AP Physics 2 - Unit 9: Thermodynamics
1/58
Earn XP
Description and Tags
"Did You ReMemBer?"
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
Heat
Energy transfered; not contained (exchanged only)
Temperature
A representation of average internal kinetic energy; contained (not exchanged)
Energy
ability to cause change
Thermal Expansion Equation Defined
Change in length (ΔL) of a solid material when heated or cooled = original length (Lo) times coefficient of linear expansion (α - alpha) for that material times change in temperature (ΔT)
Thermal Expansion Eq.
ΔL = LoαΔ𝑇
Volumetric Expansion Defined
Change in Volume (ΔV) of a solid material when heated or cooled = original volume (Vo) times coefficient of volume expansion (𝛽) of that material times change in temperature (Δ𝑇)
Volumetric Expansion Eq.
ΔV=βVoΔT
Kinetic Theory (proves ideal gases) First Law
The gas particles are VERY small (mostly empty space)
Kinetic Theory (proves ideal gases) Second Law
The number of particles is UNCOUNTABLY high
Kinetic Theory (proves ideal gases) Third Law
The collisions are ELASTIC (completely newtonian)
Kinetic Theory (proves ideal gases) Fourth Law
There are NO OTHER FORCES on the particles (no gravity)
Ideal Gas Law (Chemistry nerd verison) Eq.
P𝑉=nRT
Ideal Gas Law (Physics cool verison) Eq.
PV = NKbT
Heat Rate Eq.
(Q/t) = KAΔ𝑇/L
Conduction
Form of heat transfer; touch (cold hand holding warm hand till equilibrium)
Convection
Form of heat transfer; mass movement of particles (cold hand touching warm hand then letting go, then other cold hand touching warm hand then letting go, then another and another and another)
Radiation
Form of heat transfer; heat exchange by light
Maxwell Boltzman Distribution (shows up on AP test, otherwise useless)
Molecules per velocity VERSUS velocity.
As object gets hotter, peak gets lower and moves further right.
Area is constant thus molecules are.
Amount of Heat Transferred When Temperature Changes Eq.
Q=MCΔ𝑇
Amount of Heat to Change Structure Eq.
Q=ML
Average Kinetic Energy Eq.
½mv2
Average Kinetic Energy of an Ideal Gas Particle Eq.
3/2kbT
Relation Between Energy to Temperature
1/2mv2rms= 3/2kbT
Total Internal Energy of a Ideal Gas
N x KEavg = U OR N x 3/2kbT
0th Law of Thermo
Once the two systems are in equilibrium, they stop exchanging energy
1st Law of Thermo
Energy is conserved
1st Law of Thermo Eq.
Qin+Won=ΔU
Whose your bestest most postive friend?
Qin Won!
Whose your most meanest negative friend?
-Qout-Wby!
Isothermal Means…
Unchanging temperature (ΔT = 0)
Isothermal is a…
VERY slow process
Isothermal Eq.
Qin+Won=0
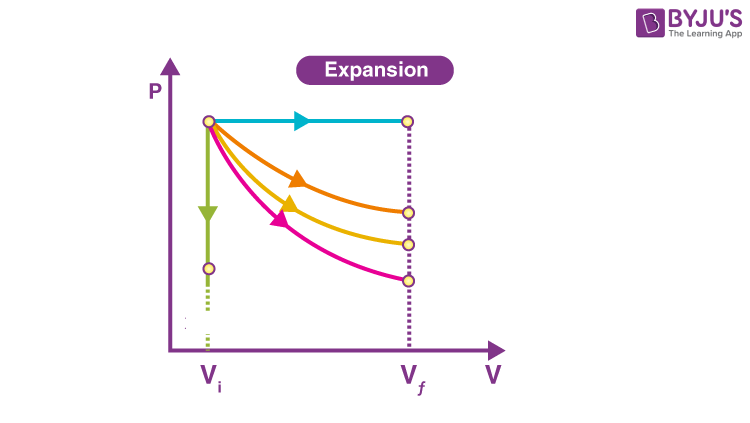
Isothermal Graph is the one in…
Orange
On a Isothermal Graph Check by…
P1V1=P2V2
Adiabatic Means…
Heat is 0 (Qin & Qout = 0)
Adiabatic is a…
VERY fast process (manipulates heat rate equation to have 0 heat added or taken)
Adiabatic Eq.
0 + Won = ΔU
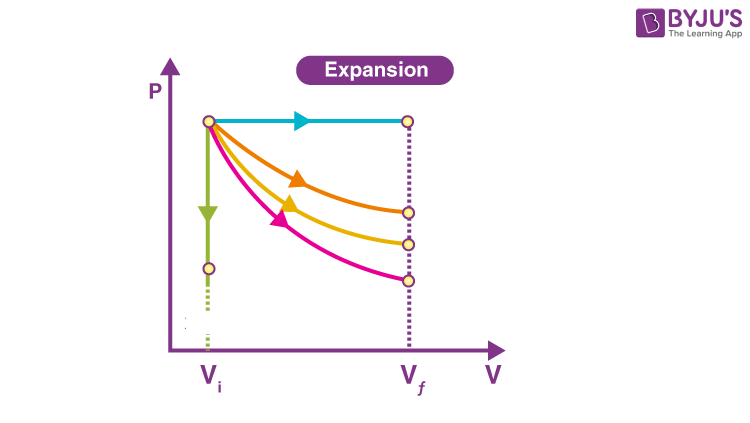
Adiabatic Graph is the one in…
Pink
On a Adiabatic Graph Check by…
NOTHING! P1V1≠P2V2
Isobaric means…
Unchanging pressure (ΔP = 0)
Isobaric Eq.
Qin + Won = ΔU
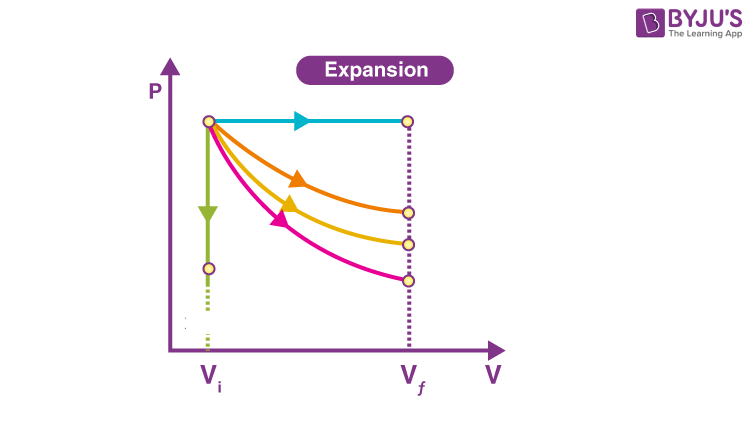
Isobaric Graph is the one in…
Blue
Isochoric means…
(Δ𝑉 = 0); Isovolumetric
Isochoric Eq.
Qin + 0 = ΔU
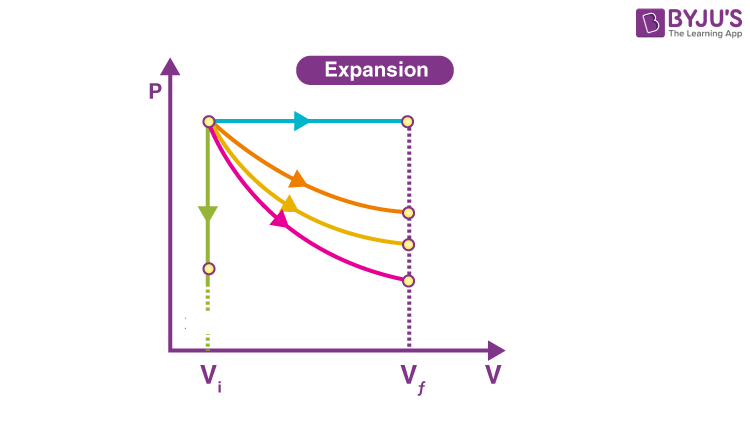
Isochoric Graph is the one in…
Green
What is the Area Under P Versus V Graph?
Work!
How do you tell if it’s Won OR Wby ?
Direction of arrow; left is on, right is by.
2nd Law of Thermo
Entropy always increases—in not isolated, decreases when discussing LOCAL entropy—(always convert some work into heat in a process)
3rd Law of Thermo
You can never reach absolute zero
Maximum Entropy is at…
Thermal equilibrium
Entropy is…
The tendency of energy to spread out
Entropy is Based Off of…
Inital and final states ONLY
An Open System is…
Energy + matter in & out
A Closed System is…
Energy in & out ONLY
An Isolated System is…
Nothing in, nothing out
Efficiency Eq.
(Qhot - Qcold)/Qhot = e
Pressure is…
Same in ALL directions
Change in Total Internal Energy =… (Chemistry)
3/2nRΔT
Change in Total Internal Energy =… (Physics)
3/2NKbΔT