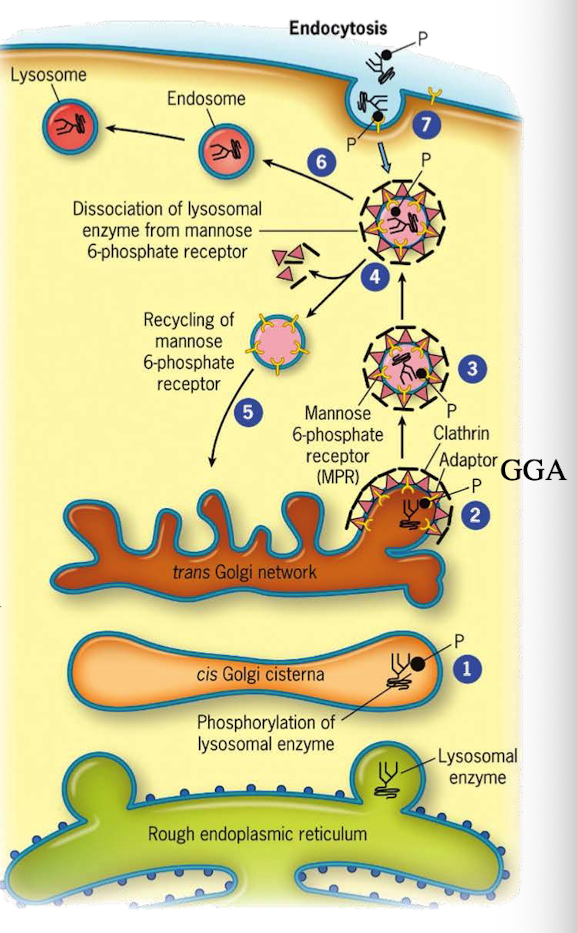
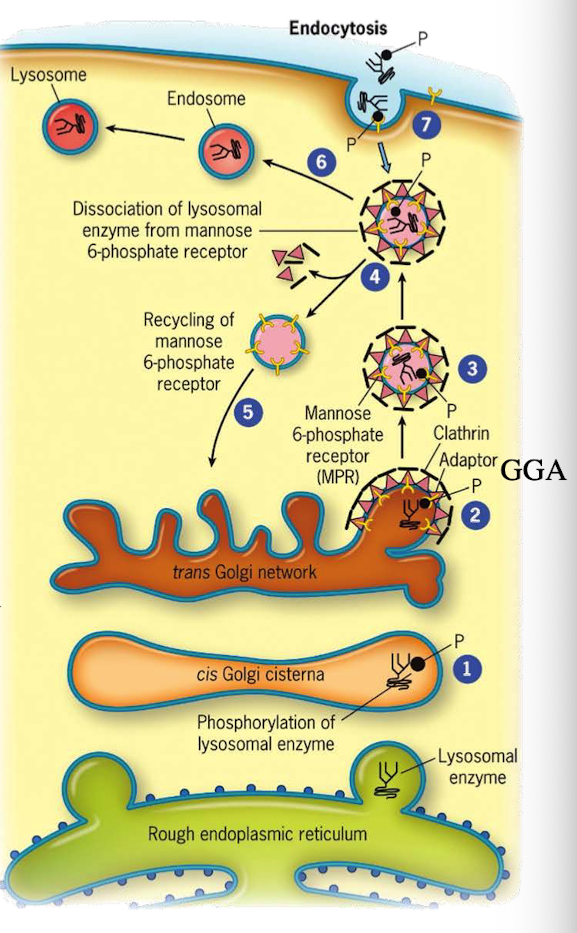
How do lysosomal proteins avoid being secreted by default?
They carry an acid patch or signal patch recognized in the cis-Golgi for mannose-6-phosphate (M6P) tagging.
Where does lysosomal protein synthesis begin?
In the cytoplasm; translation is directed into the ER lumen.
What enzyme in the cis-Golgi adds the M6P tag to lysosomal proteins?
Phosphotransferase
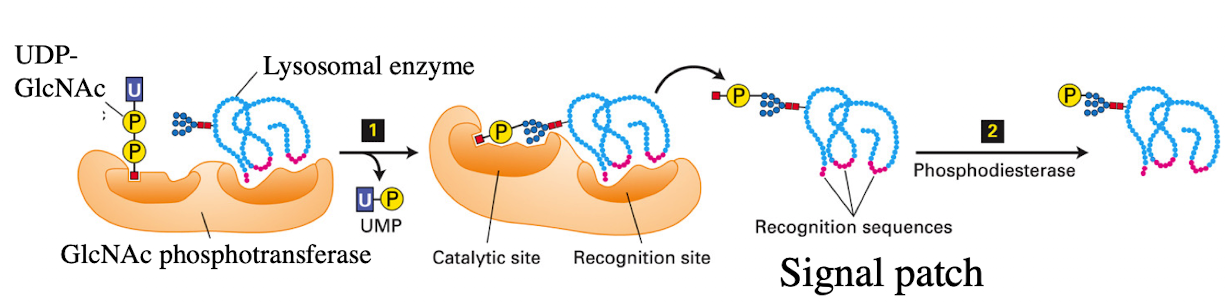
What does phosphotransferase recognize in the lysosomal protein?
Acid patch or signal patch sequences.
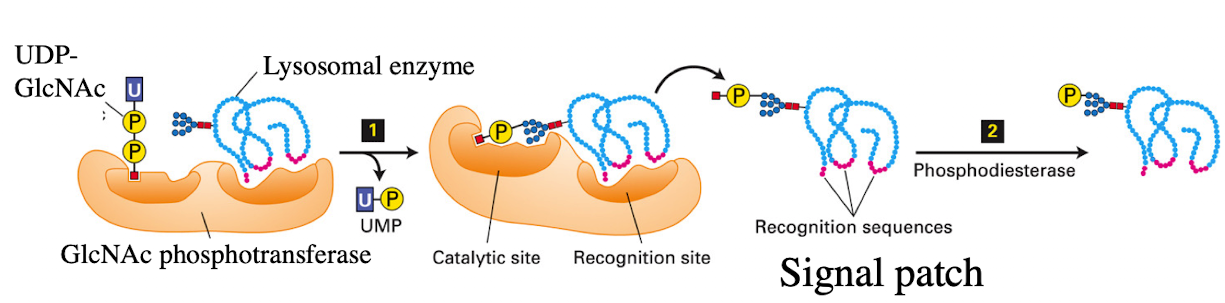
What modification does phosphotransferase perform?
Transfers a phosphate to the 6th carbon of mannose using UDP.
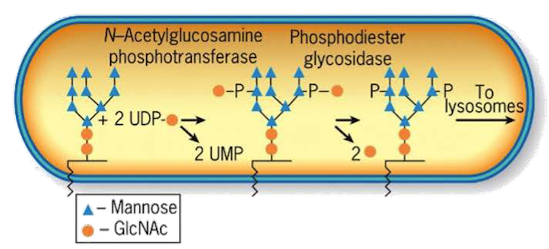
What happens after phosphate is transferred?
Phosphodiesterase cleaves the sugar-phosphate bond to release the modified protein.
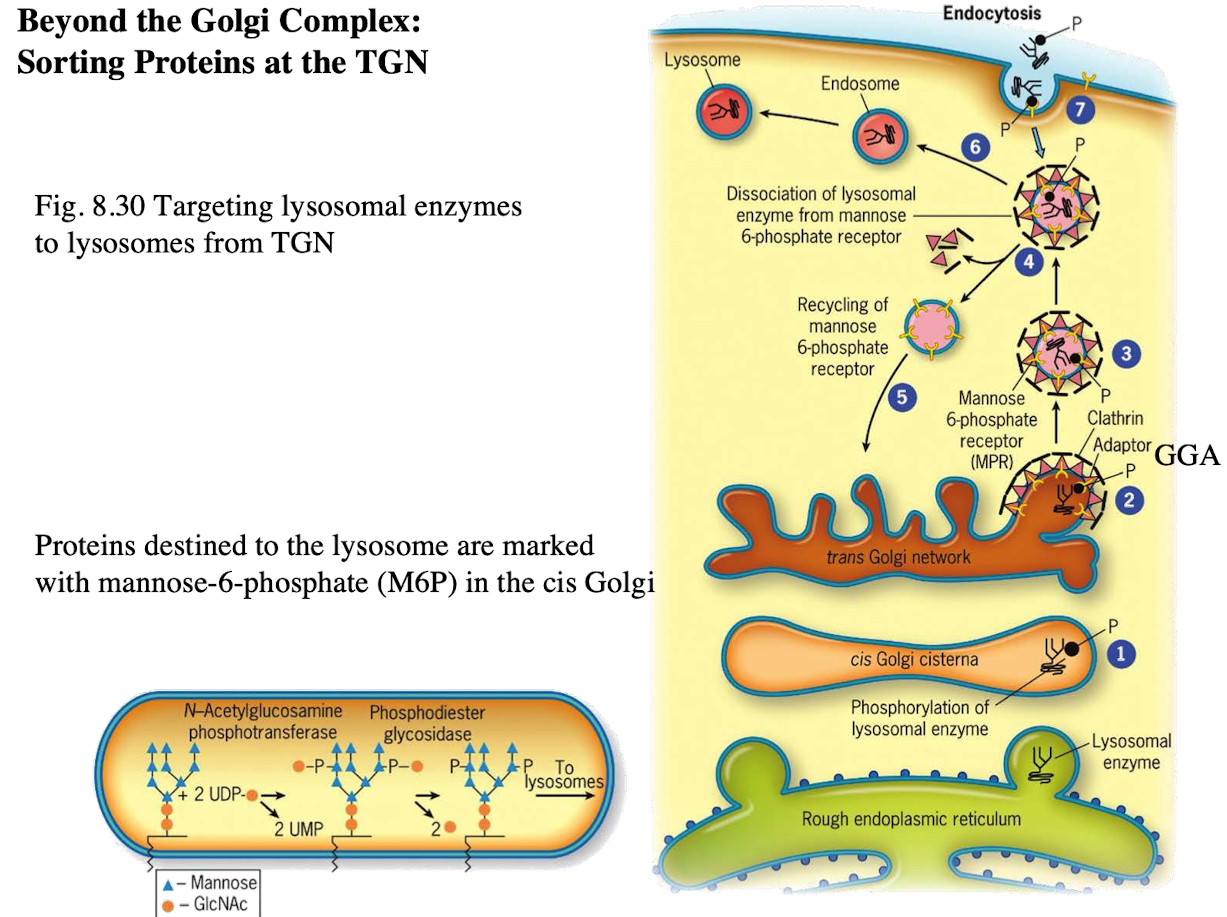
Why is M6P tagging important?
It targets proteins to the lysosome instead of the secretory pathway.
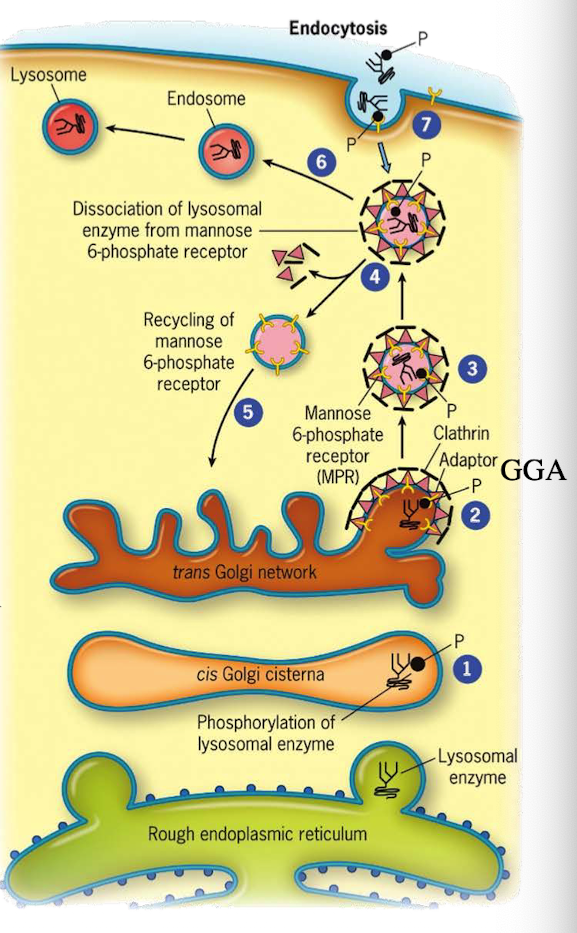
What happens to M6P-tagged proteins at the TGN?
They are recognized by the M6P receptor, which recruits GGA adaptor protein.
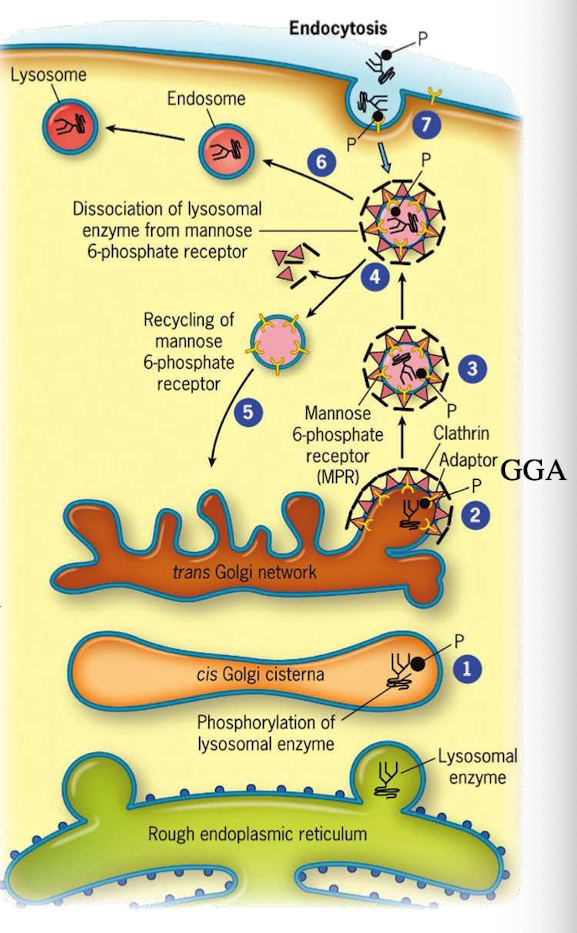
What does the GGA adaptor do?
Recruits clathrin to form a clathrin-coated vesicle.
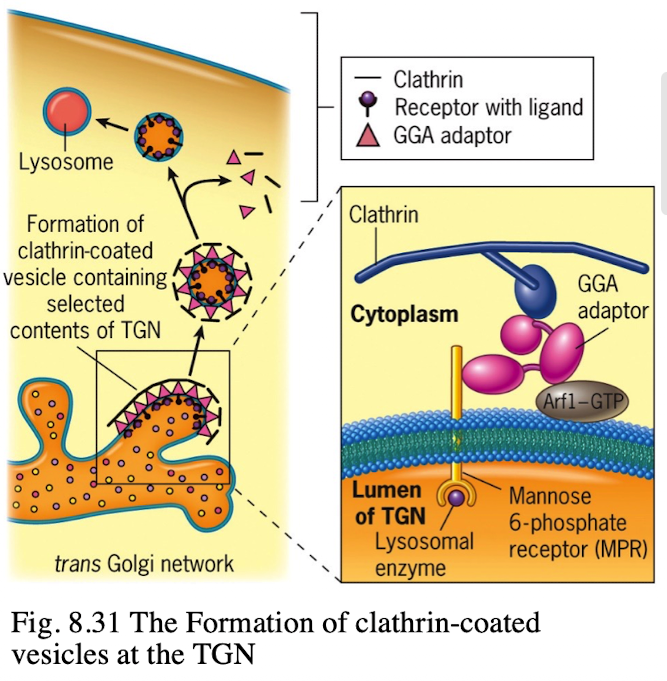
Where is the M6P receptor located?
On the membrane of the trans-Golgi network.
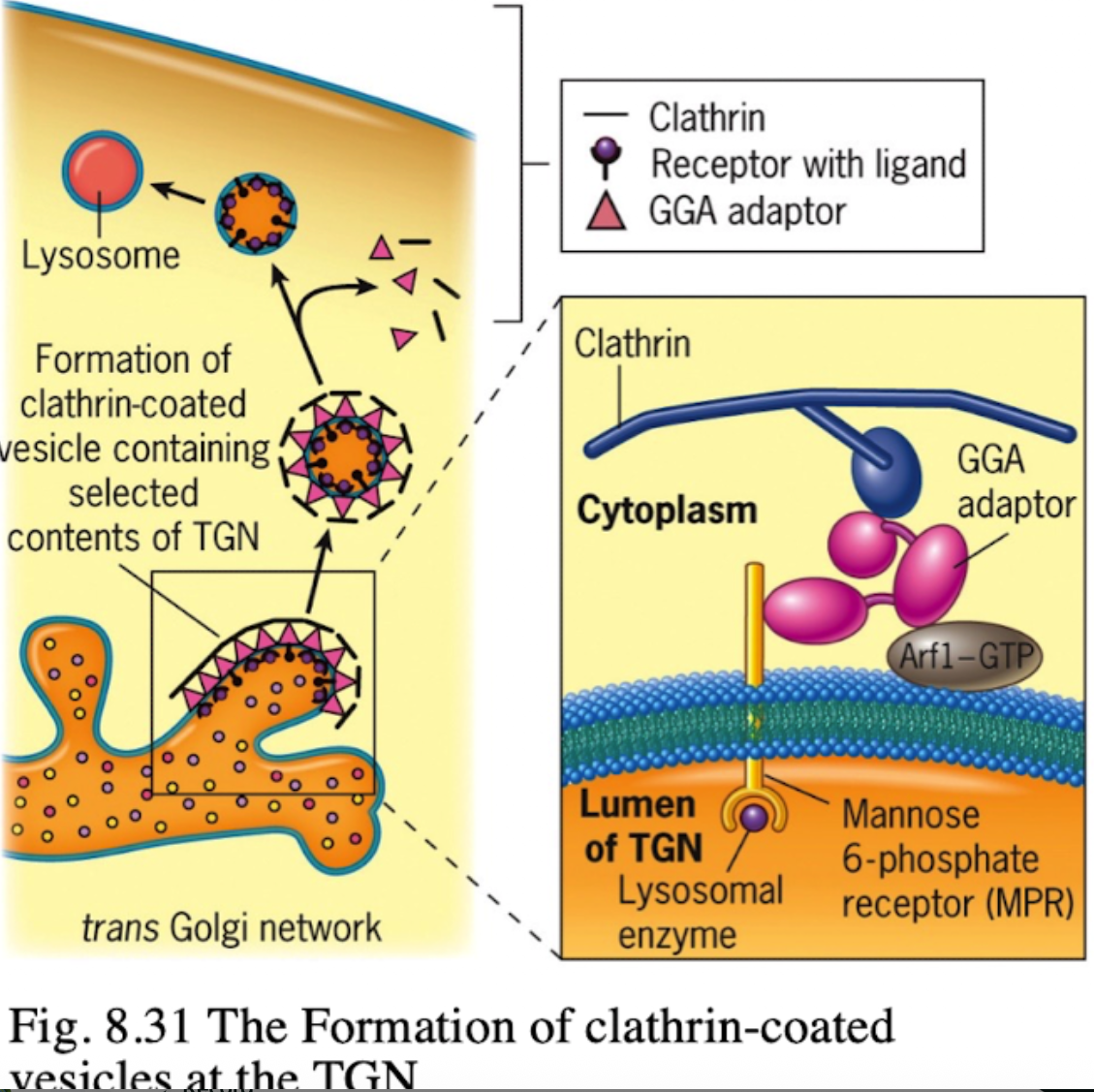
What does clathrin do in vesicle formation?
Provides the structural coat
What happens after the clathrin-coated vesicle forms?
It uncoats and becomes an early endosome.
What role does acidic pH play in endosomes?
Causes M6P-protein to dissociate from the receptor.
What happens to the receptor after dissociation?
It is recycled back to the TGN.
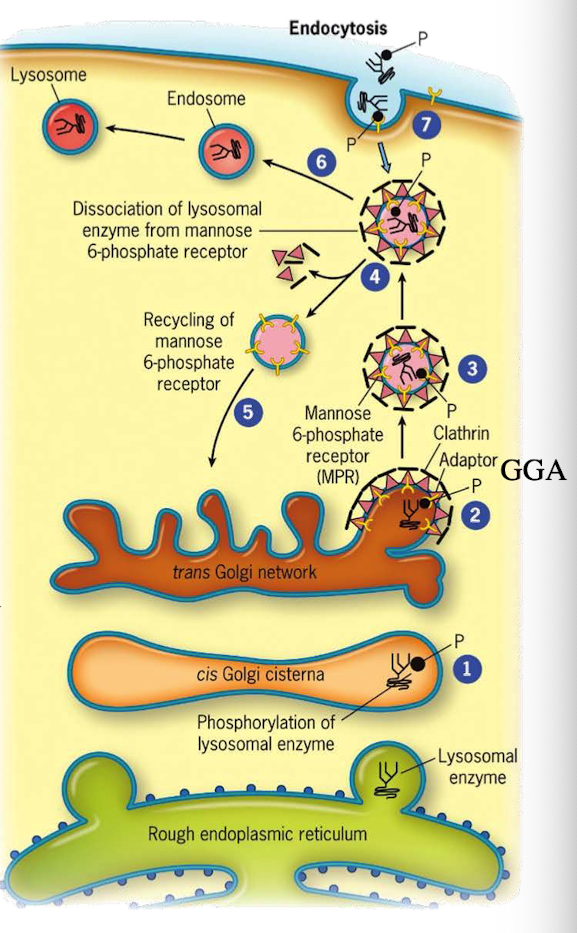
What happens to the endosome containing M6P-protein?
It fuses with a lysosome, delivering the enzyme cargo.
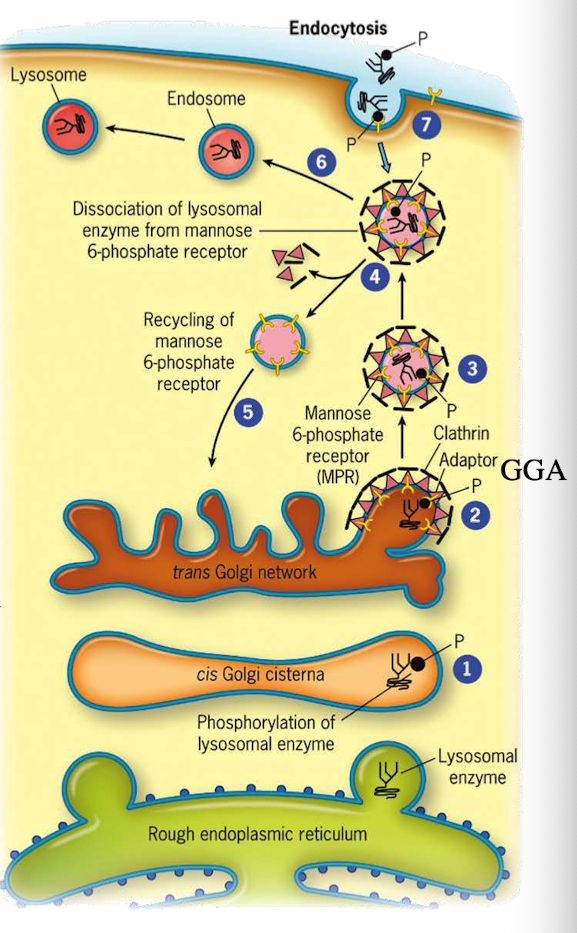
What two signals are required for lysosomal targeting?
Signal sequence for ER entry and acid patch for M6P tagging. If you don’t have it, then there is no way to modify protein
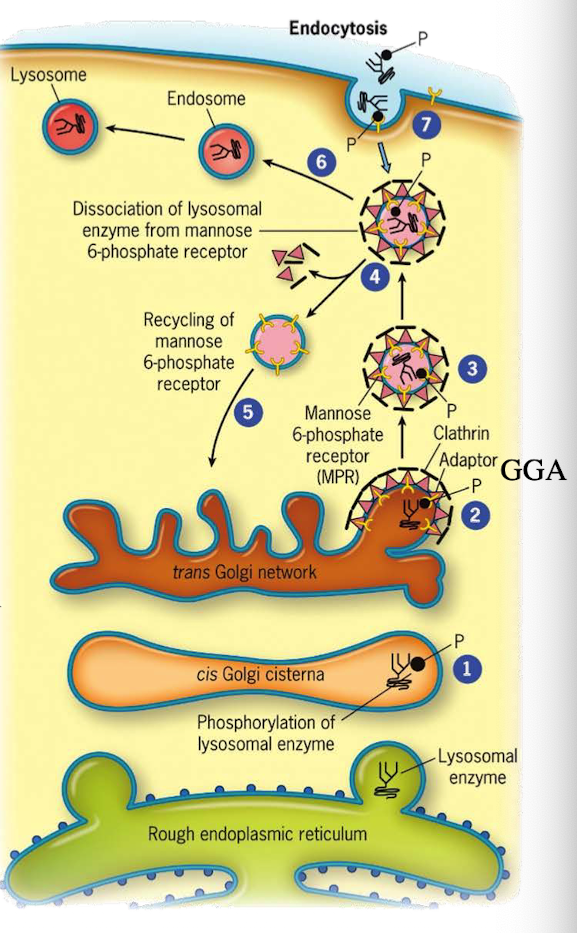
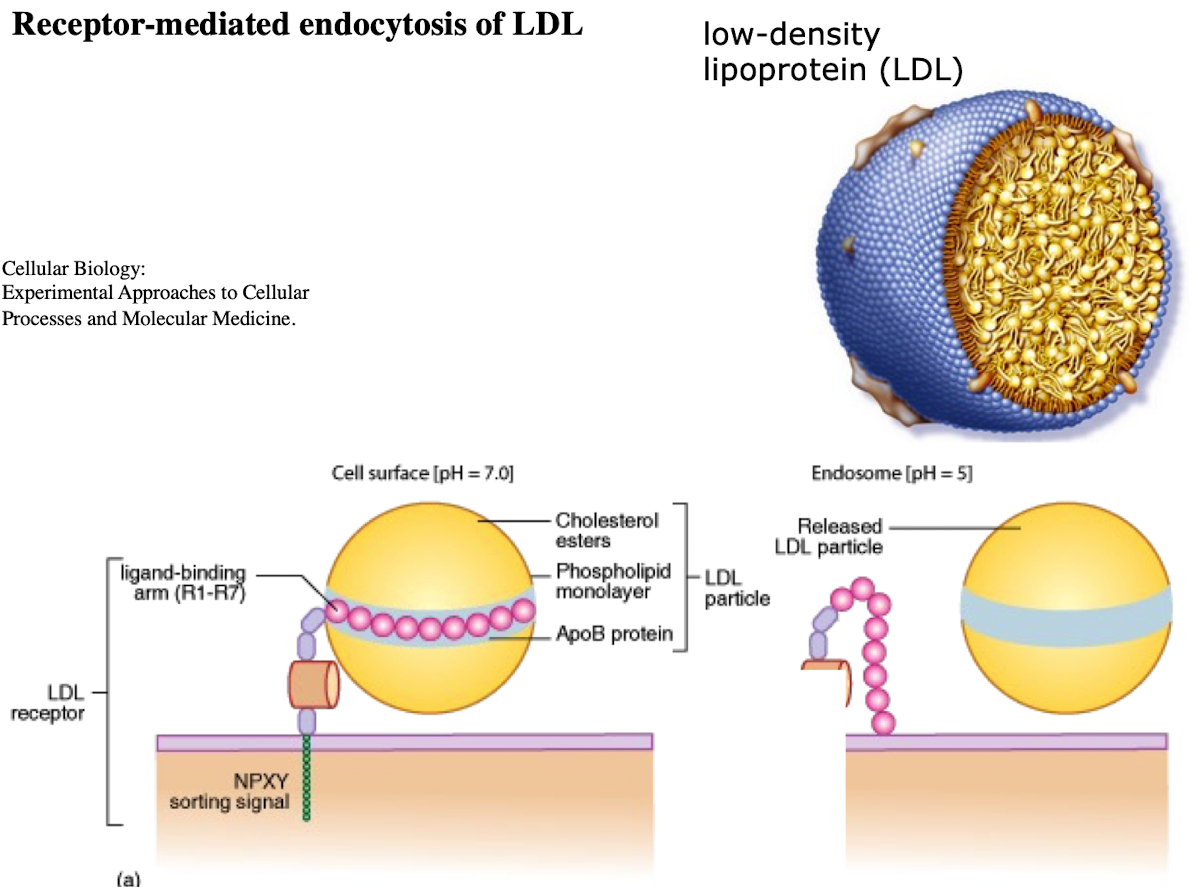
What is LDL and what does it contain?
Low-density lipoprotein; contains ~1,500 cholesterol molecules and ApoE protein
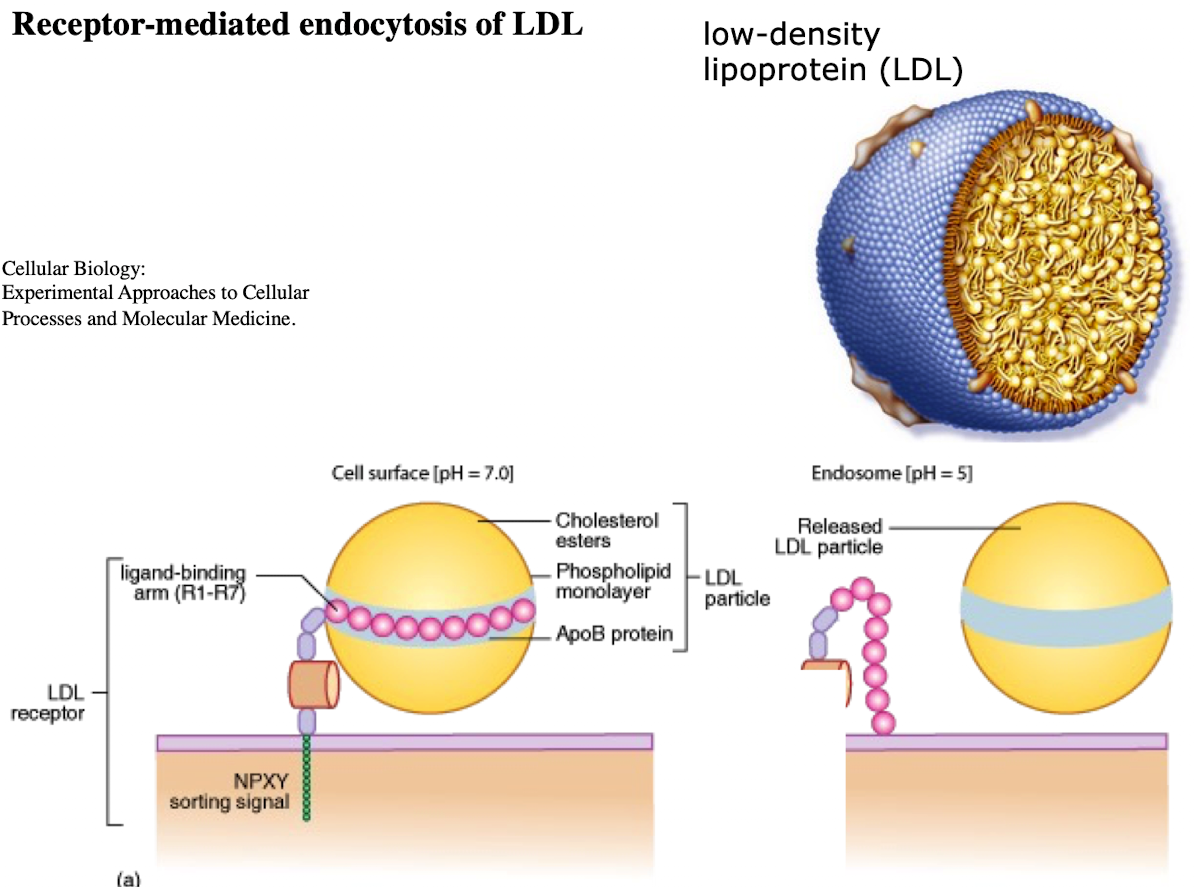
How does LDL enter cells?
Through receptor-mediated endocytosis.
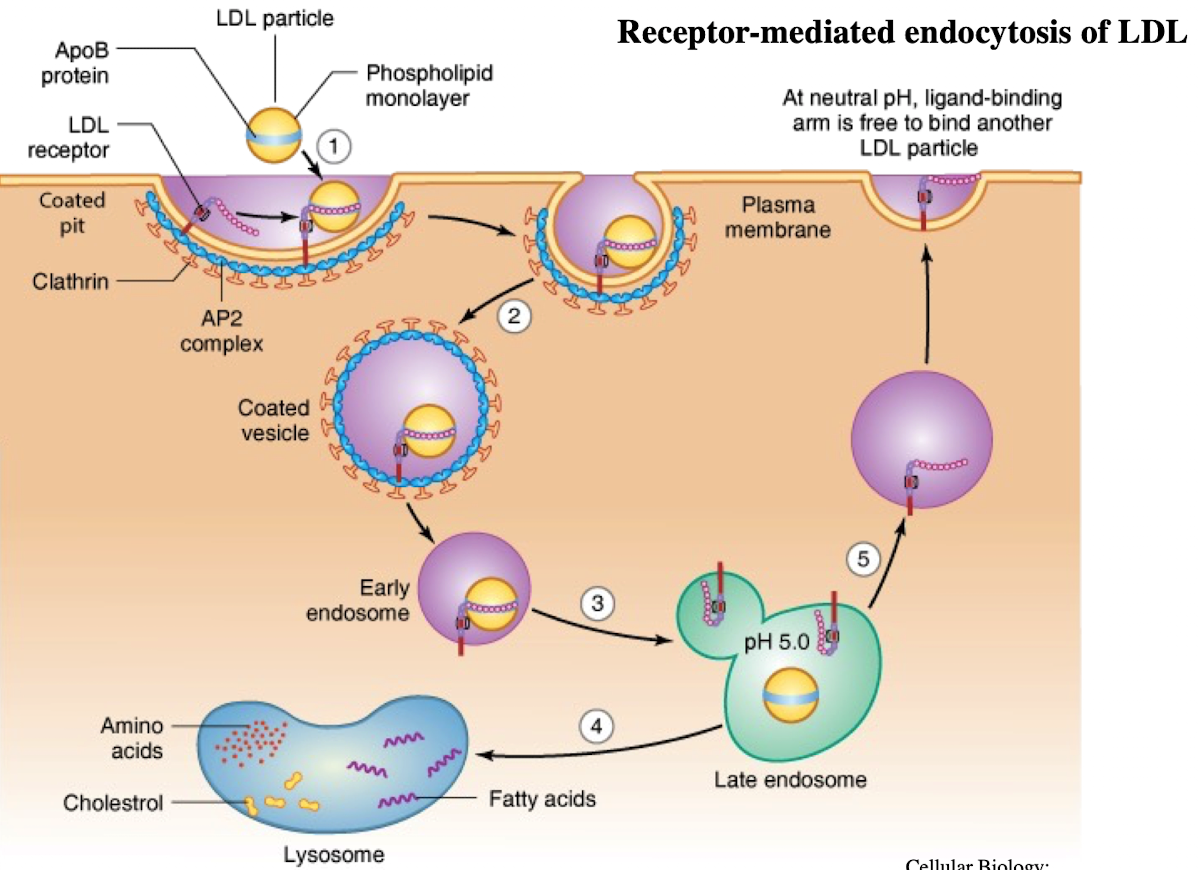
What receptor binds LDL?
LDL receptor on the plasma membrane.
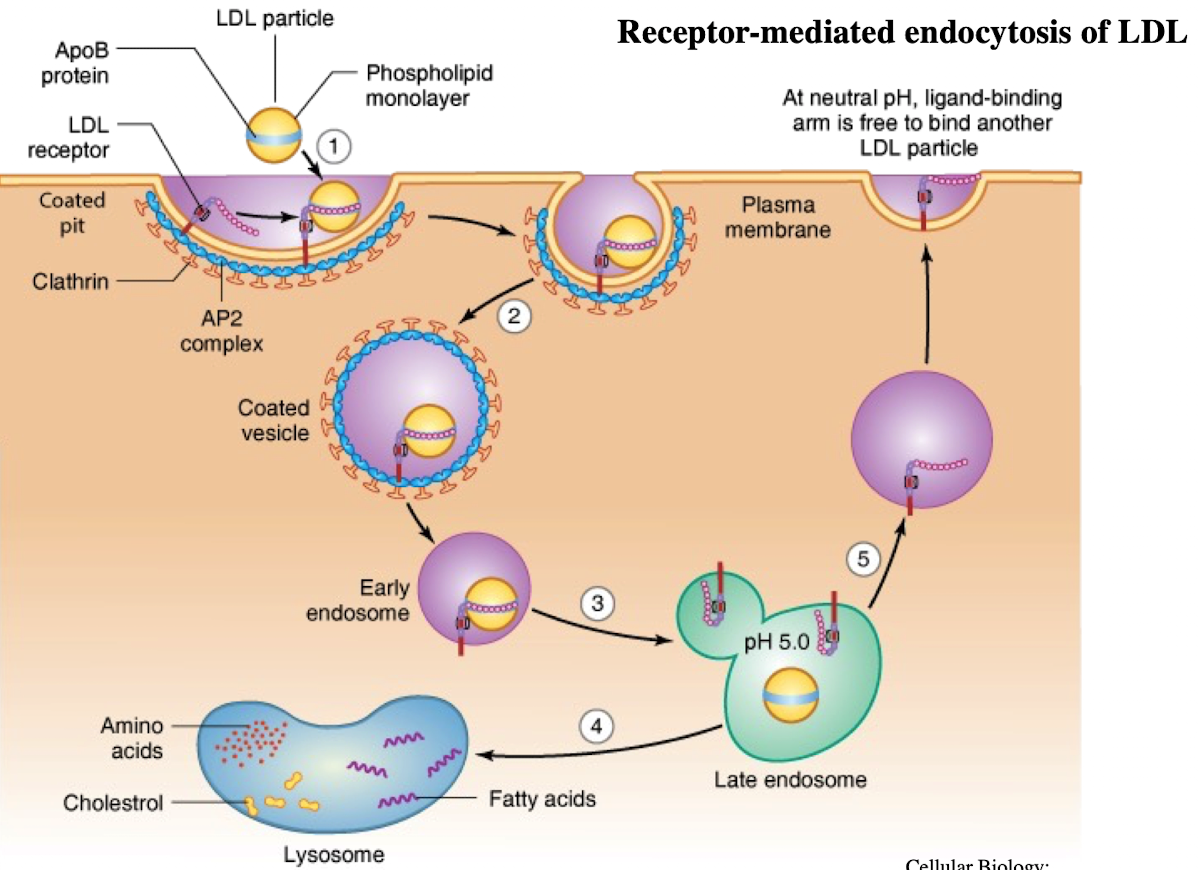
What is the NPXY motif and why is it important?
A sequence on the receptor’s cytoplasmic tail (Asn-Pro-X-Tyr) needed to recruit AP2 adaptor protein.
What does AP2 do after binding NPXY?
Recruits clathrin to form a clathrin-coated vesicle.
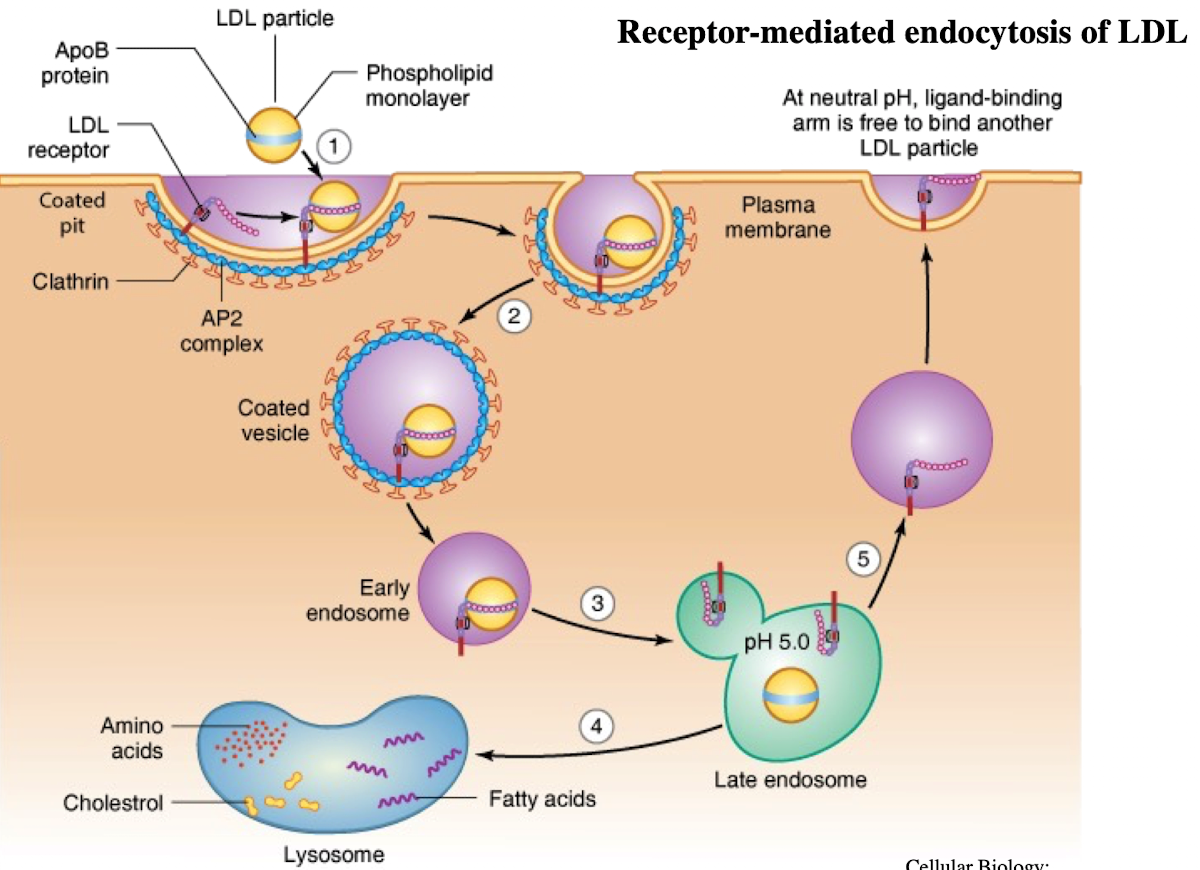
What happens after vesicle formation?
It uncoats and acidifies into an endosome.
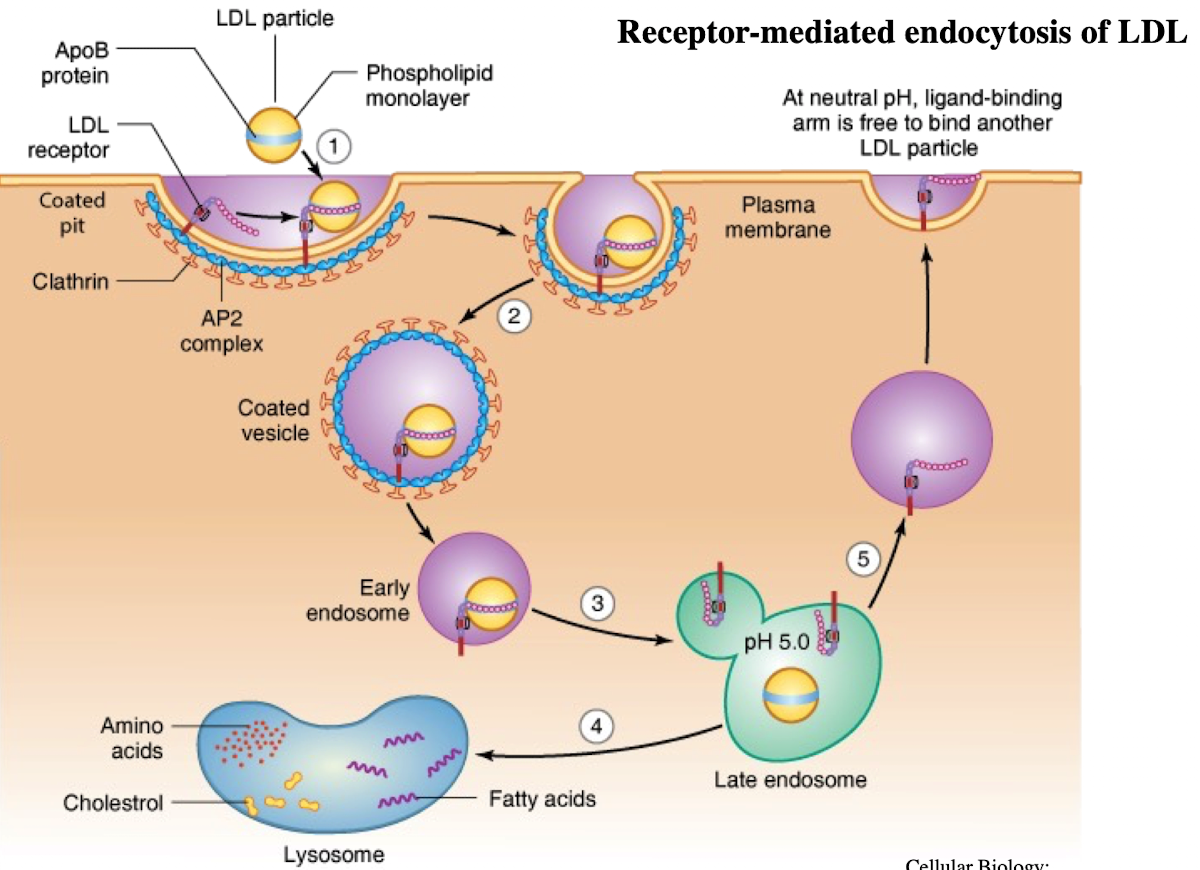
What does the low pH in endosomes cause?
Dissociation of LDL from its receptor.
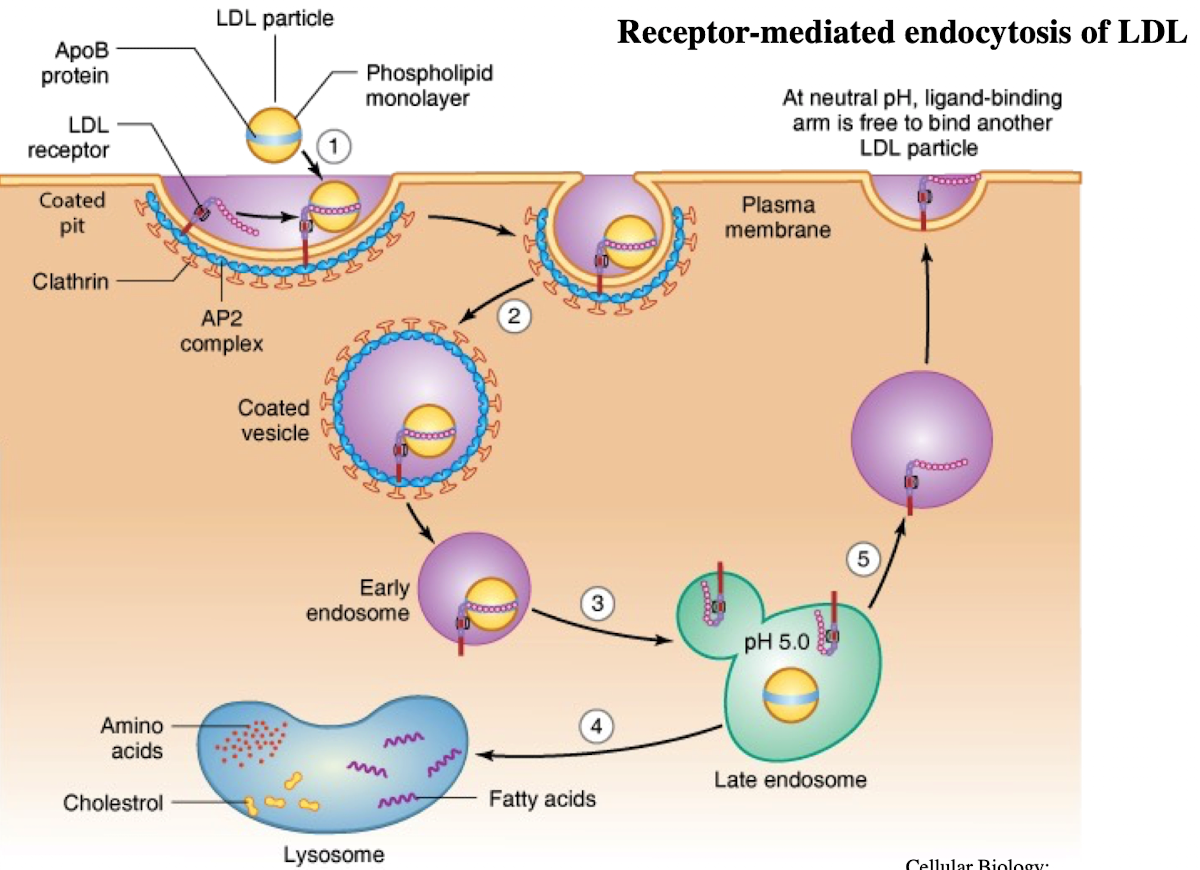
What happens to the LDL receptor?
It is recycled back to the plasma membrane.
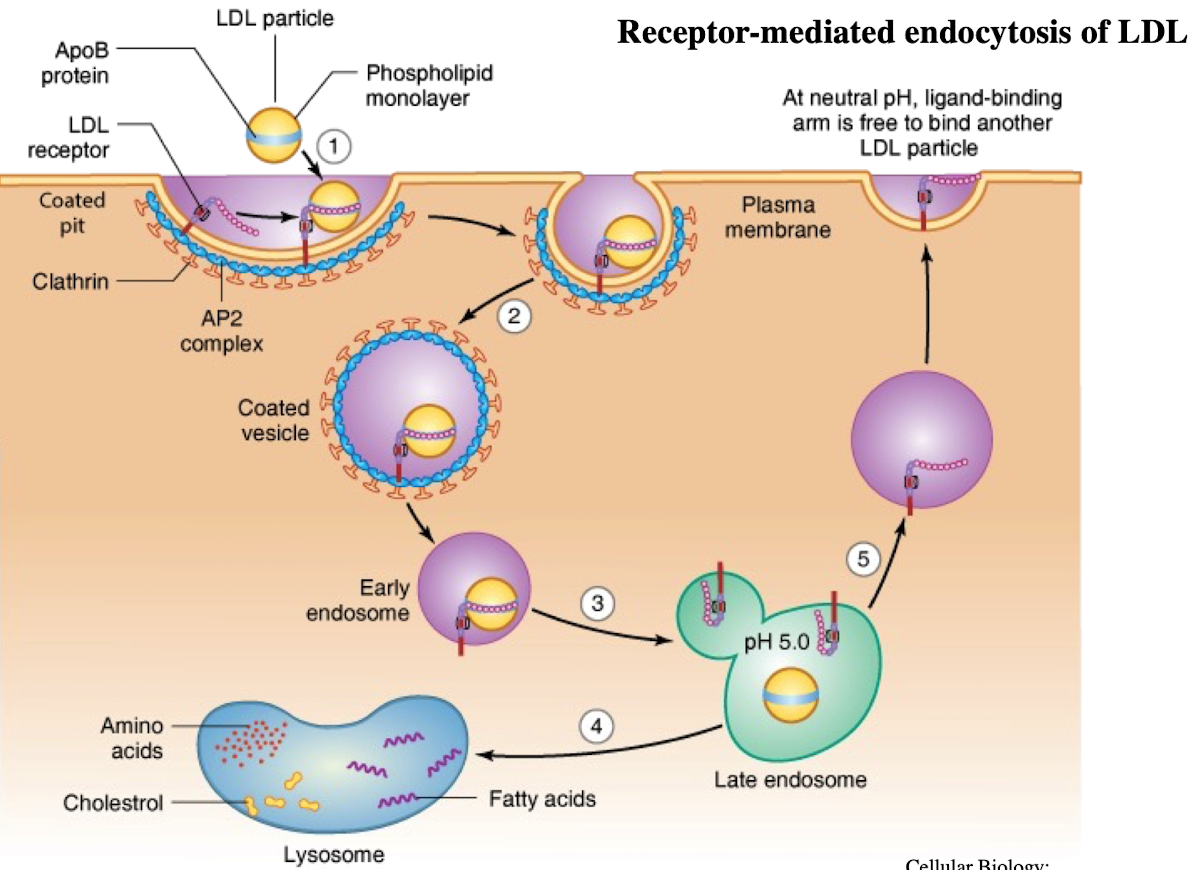
Where does the LDL-containing endosome go?
It fuses with the lysosome.
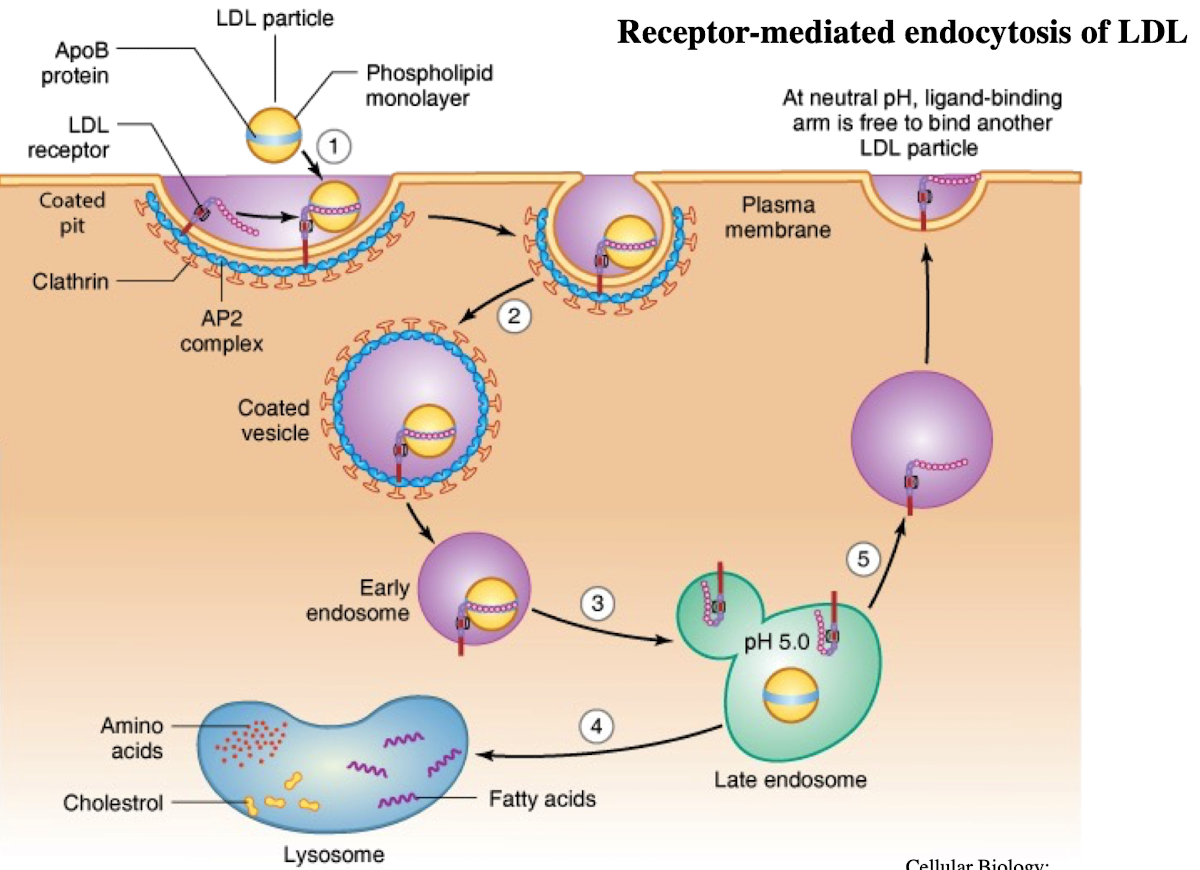
What does the lysosome do with LDL?
Breaks it down into cholesterol, amino acids, and phospholipids.
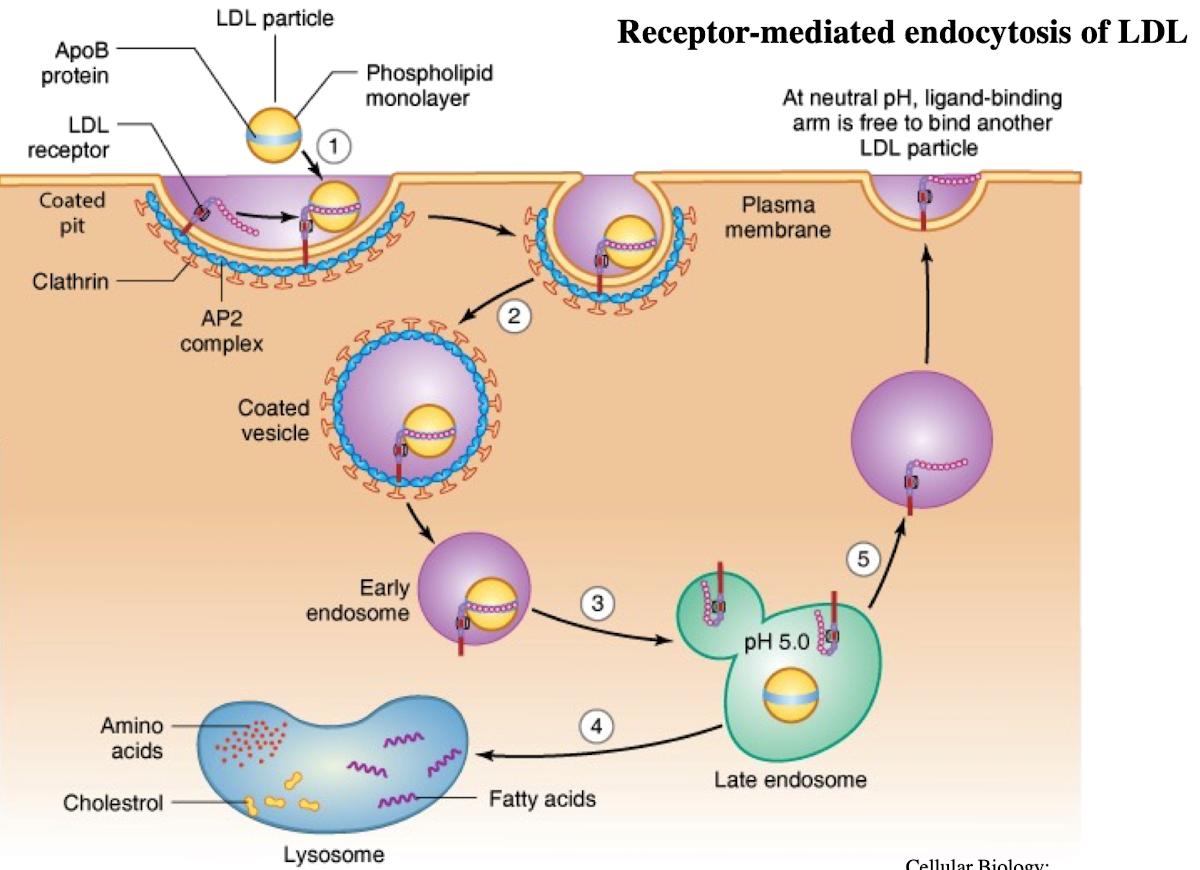
What causes familial hypercholesterolemia?
A mutation in the NPXY motif (e.g., tyrosine to cysteine) of the LDL receptor, preventing AP2 binding and LDL uptake.
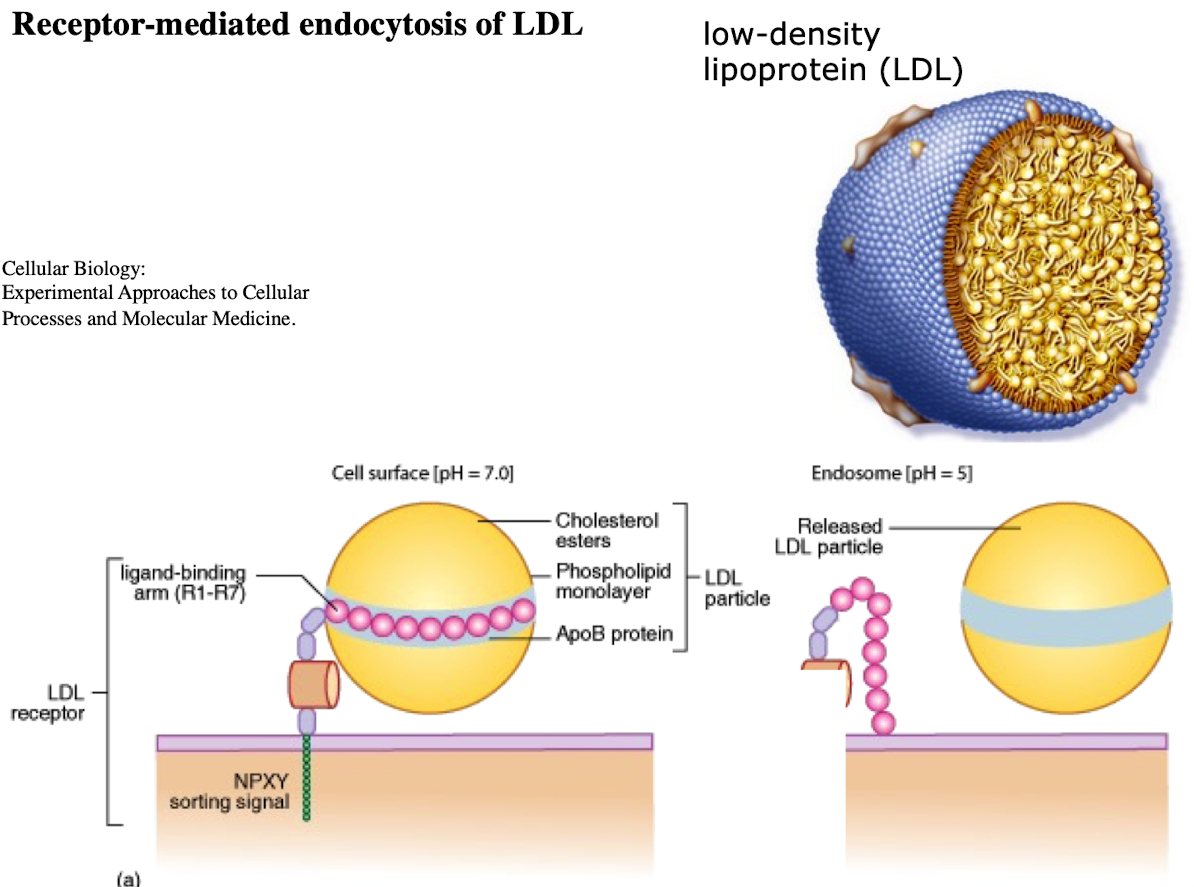
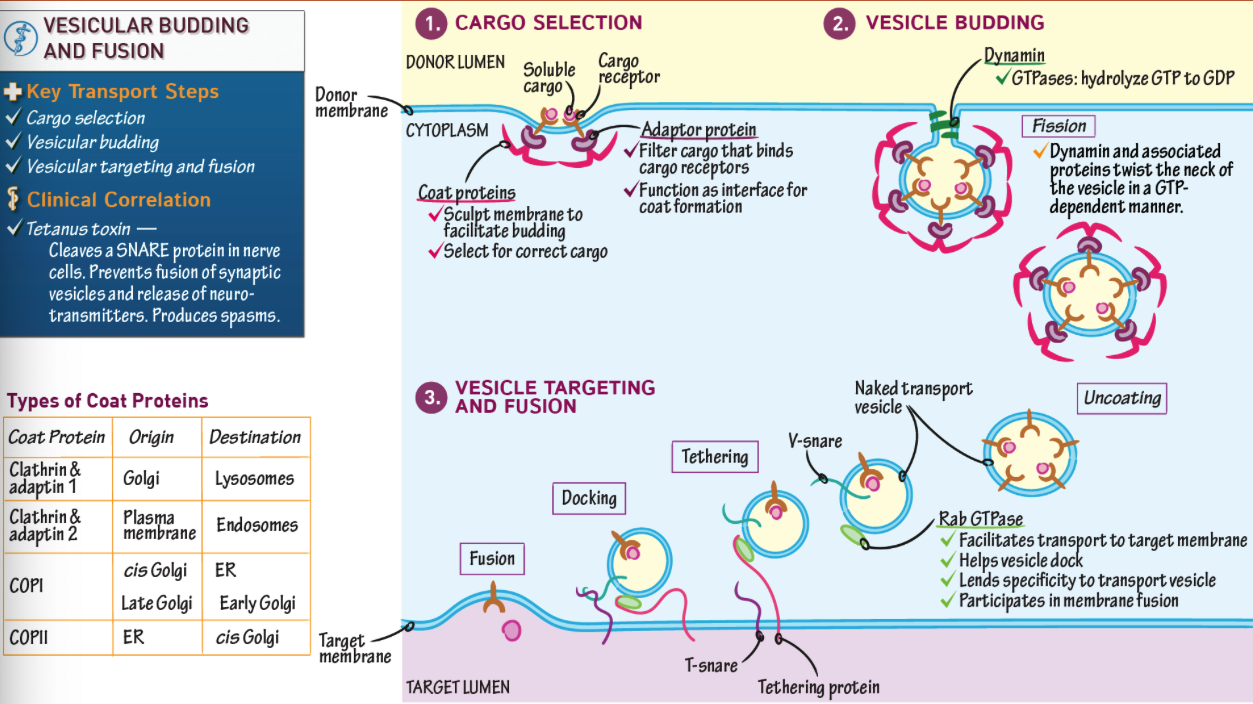
What provides specificity in vesicle formation: the adaptor or clathrin?
The adaptor protein (AP2 or GGA); clathrin is nonspecific.
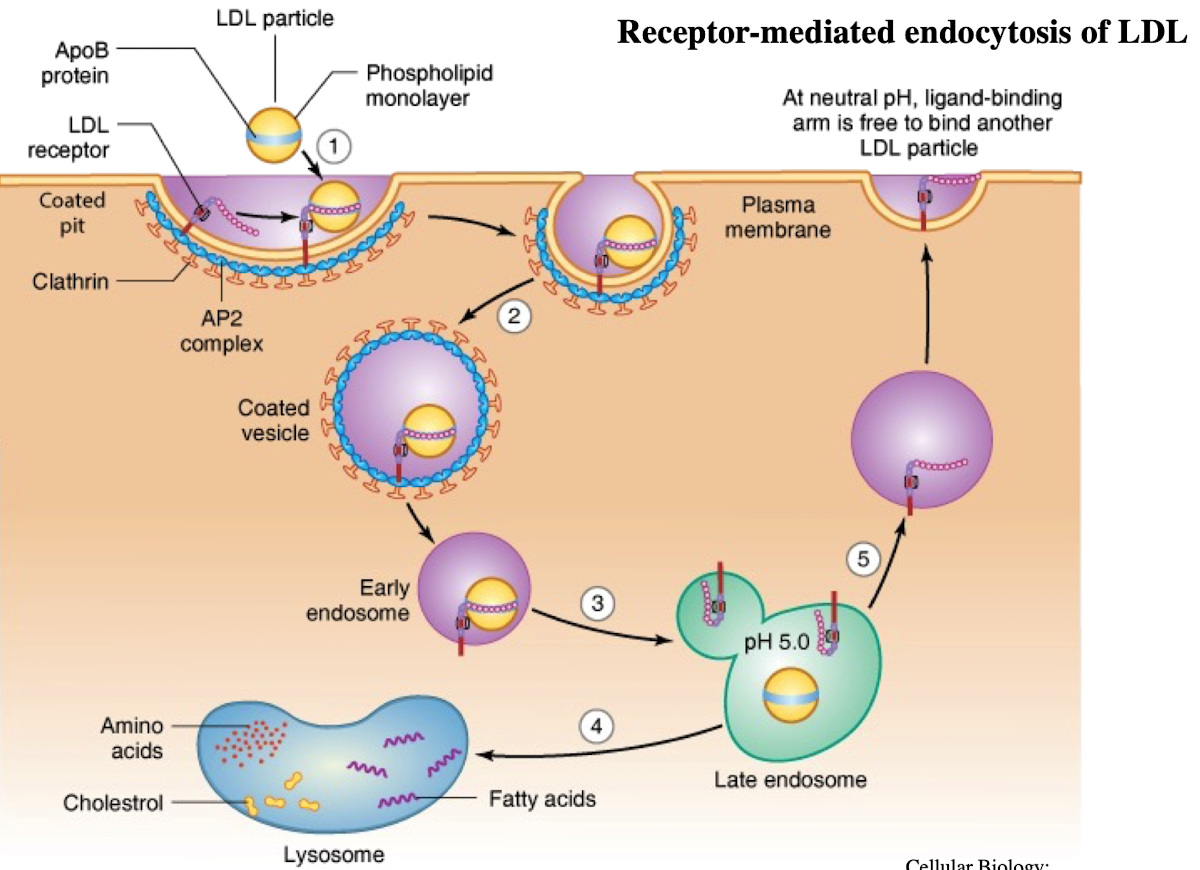
How are clathrin-coated vesicles formed?
Adaptor protein binds receptor → recruits clathrin → membrane bends → vesicle pinches off.
iClicker
What would happen if the enzyme that adds phosphate groups to the appropriate mannose residues on the carbohydrate chains of lysosomal enzymes were defective?
A. Lysosomal enzymes would be localized to lysosomes
B. Lysosomal enzymes would be localized to the plasma membrane
C. Lysosomal enzymes would be secreted
D/ Lysosomal enzymes would be degraded
E. Lysosomal enzymes would be in cis-Golgi
C
iClicker
A Familial Hypercholesterolemia patient is unable to internalize LDL from the blood. Tests determine that she makes LDL receptors, but they are mutant. The LDL receptors efficiently bind LDL on the plasma membrane but are not observed in coated pits. The mutation in the receptor is in ____.
A. The extracellular domain of the receptor
B. The clathrin binding domain
C. The GGA binding domain
D. The AP-2 binding domain
E. A Tyrosine that is phosphorylated by a protein kinase
D
Lecture 5/19:
Cell Signaling & Signal Transduction: Communication btwn Cells
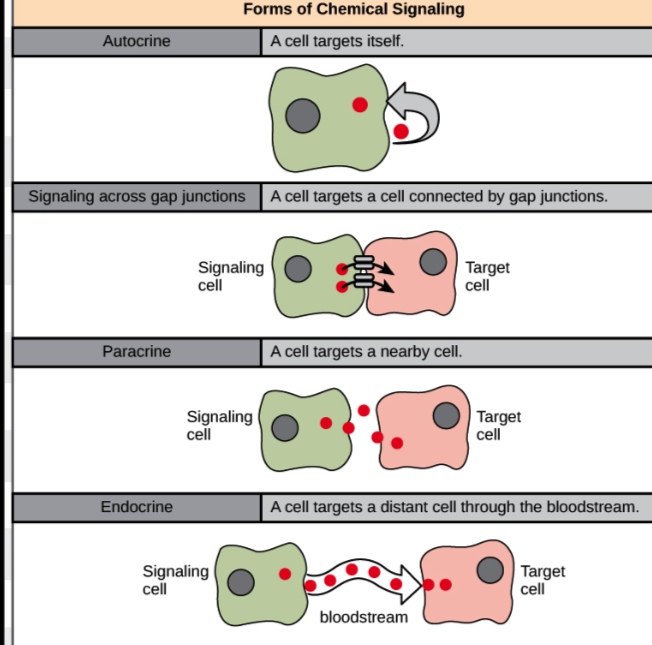
What are the four main types of cell signaling?
Endocrine (long-distance), Paracrine (short-distance), Autocrine (same cell sends and receives), and Direct cell-to-cell (adjacent cells communicate via contact).
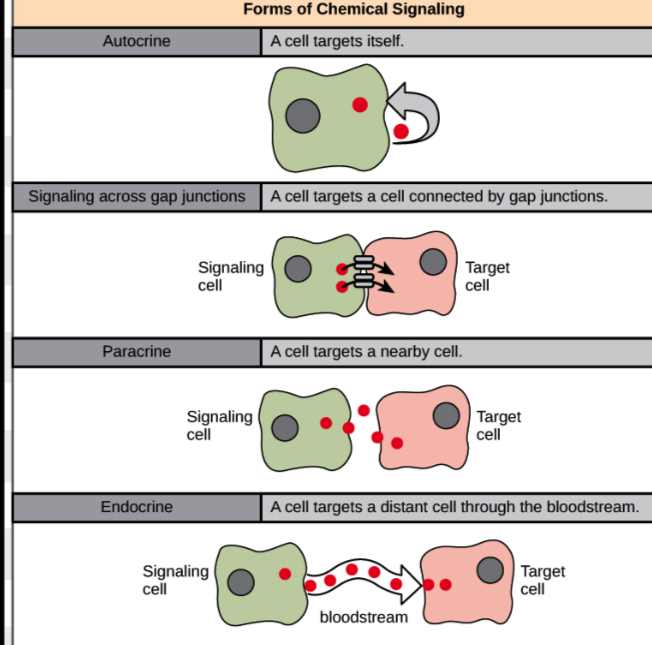
What is the key difference between autocrine and direct cell-to-cell signaling?
Autocrine involves one cell both producing and receiving the signal, while direct signaling involves two cells in physical contact.
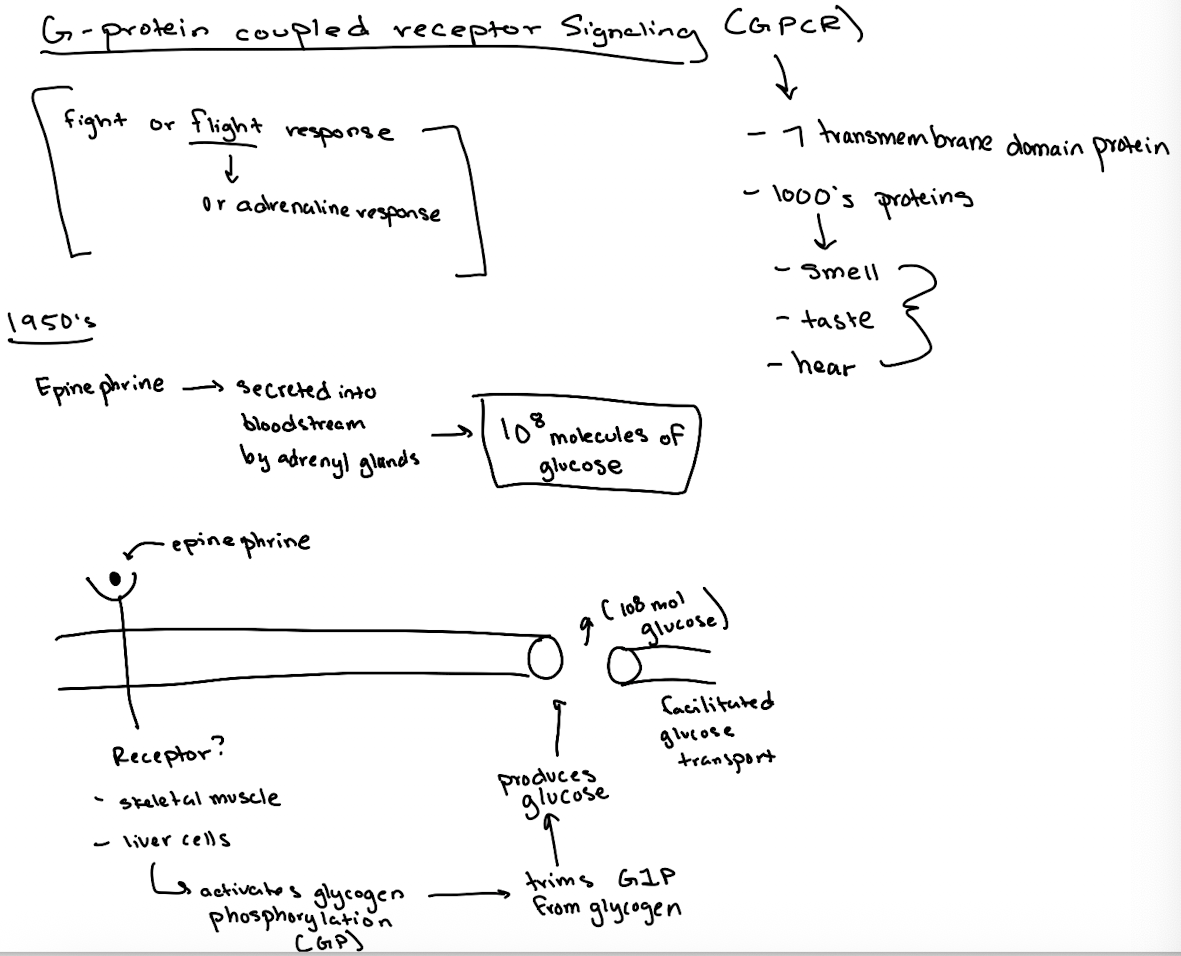
What are G-protein coupled receptors (GPCRs) and why are they important?
7-transmembrane domain receptors involved in senses like taste, smell, hearing, and are major drug targets.
What signaling process is GPCR critical?
epinephrine (adrenaline) fight-or-flight response, which triggers glucose release.
What did researchers originally observe about epinephrine's effect?
One molecule of epinephrine could cause the production of 10⁸ glucose molecules by activating glycogen breakdown.
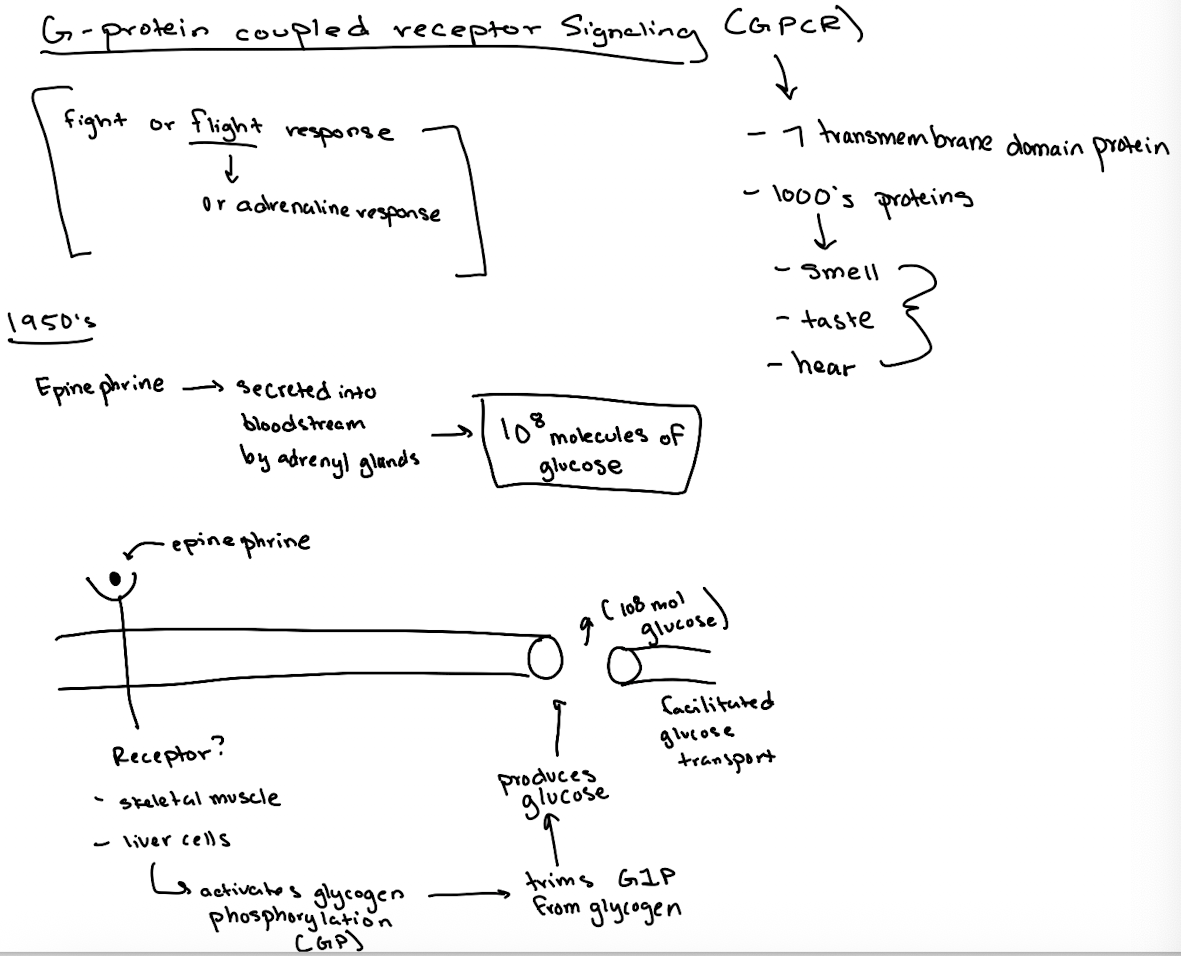
What enzyme does epinephrine ultimately activate to break down glycogen?
Glycogen phosphorylase (GP).
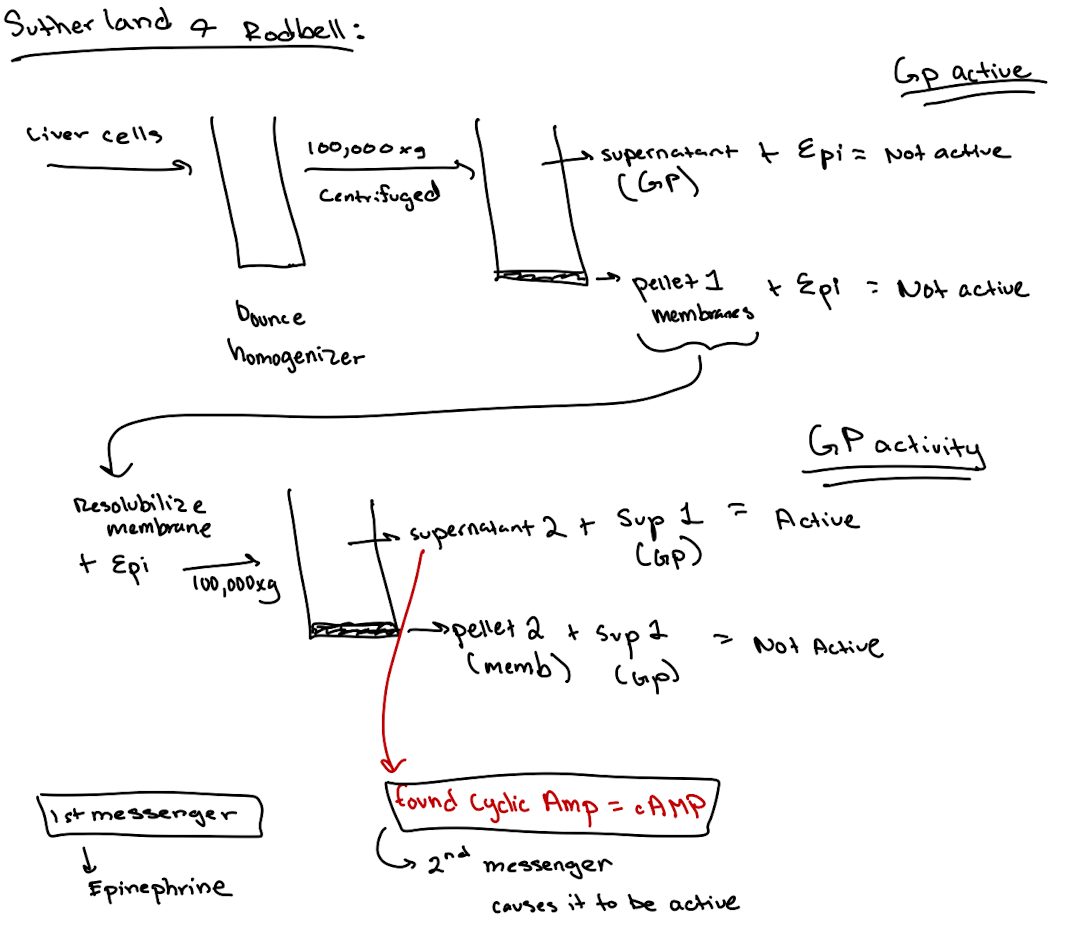
How did scientists discover a second messenger was involved?
They found that incubating membrane fractions with epinephrine produced a soluble factor (later identified as cAMP) that activated GP in another fraction.
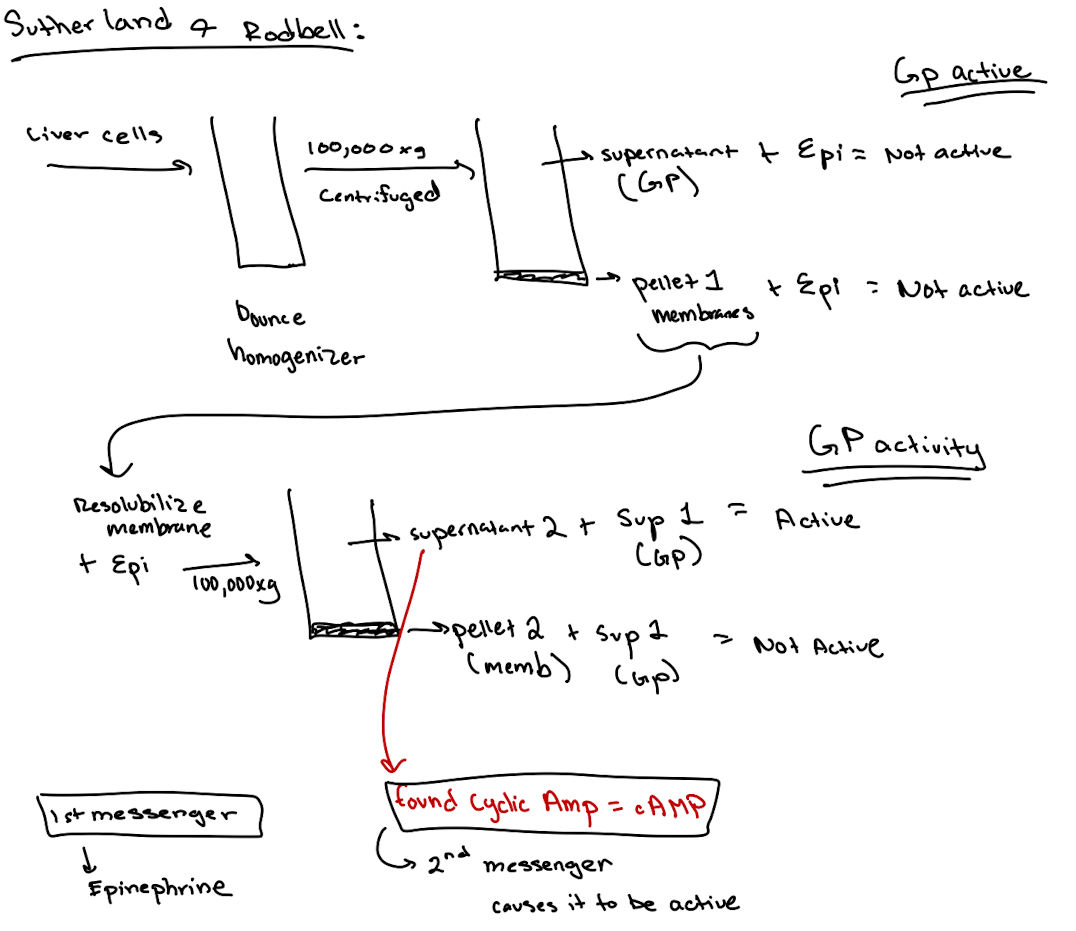
What is a second messenger? What was the first one discovered?
1st messenger = Epinephrine
2nd messenger = Cyclic AMP (cAMP)
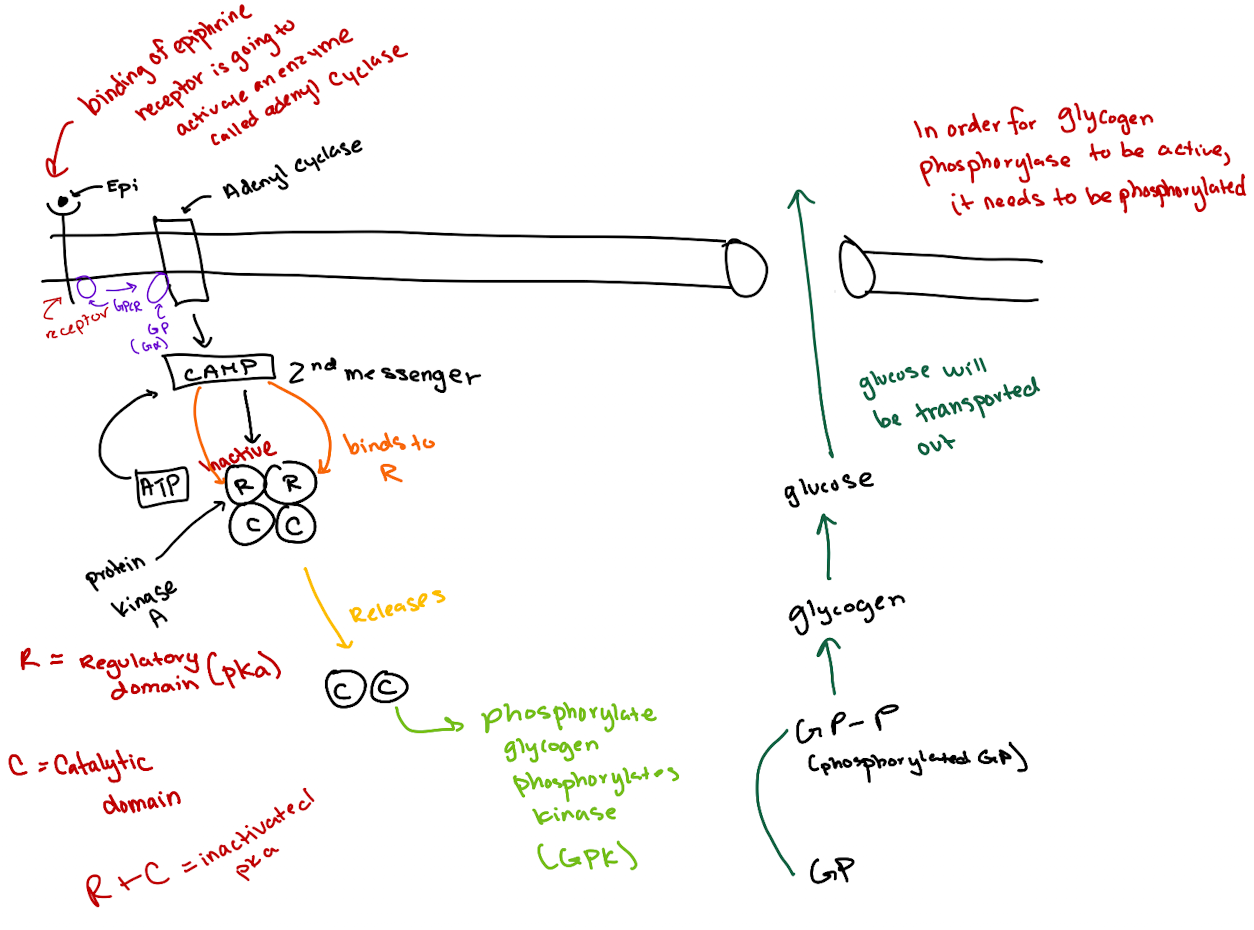
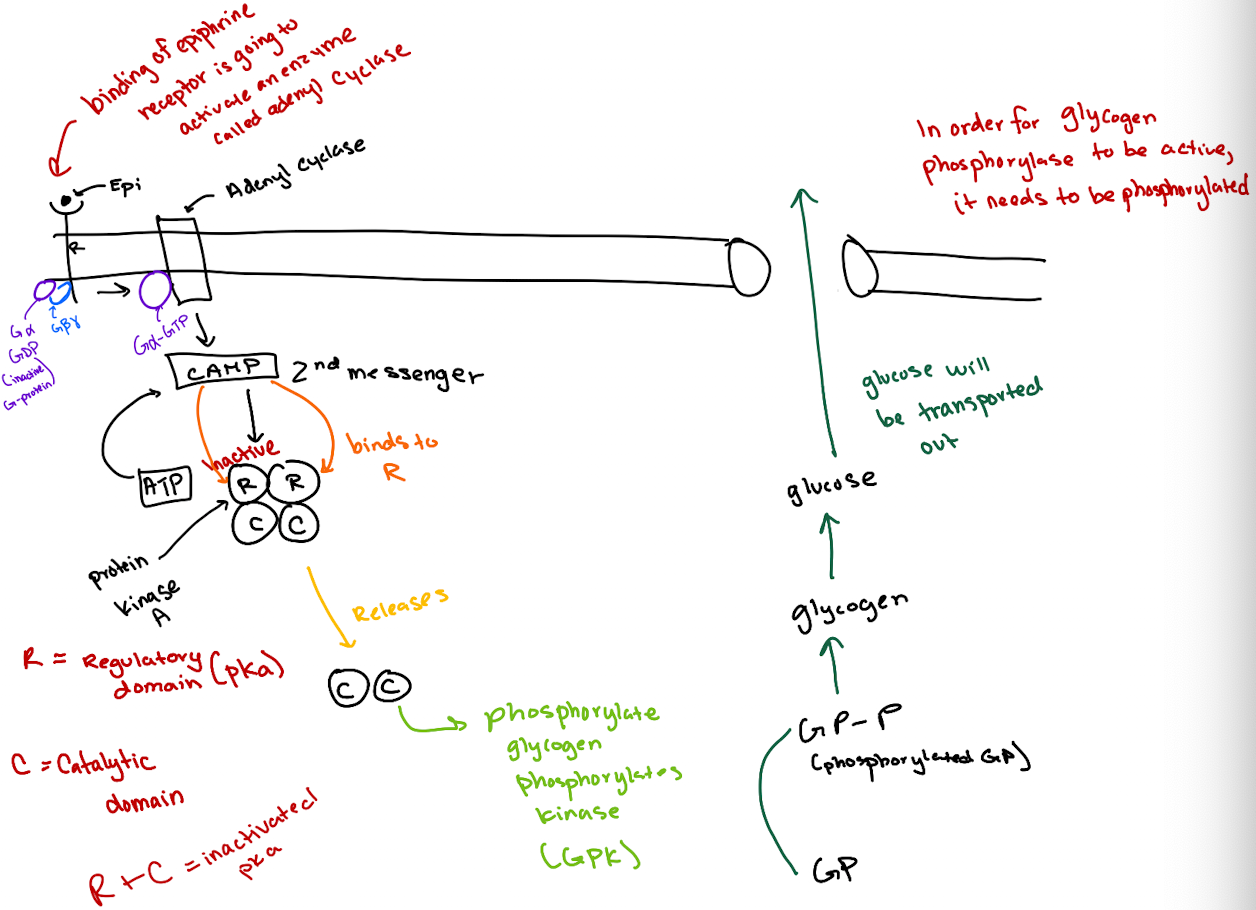
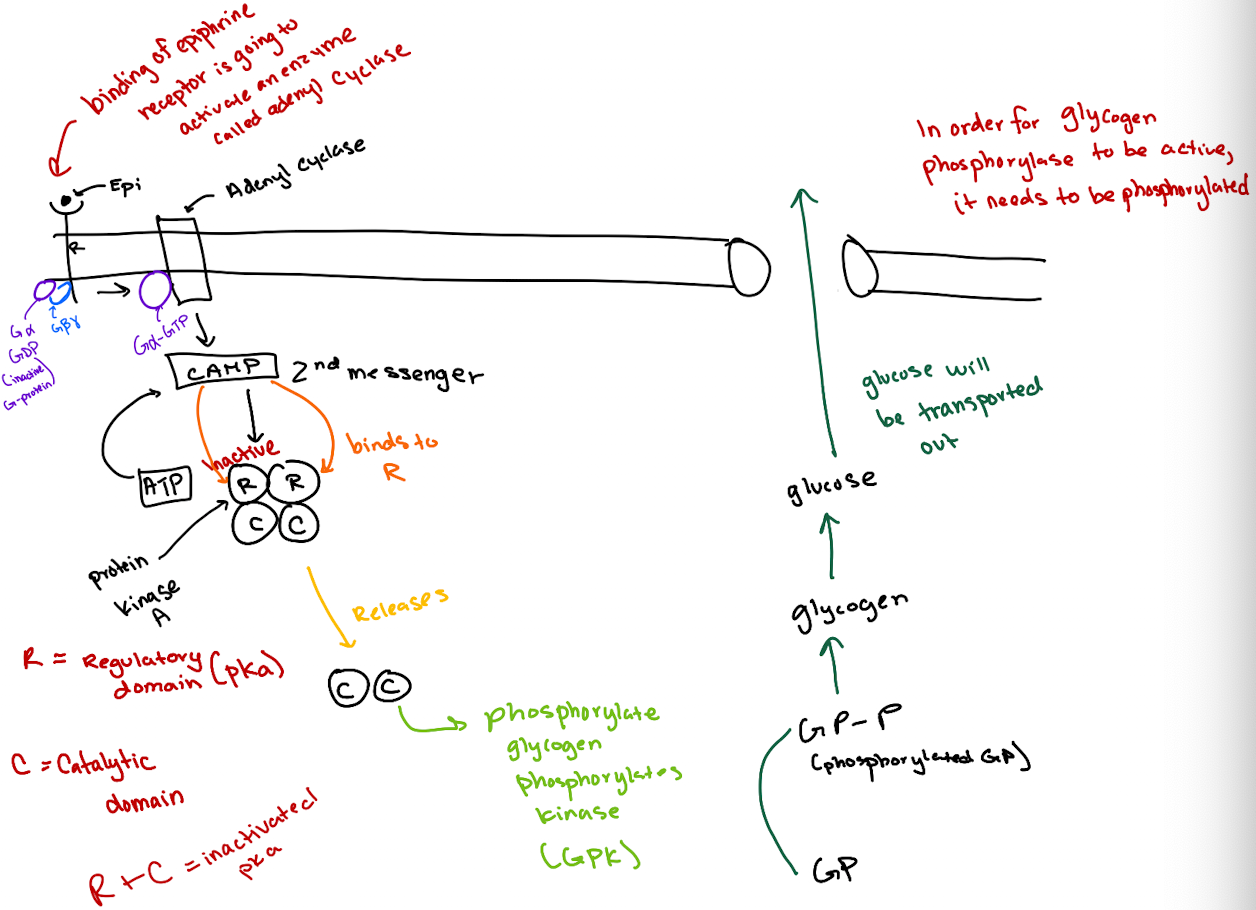
What is the correct order of the epinephrine signaling pathway?
Epinephrine → GPCR → G-protein (Gα) → Adenylyl cyclase → cAMP → PKA → GP kinase → Glycogen phosphorylase → Glucose release.
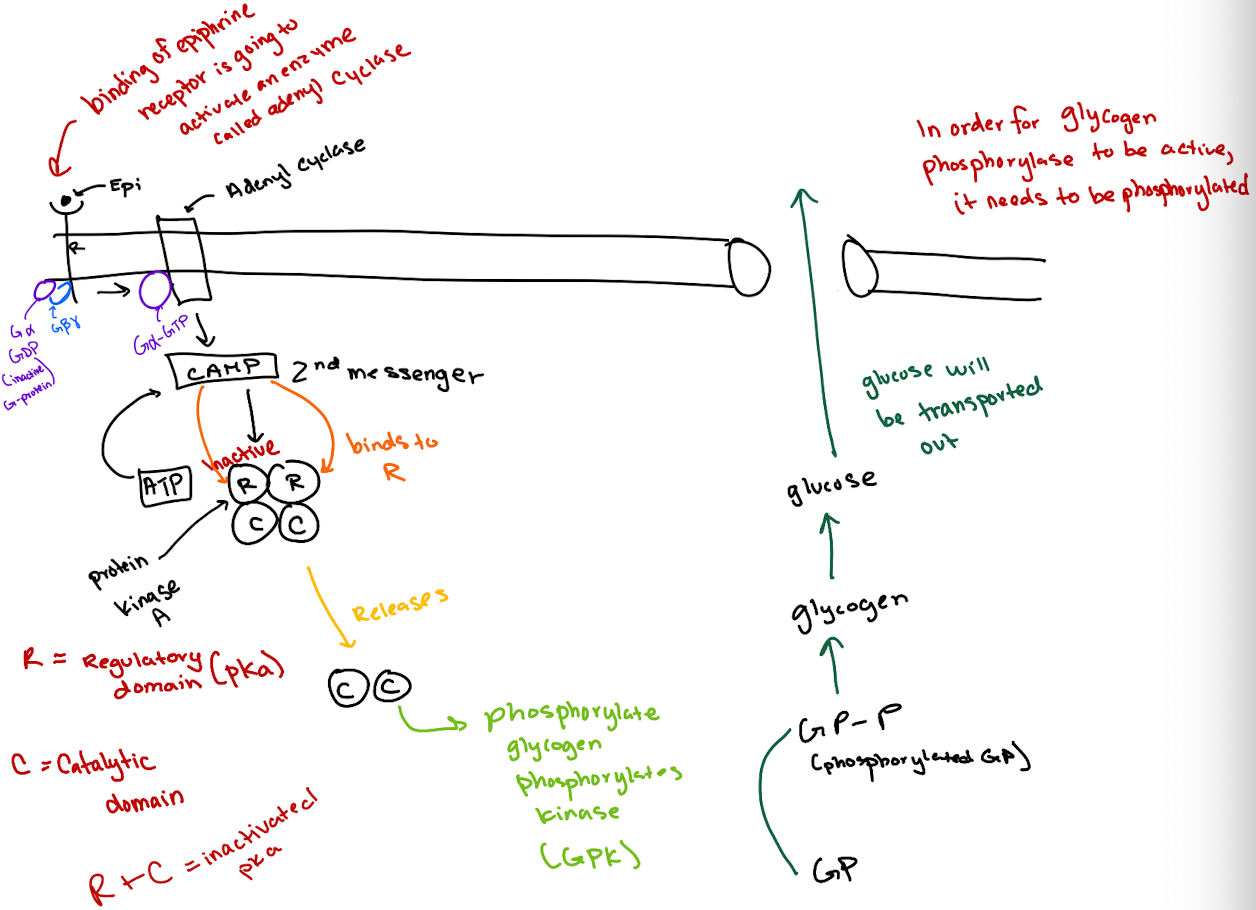
How does cAMP activate Protein Kinase A (PKA)?
cAMP binds to the regulatory (R) subunits of PKA, releasing the catalytic (C) subunits to phosphorylate targets.
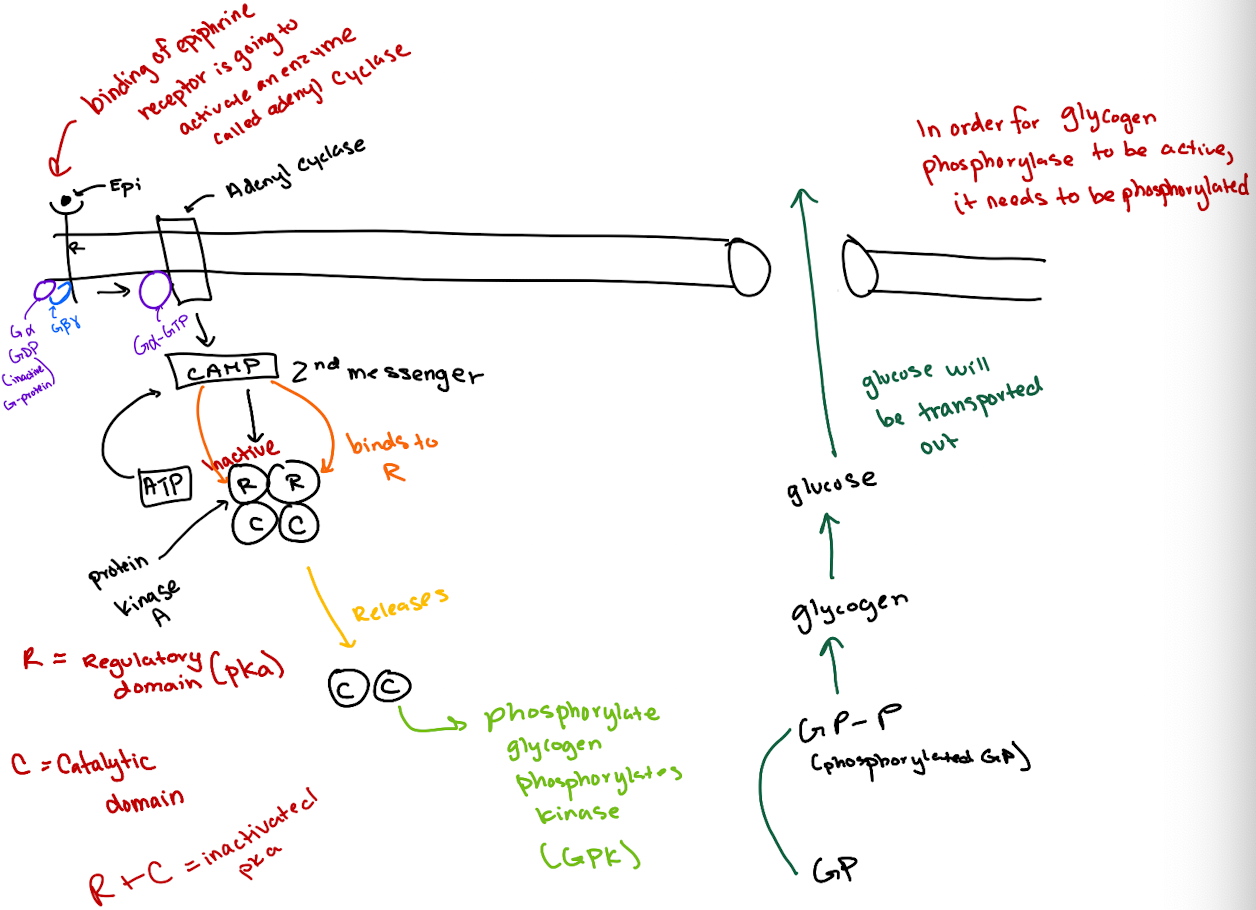
What is the structure of a heterotrimeric G-protein?
It has three subunits: Gα (binds GTP and has GTPase activity), Gβ, and Gγ.
What activates adenylyl cyclase in this pathway?
GTP-bound Gα subunit, after being activated by the GPCR.
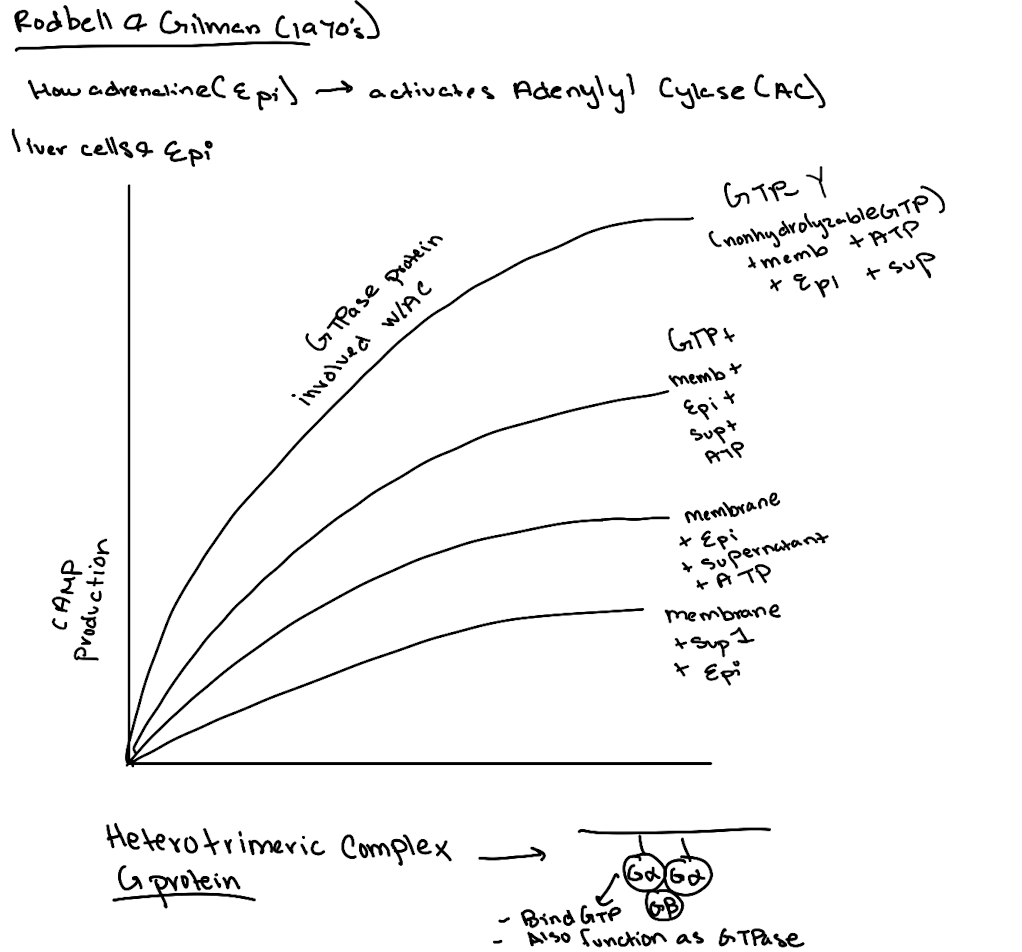
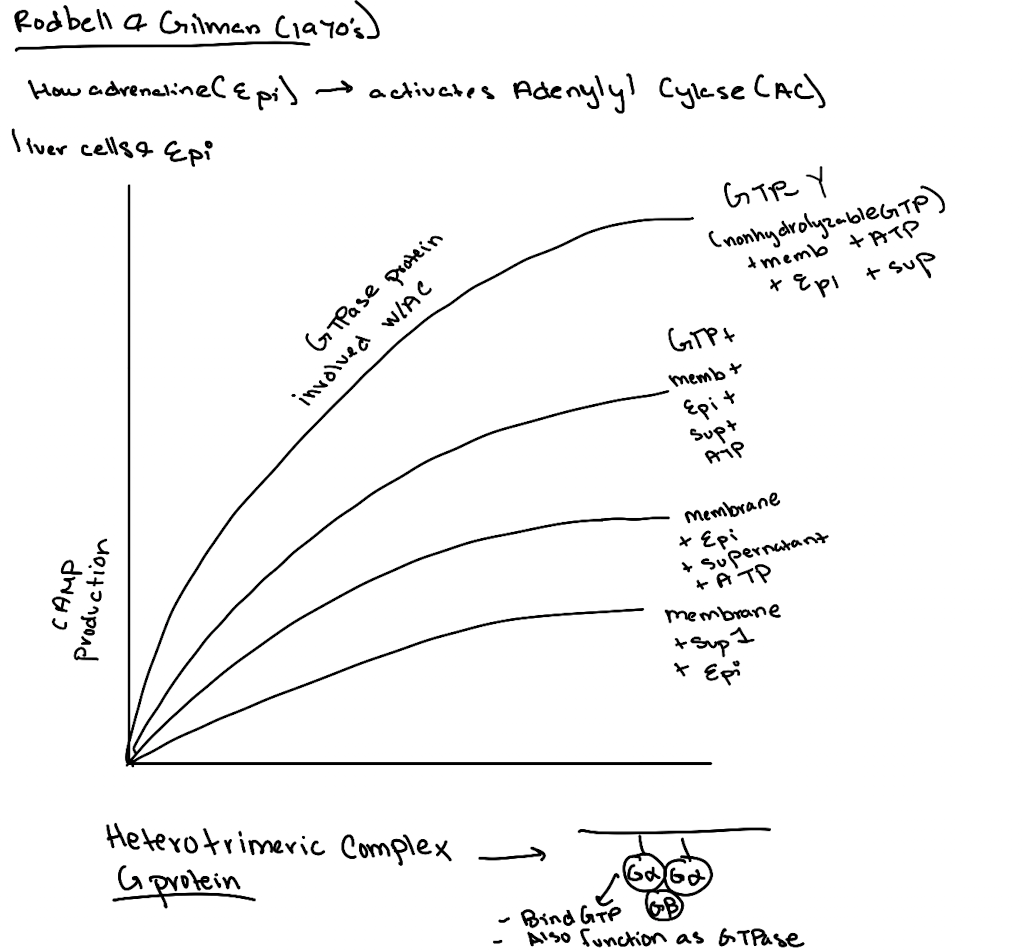
What did the addition of GTP do in experiments?
It boosted cAMP production, suggesting a GTP-binding protein was involved.
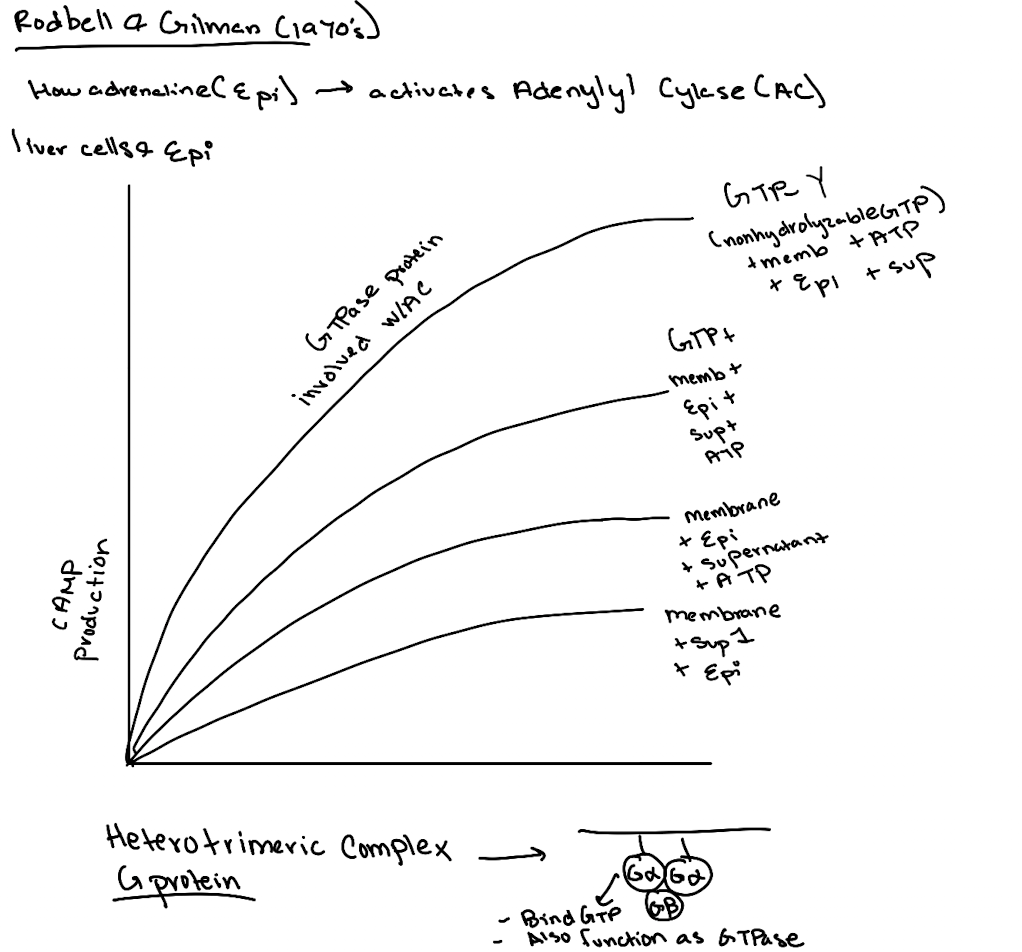
What is GTPγS and why was it important?
GTPγS is a non-hydrolyzable GTP analog. Its use showed the G-protein was a GTPase, not just a binding protein, since it locked Gα in the active state.
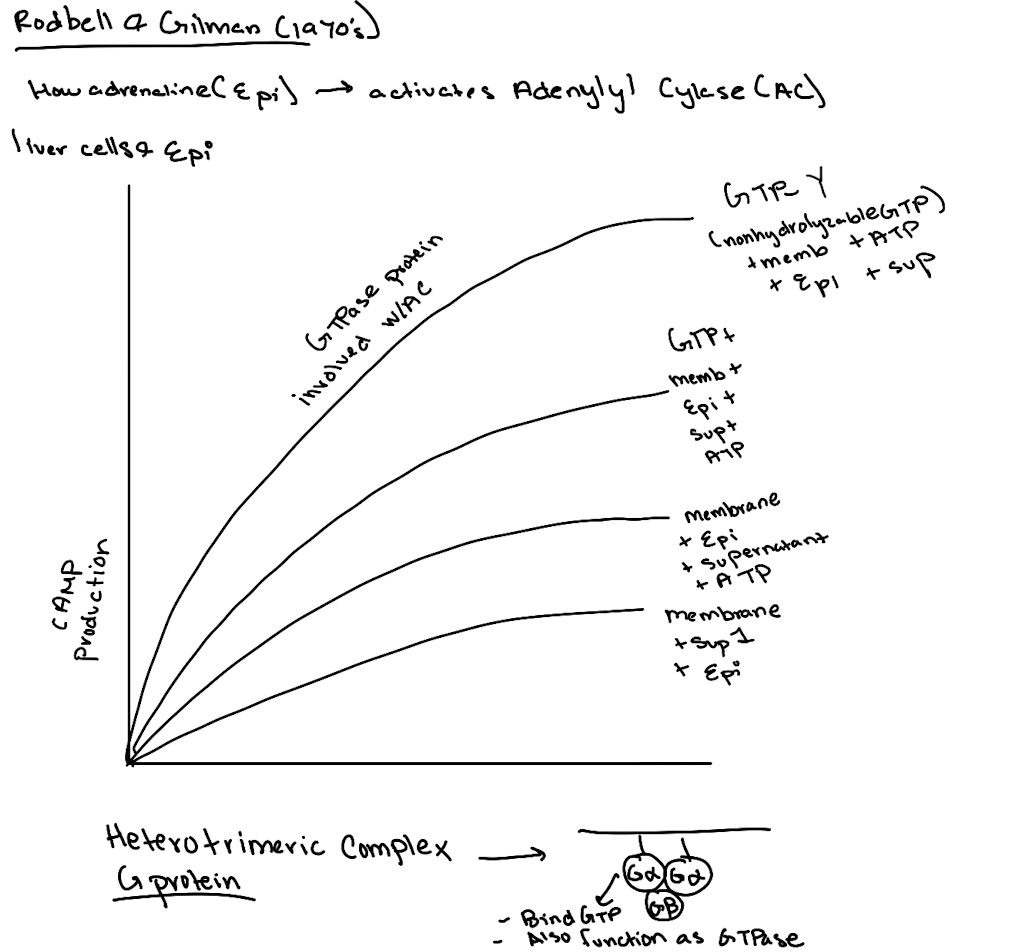
What was the importance of using ATP and GTP as controls in these experiments?
ATP showed the substrate was needed for cAMP production. GTP revealed a GTPase was essential for activating adenylyl cyclase.
What was ultimately discovered by Gilman and Rodbell?
A membrane-associated G-protein complex (Gαβγ) that links receptor activation to cAMP production.
Lecture 5/21
What type of receptor is the epinephrine receptor and where does epinephrine bind?
It’s a GPCR (7-transmembrane receptor); epinephrine binds between transmembrane domains 3 and 4.
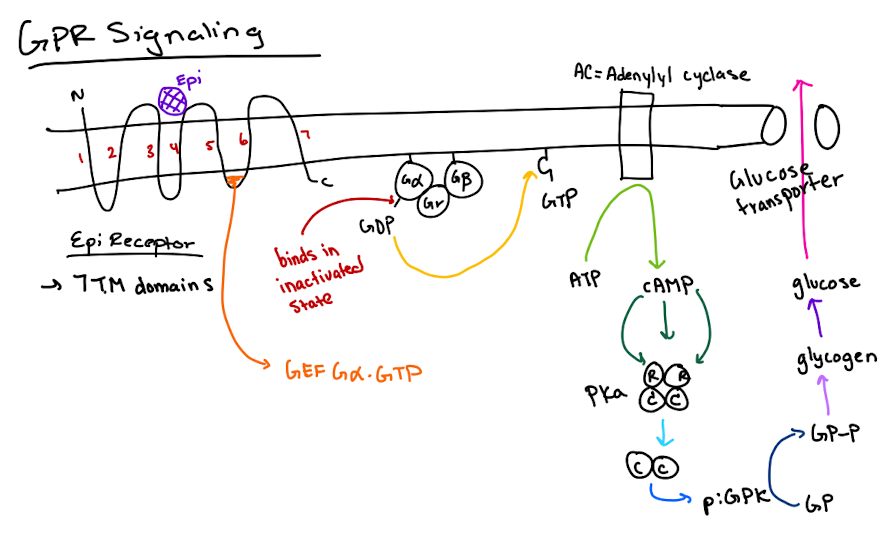
What happens after epinephrine binds to the GPCR?
The GPCR’s GEF region activates Gα by exchanging GDP for GTP.
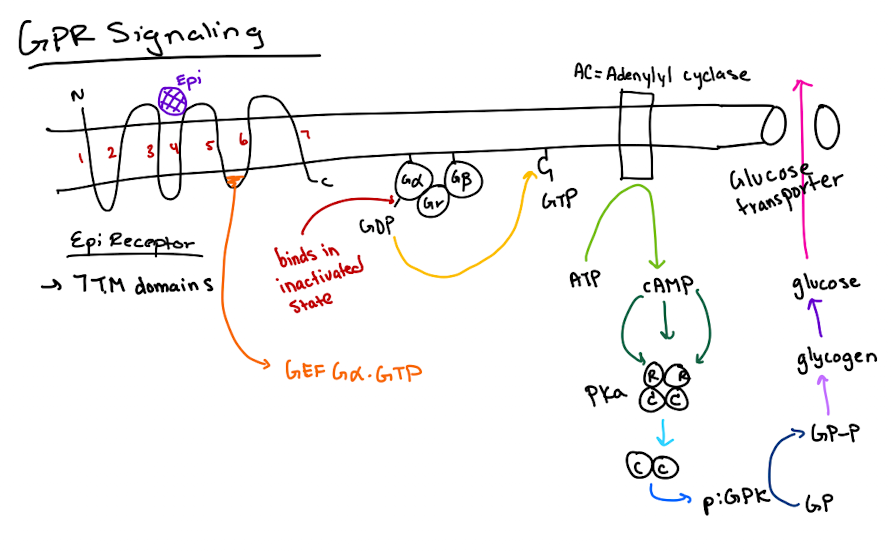
What does GTP-bound Gα do?
It activates adenylyl cyclase, which converts ATP to cyclic AMP (cAMP).
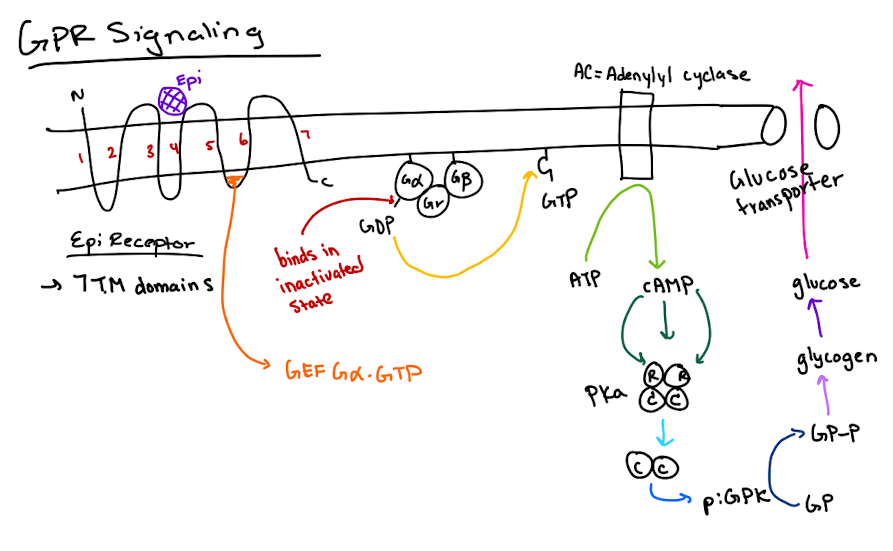
How does cAMP lead to glucose release?
cAMP activates PKA → PKA phosphorylates glycogen phosphorylase kinase (GPK) → GPK phosphorylates glycogen phosphorylase (GP) → GP breaks down glycogen to glucose.
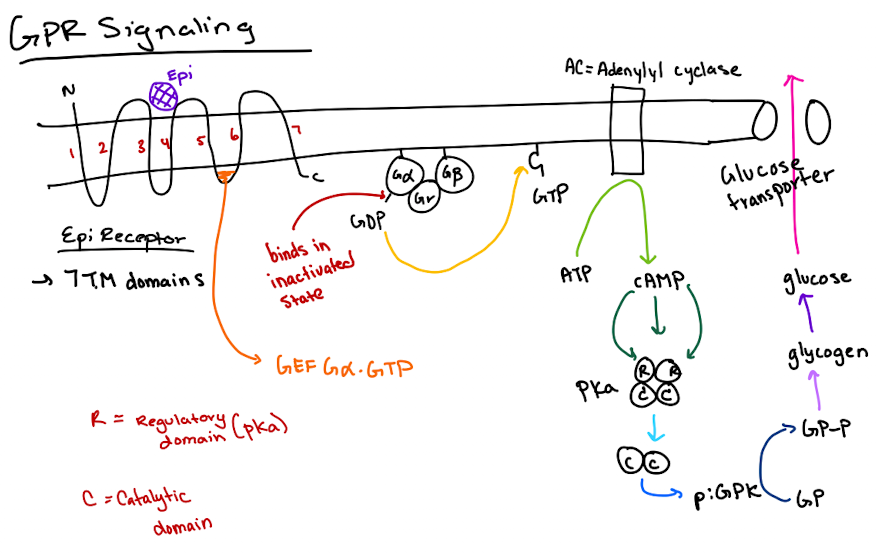
What are the two fastest ways to deactivate GPCR signaling?
1) Gα hydrolyzes GTP to GDP.
2) cAMP is degraded by phosphodiesterase.
What is the slower way to deactivate GPCR signaling?
Internalization and degradation of the GPCR via receptor-mediated endocytosis.
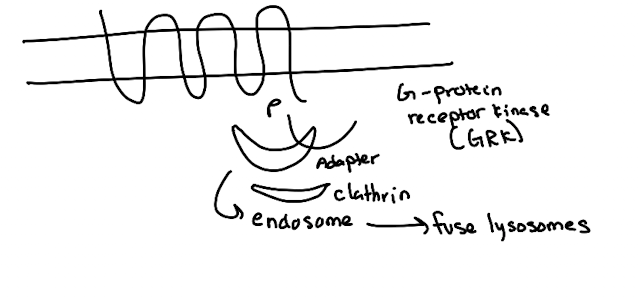
What are the steps in GPCR endocytosis?
GRK (GPCR kinase) phosphorylates GPCR C-terminus.
Arrestin binds phosphorylated receptor (GPCR)
Arrestin recruits clathrin → endocytosis.
Vesicle → endosome → lysosome degradation.
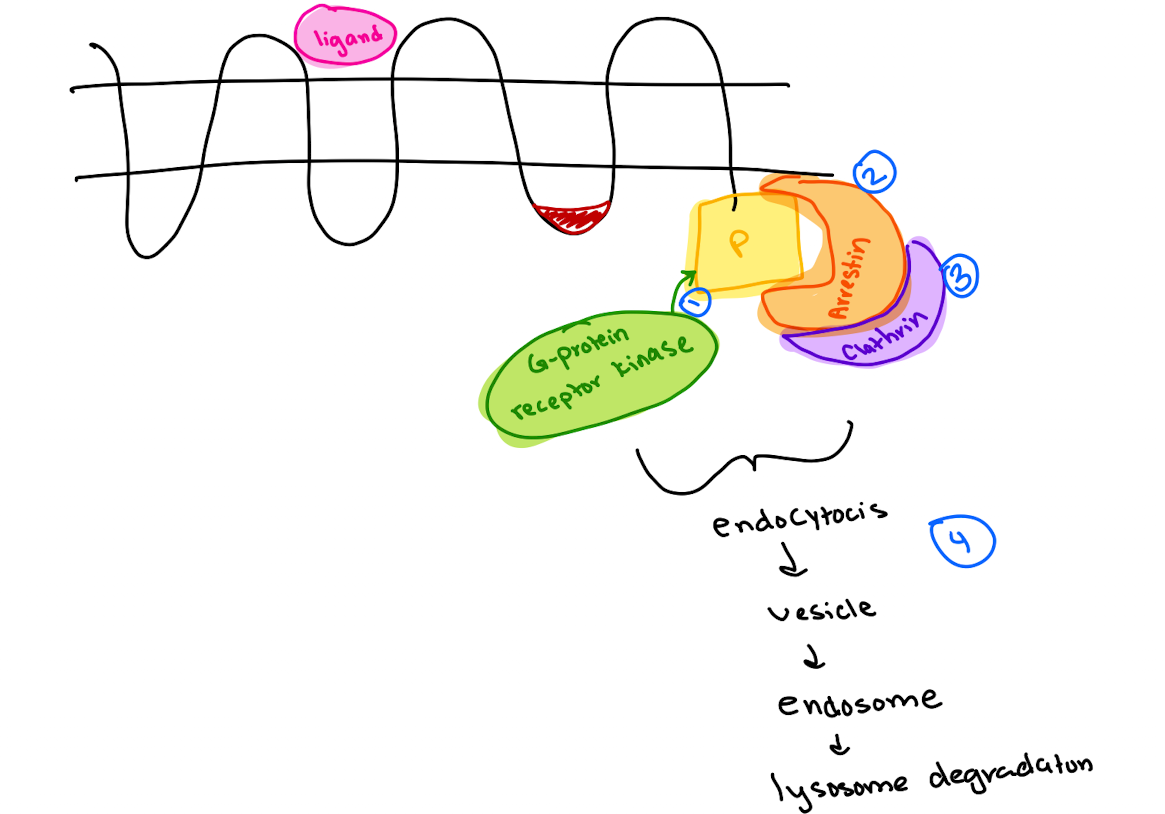
What adaptor proteins recruit clathrin in different pathways?
Arrestin (GPCR),
AP2 (LDL receptor),
GGA (M6P receptor from trans-Golgi to lysosome).
What common feature allows clathrin recruitment?
Adaptor proteins recognize phosphorylated or specific motifs on receptors to recruit clathrin (which has no specificity on its own).
What happens when EGF binds to its receptor (EGFR)?
EGFR dimerizes → tyrosine kinase domains cross-phosphorylate → recruits downstream signaling molecules.
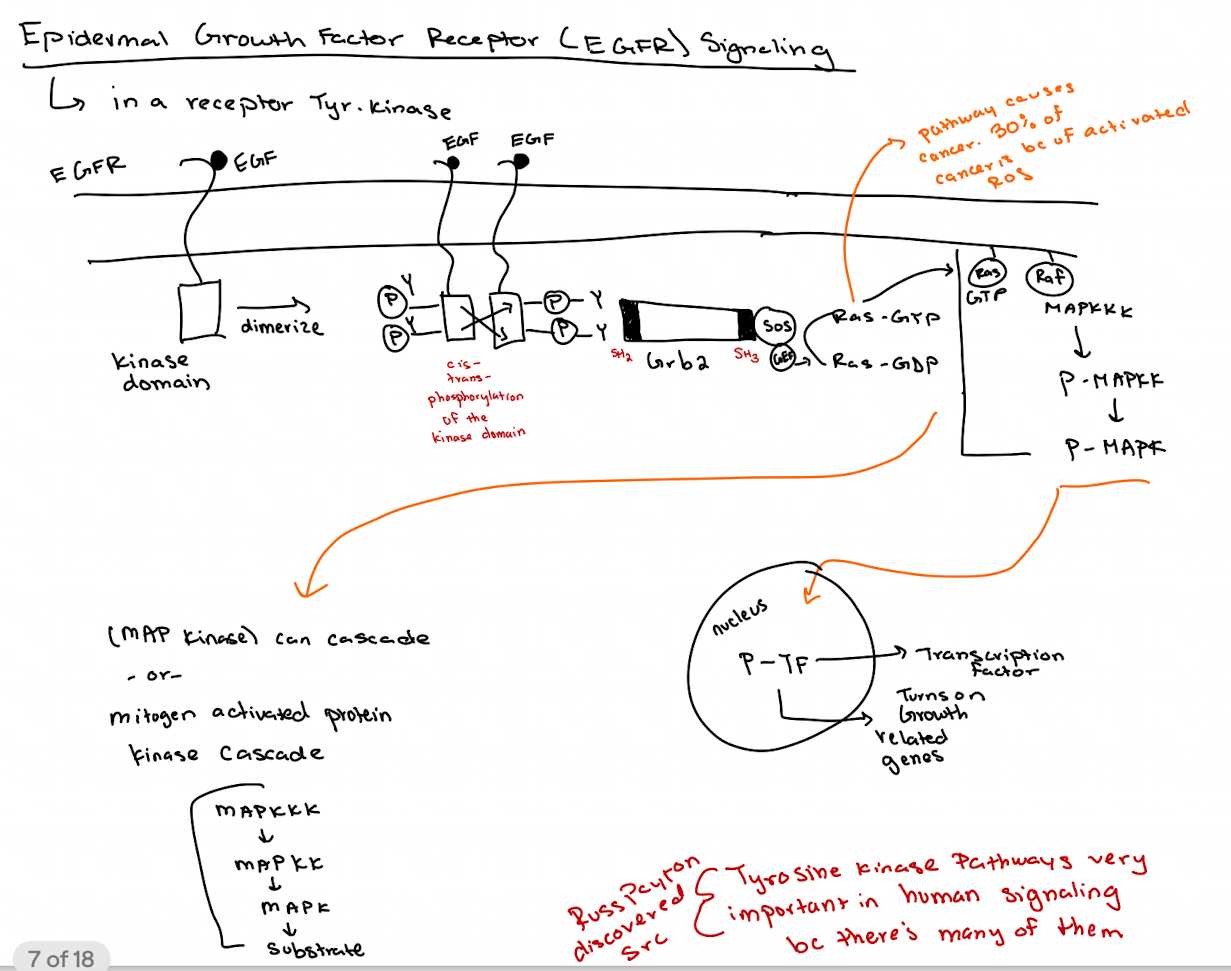
How does GRB2 interact with EGFR?
GRB2 binds phospho-tyrosines on EGFR via its SH2 domain and recruits SOS via its SH3 domain.
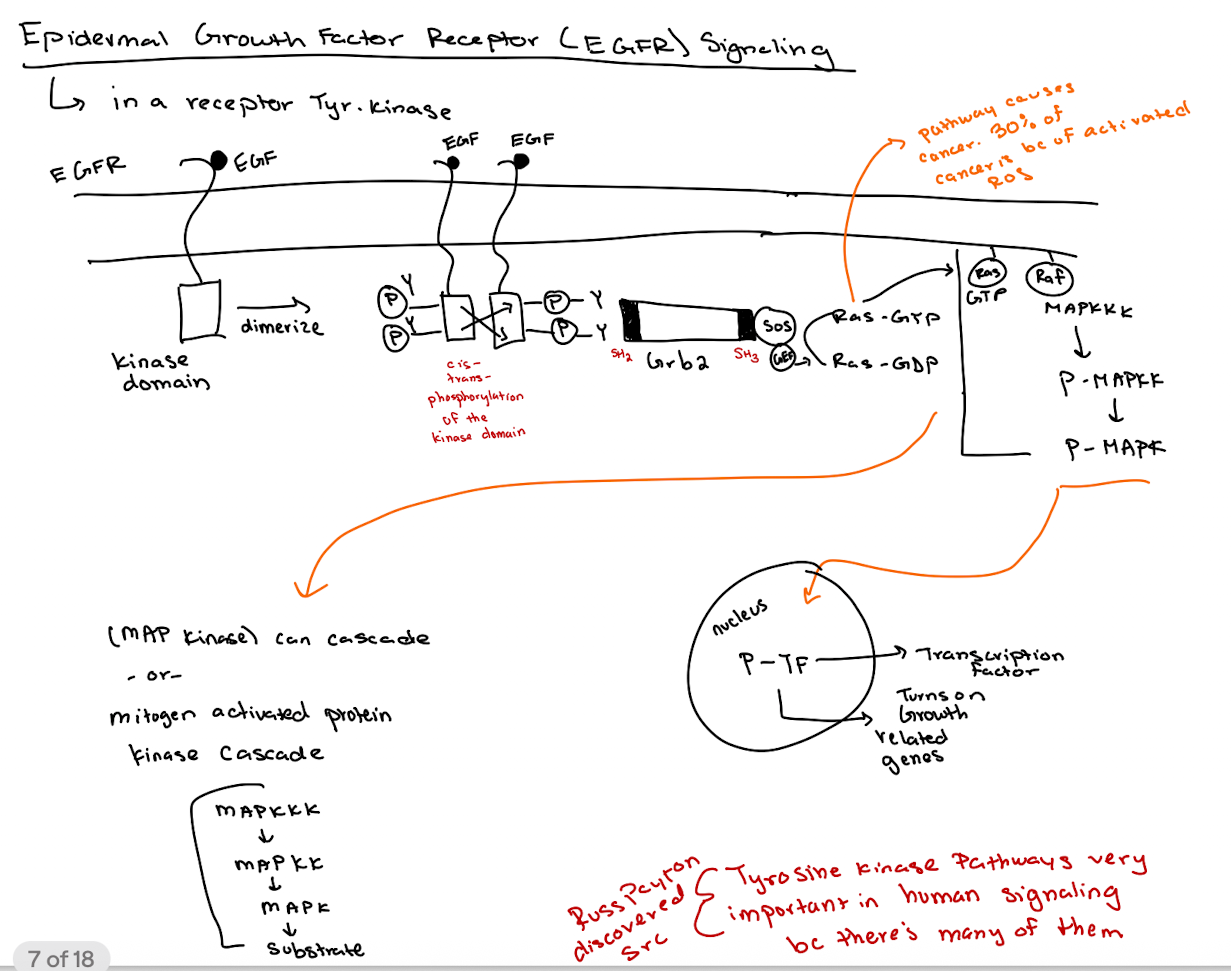
What is the role of SOS in the pathway?
SOS acts as a GEF for Ras, converting Ras-GDP to Ras-GTP.
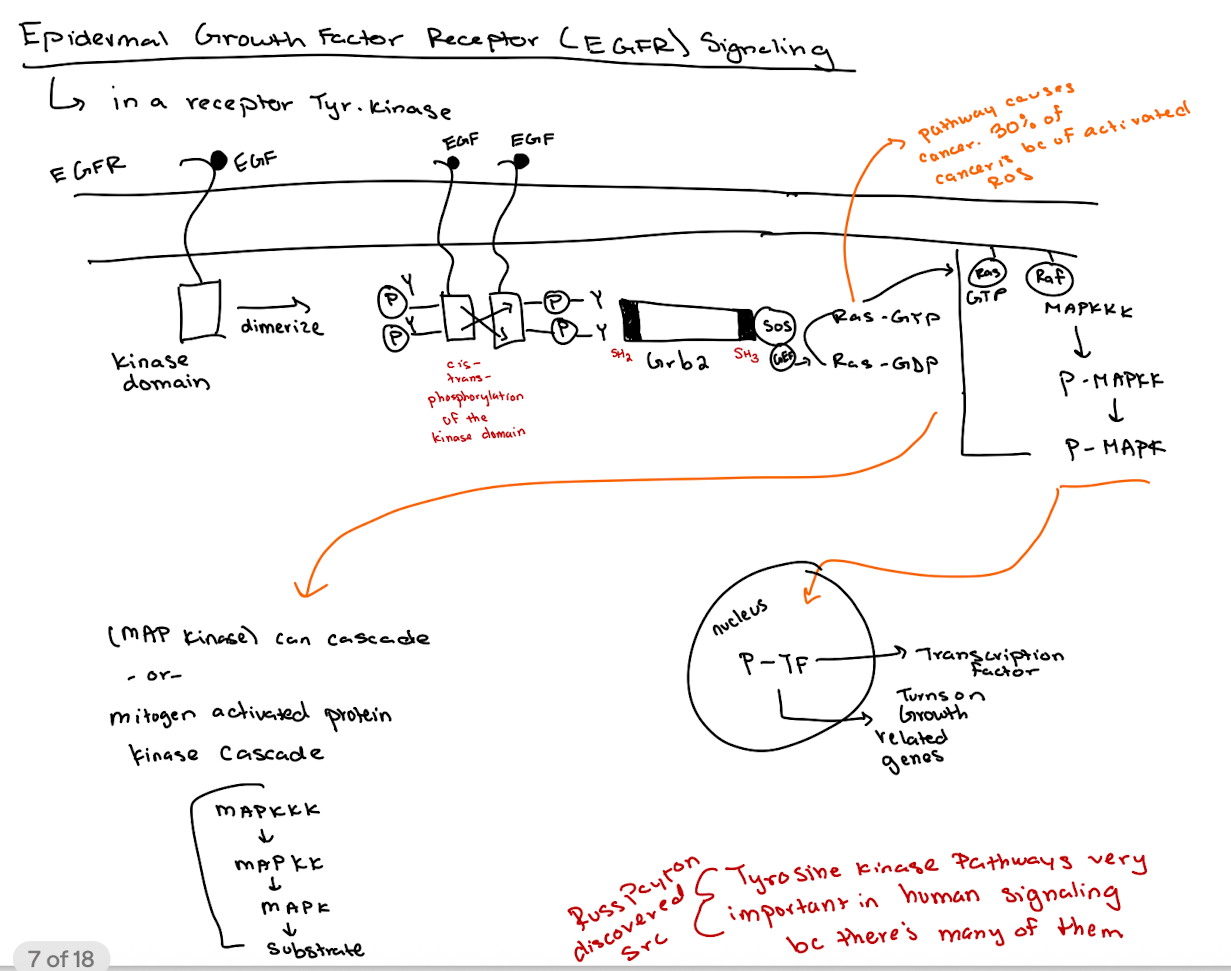
What happens after Ras is activated (Ras-GTP)?
Ras-GTP activates RAF → RAF phosphorylates MAPKKK → P-MAPKK phosphorylates MAPK → P-MAPK enters nucleus to activate transcription factors (thru phosphorylation → P-TF)
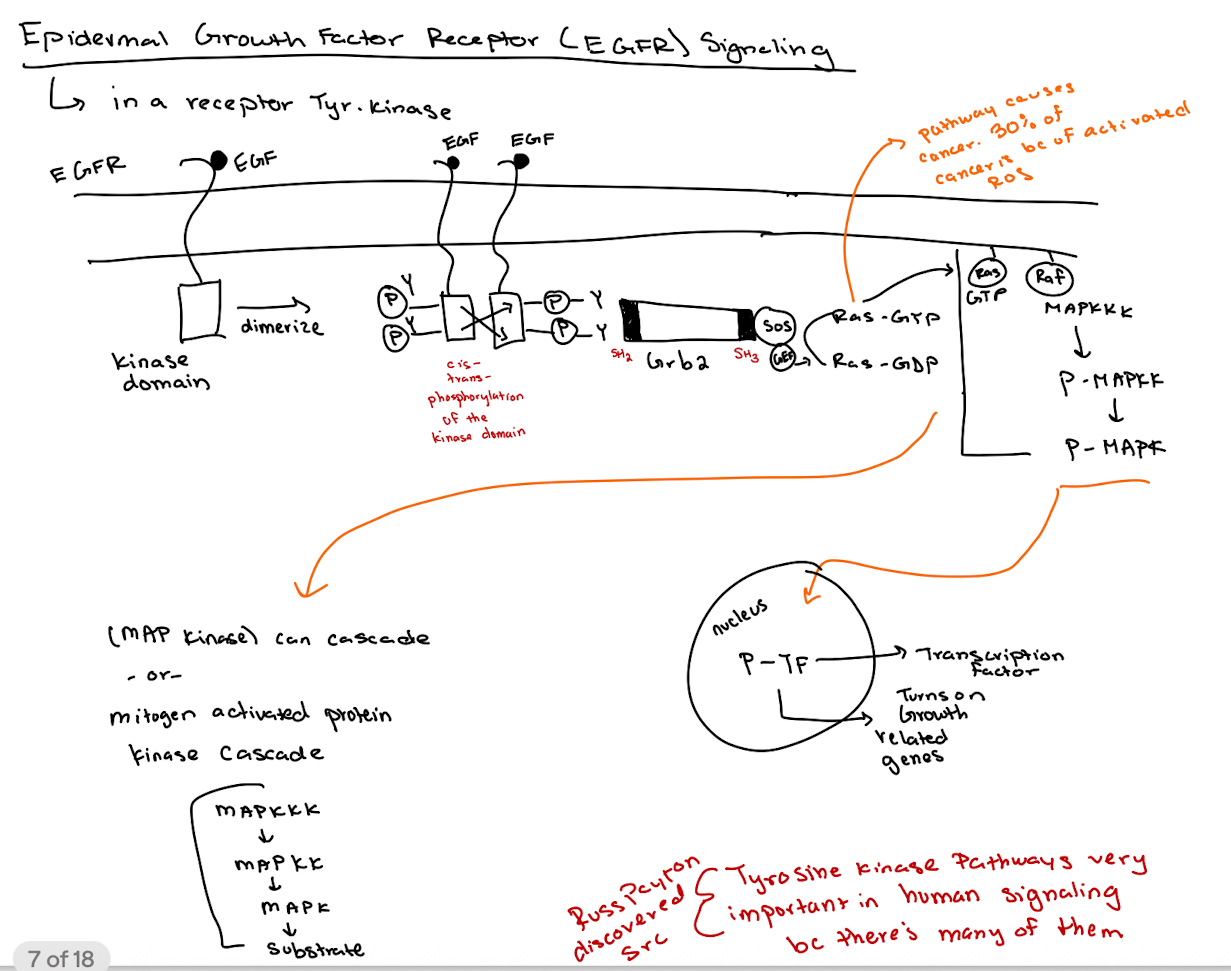
What is the overall purpose of the MAP kinase (MAPK) cascade?
To regulate gene expression related to cell growth and development.
What is the key difference between cellular Src (c-Src) and viral Src (v-Src)?
c-Src is regulated (inhibited by Y527 phosphorylation);
v-Src lacks Y527 and has phosphorylated Y416, making it constitutively active.
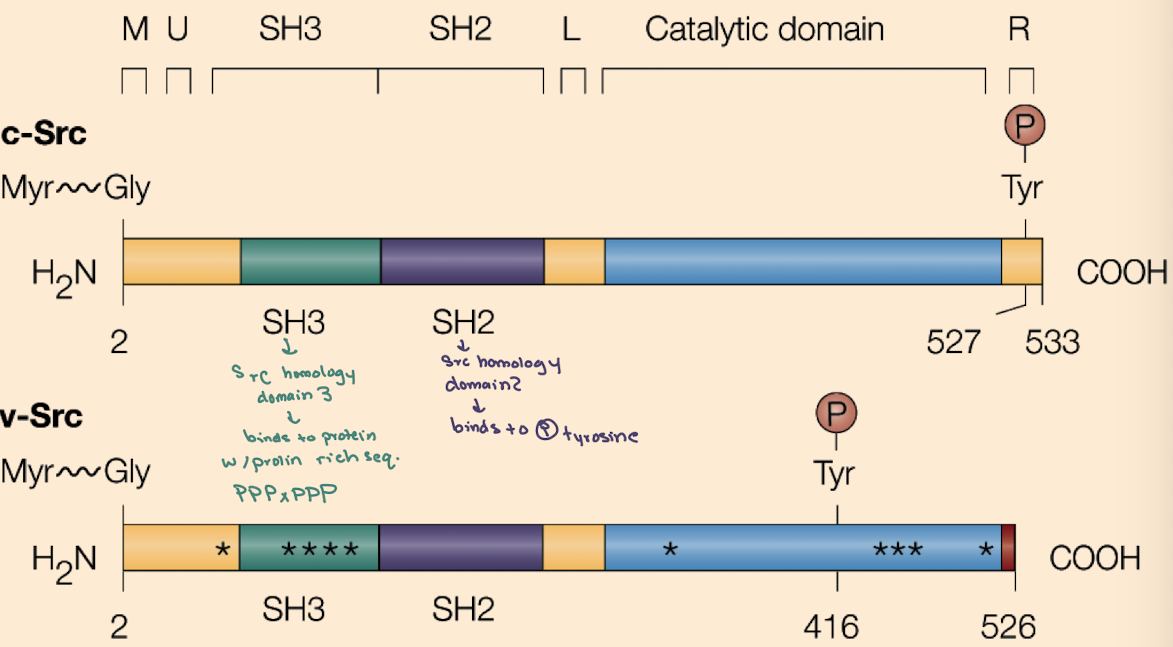
Why is v-Src oncogenic (cancerous) but c-Src is not (under normal conditions)?
v-Src is always active due to loss of inhibitory control, driving unregulated cell proliferation (lots of growth & division)
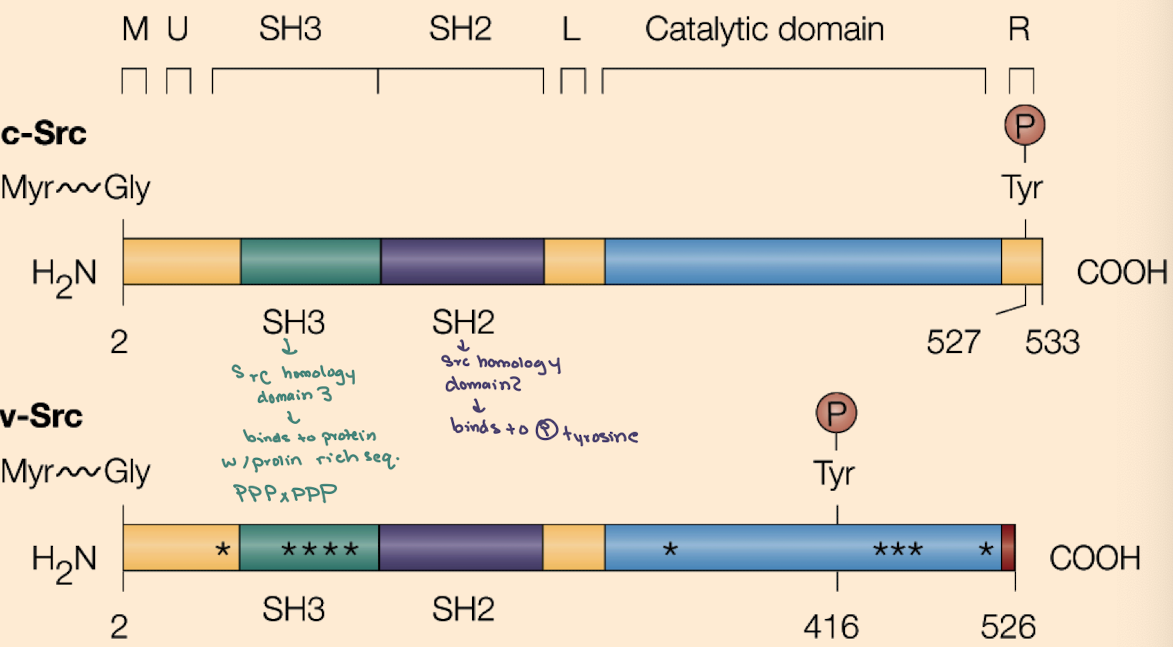
What domains are found in Src proteins and what do they do?
SH2 (binds phospho-tyrosine),
SH3 (binds proline-rich sequences),
Kinase domain (phosphorylates tyrosines),
Myristoylation site (membrane anchoring).
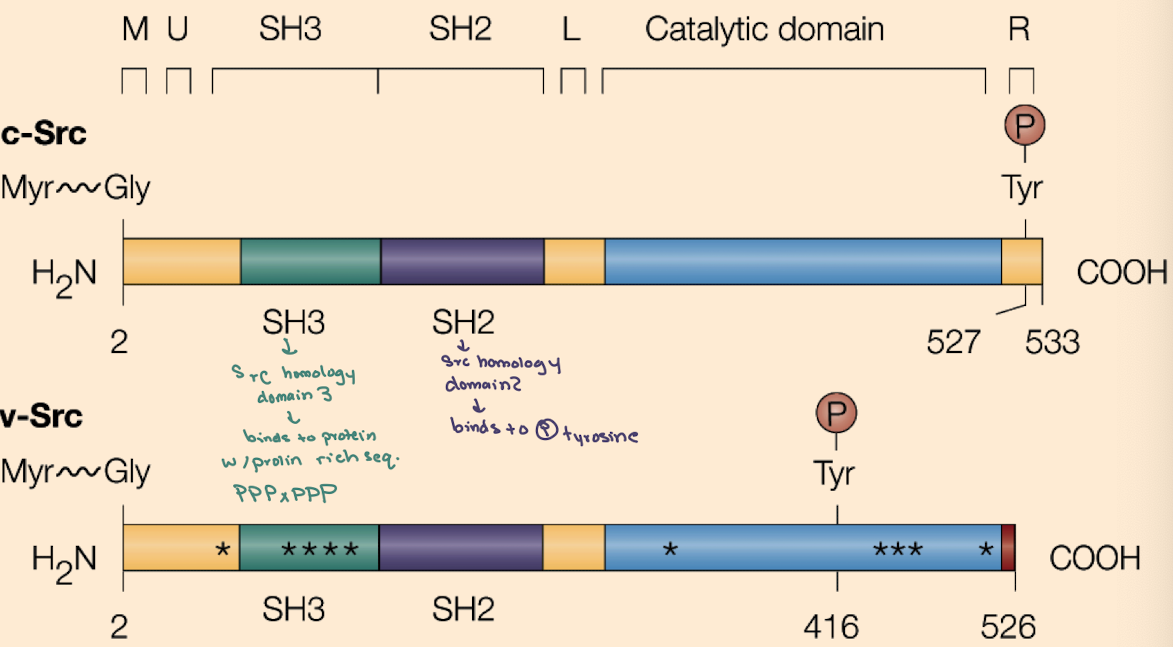
iClicker
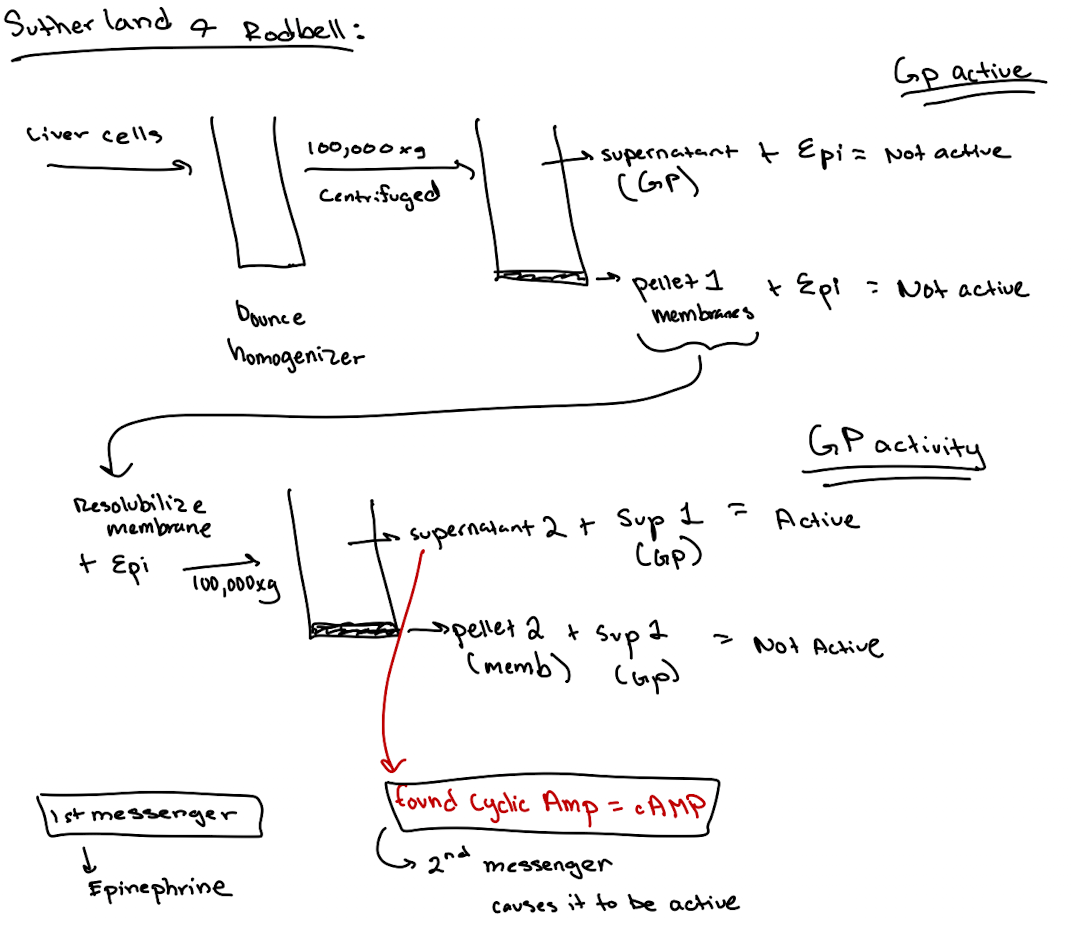
Earl Sutherland conducted a set of experiments that we discussed in class. He broke open liver cells and separated soluble supernatant 1 from the membrane pellet. Then he resuspended the membrane, added epinephrine, and spun again to get a supernatant 2. He found that supernatant 2, when combined with supernatant 1. efficiently stimulated the breakdown of glycogen. What essential signaling component was in Supernatant 1?
A. the beta-adrenergic receptor
B. Epinephrine
C. Glycogen Phosphorylase Kinase
D. cAMP
E. None of the above
C
What’s found in Supernatant 1 in the Rodbell experiment?
Cytoplasmic components like GP, GPK, PKA—but not cAMP (because no epinephrine stimulation yet).
iClicker
Propose a specific type of mutation in the gene that encodes protein kinase A (PKA) that could lead to a permanently active PKA.
A. Alter the regulatory subunit so that it cannot bind the catalytic subunit
B. Alter the catalytic subunit so that it can bind tightly to the regulatory subunit
C. Alter the regulatory subunit so that it could not bind cAMP
D. Alter the catalytic subunit so that it could not bind cAMP
E. None of the above
A
What activates PKA without epinephrine?
Direct addition of cAMP to the system will activate PKA by releasing its catalytic subunits.
Lecture 5/23
iClicker
As we discussed, Grb2 is required in the EGF receptor (EGFR) pathway. You have been given a mutant cell line that does not express Grb2 (all other proteins are fine) and is therefore unable to respond to EGF. What new protein could you engineer and express to make the cell respond to EGF ligand?
A. Add more tyrosine residues to EGFR
B. Add a stretch of proline residues to Ras
C. Add GEF to the EGFR
D. Add an SH2 domain to SOS
E. Add an SH3 domain to SOS
D
iClicker
v-Src from Rous Sarcoma Virus causes cancer. What will be the effective drug target in the v-Src protein to inactivate its function?
A. A drug that inhibits Y527 phosphorylation
B. A drug inhibits the SH2 domain-contains Grb2 protein
C. A drug that inhibits EGF receptor dimerization
D. A drug that blocks Y416 phosphorylation
E. A drug that blocks the interaction Between Y527 and SH2 domain
D