A level Chem 1.1 Atomic Structure
1/44
Earn XP
Description and Tags
Pls rate it 5 stars if you find it helpful :)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
A sample of bromine (Br2) is analysed in a mass spectrometer. The sample is ionised using electron impact ionisation.
Give an equation, including state symbols, for the process that occurs during the ionisation of bromine. (1)
Br₂(g) + e⁻ → Br₂⁺(g) + 2e⁻ (1)
State how the detector enables the relative abundance of each ion to be determined(1)
The (relative) abundance is proportional to the size of the current. (1)
Tellurium has a relative atomic mass of 127.6 Iodine has a relative atomic mass of 126.9 Define relative atomic mass. Suggest one property of tellurium that justifies its position before iodine in the modern Periodic Table. (3)
Definition : Average mass of one atom of an element / 1/12 mass of one atom of carbon 12 (2)
Property- Te has one fewer proton than I (1)
Tellurium has several other isotopes. Two of these isotopes are 126Te and 124Te A different sample of tellurium is analysed using a TOF mass spectrometer. Which statement about kinetic energy (KE) is correct?
The KE of 126Te+ is the same as the KE of 124Te+
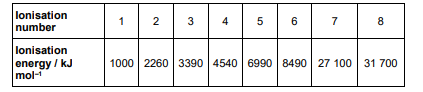
The table below shows some successive ionisation energies for an element in Period 3
Identify the Period 3 element. Explain your answer. (3)
sulfur/ S (1)
large difference between ionisation energy 6 and 7 (1)
electron removed from the second shell which is closer to the nucleus (1)
State how the ions are detected, and how the abundance of each ion is measured, in a TOF mass spectrometer.(2)
(ions hit a detector and) each ion gains an electron (generating a current) (1)
(the abundance is) proportional to (the size of) the current (1)
State the meaning of the term electron impact ionisation. (1)
(High energy) electrons (from an electron gun) are used to knock out an electron (from each molecule or atom.) (1)
Define the term relative atomic mass
average/mean mass of 1 atom (of an element) /1/12 mass of one atom of 12C
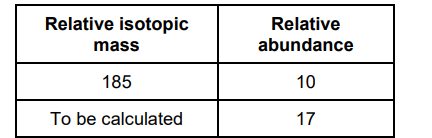
The relative atomic mass of a sample of rhenium is 186.3 The table below shows information about the two isotopes of rhenium in this sample
Calculate the relative isotopic mass of the other rhenium isotope. Show your working.
(185×10) +( X x 17) /27 =187.1 (2)
State how the relative abundance of 185Re+ is determined in a TOF mass spectrometer. (2)
at the detector/(negative) plate the ions/Re+ gain an electron (1)
(relative) abundance depends on the size of the current(1)
Define relative molecular mass (1)
average mass of one molecule x 12 /mass of a 12C (1)
State the equation that links number of particles, no. of moles and avogardro’s constant (1)
N=nL (1)
State the equation that links the mass of one ion, relative isotopic mass and avogardro’s constant
mass of an ion(kg) = relative isotopic mass x 10^-3 / 6.022 x10²³ (1)
What are four factors that affect the size of Ionisation energy?(4)
size of nuclear charge (1)
distance of outer electrons from the nucleus (1)
shielding effect of inner electrons (1)
Spin-pair repulsion (1)
What is spin-pair repulsion ? (1)
It is the repulsive force experienced between two electrons when they’re forced to occupy the same atomic model. (1)
What happens to IE across a period ? (1)
IE increases (1)
What happens to IE down a group? (1)
IE decreases (1)
State the equation in electron impact (1)
X(g) → X+(g) + e⁻ (1)
State the equation in electrospray ionisation (1)
X(g)+H+ → XH+(g) (1)
Name four stages of mass spectrometry (4)
Ionisation (1)
Acceleration (1)
Ion drift (1)
Detection (1)
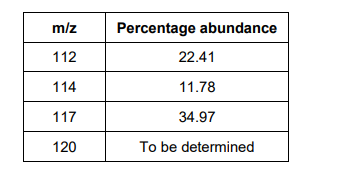
A sample of tin is analysed in a time of flight mass spectrometer. The sample is ionised by electron impact to form 1+ ions. The table below shows data about the four peaks in this spectrum.
Give the symbol, including mass number, of the ion that reaches the detector first. Calculate the relative atomic mass of tin in this sample. Give your answer to 1 decimal place. (4)
112Sn+ (1)
missing abundance = 30.84% (1)
RAM: (112×22.41+114×11.78+117×34.97) (1)
116.5 (1)
What is the electron configuration of V2+ in the ground state?
1s2 2s2 2p6 3s2 3p6 3d3 (1)
A sample of titanium contains four isotopes, 46Ti, 47Ti, 48Ti and 49Ti This sample has a relative atomic mass of 47.8 In this sample the ratio of abundance of isotopes 46Ti, 47Ti and 49Ti is 2:2:1 Calculate the percentage abundance of 46Ti in this sample. (3)
Let the abundance of 49Ti be x
Let the abundance of 48Ti be y.
Since abundances add to 100%,
2x+2x+y=100
5x+y=100
RAM =( (46 × 2x) +(47× 2x) +48y+49x )/100
47.8 = ( (46 × 2x) +(47× 2x) +48y+49x )/100
4780= 235x + 48y
y=100-5x
4780=4800-5x
5x=20
x=4
Abundance of 46Ti=2x=2×4=8%
A sample of chromium containing the isotopes 50Cr, 52Cr and 53Cr has a relative atomic mass of 52.1 The sample contains 86.1% of the 52Cr isotope. Calculate the percentage abundance of each of the other two isotope (4)
% of 50Cr and 53Cr = 13.9%
Let % of 53Cr = x% and Let % of 50Cr=(13.9-x )% (1)
52.1 =( 50(13.9-x) + 52×86.1 + 53x)/100
3x=37.8 (1)
x= % of 53Cr =12.6% (1)
% of 50Cr = 1.3% (1)
The sample of chromium is analysed in a time of flight (TOF) mass spectrometer.
Give two reasons why it is necessary to ionise the isotopes of chromium before they can be analysed in a TOF mass spectrometer. (2)
Ions create a current when hitting the detector (1)
(Ions will interact with and) be accelerated (by an electric field/negative plate) (1)
Describe how the molecules are ionised using electrospray ionisation. (3)
(Sample is) dissolved (in a volatile solvent) (1)
(Injected through) needle at high voltage/positively charged (1)
Each molecule/particle gains a proton/H+(1)
Time of flight (TOF) mass spectrometry is an important analytical technique. A mixture of three compounds is analysed using a TOF mass spectrometer. The mixture is ionised using electrospray ionisation.
The three compounds are known to have the molecular formulas:
C3H5O2N
C3H7O3N
C3H7O2 NS
Give the formula of the ion that reaches the detector first in the TOF mass spectrometer. (1)
C3H5O2N+ (1)
The first ionisation energies of the elements in Period 2 change as the atomic number increases.
Explain the pattern in the first ionisation energies of the elements from lithium to neon. (6)
Stage 1: General Trend (Li → Ne)
1a. 1st IE increases
1b. More protons/increased nuclear charge
1c. Electrons in same energy level / shell
1d. No extra/similar shielding
1e. Stronger attraction between nucleus and outer e OR outer e closer to nucleus
Stage 2: Deviation Be → B
2a. B lower than Be
2b. Outer electron in (2)p
2c. higher in energy than (2)s
Stage 3: Deviation N → O
3a. O lower than N
3b. 2 electrons in (2)p need to pair
3c. pairing causes repulsion (do not award if it is clear reference to repulsion is in s orbital)
Deduce the formula of the compound formed when nitrogen reacts with the Group 2 metal in the same period as nitrogen (1)
Be3N2 (1)
Write an equation, including state symbols, to represent the process that occurs when the third ionisation energy of manganese is measured. (1)
Mn2+(g) → Mn3+(g) + e- (1)
State which of the elements magnesium and aluminium has the lower first ionisation energy. Explain your answer. (3)
Al (1)
(Outer) electron in 3p sublevel/orbital (1)
Higher in energy/further from the nucleus so easier to remove (1)
Chlorine exists as two isotopes 35Cl and 37Cl in the ratio 3:1 Which statement about peaks in the mass spectrum of Cl2 is correct? (1)
Peaks at m/z = 70, 72 and 74 in the ratio 9:6:1 (1)
Use the Periodic Table to deduce the full electron configuration of calcium. (1)
1s22s22p63s23p64s2 (1)
Write an ionic equation, with state symbols, to show the reaction of calcium with an excess of water (1)
Ca(s)+ 2H2O(l) → Ca2+(aq) + 2OH– (aq) + H2(g)
State the role of water in the reaction with calcium. (1)
Oxidising agent (1)
State and explain the trend in the first ionisation energies of the elements in Group 2 from magnesium to barium.
Trend
Explanation (3)
Decrease (1)
Ions get bigger/more (energy) shells (1)
Weaker attraction of ion to lost electron (1)
A sample of the element Q consists of several isotopes. All of the Q+ ions in the sample of Q that has been ionised in a TOF mass spectrometer have the same kinetic energy.
kinetic energy of each ion : 1/2mv2
v=d/t
The time of flight of a 82Q+ ion is 1.243 × 10−5 s.
Calculate the time of flight of the 86Q+ ion. (3)
All the Q+ions have the same kinetic energy, so
1/2m1v1=1/2m2v2
m1v1=m2v2
m1 (d/t1)2 = m2 (d/t2)2- d2 cancels
m1/(t1)2=m2(t2)2
t2=t1√(m2/m1 )
t2=(1.243×10−5)×8286
t21.273×10−5s
A sample of ethanedioic acid was treated with an excess of an unknown alcohol in the presence of a strong acid catalyst. The products of the reaction were separated and analysed in a time of flight (TOF) mass spectrometer. Two peaks were observed at m / z = 104 and 118.
Identify the species responsible for the two peaks.(2)
[CH3OCOCOOH]+(1)
[CH3OCOCOOCH3] +(1)
A sample of ethanedioic acid was treated with an excess of an unknown alcohol in the presence of a strong acid catalyst. The products of the reaction were separated and analysed in a time of flight (TOF) mass spectrometer. Two peaks were observed at m / z = 104 and 118.
Outline how the TOF mass spectrometer is able to separate these two species to give two peaks. (4)
Positive ions are accelerated by an electric field (1)
To a constant kinetic energy (1)
The positive ions with m / z of 104 have the same kinetic energy as those with m / z of 118 and move faster (1)
Therefore, ions with m / z of 104 arrive at the detector first (1)
What happens during stage 2 in Mass spectrometry (2)
Acceleration-The positive ions are attracted to the negatively-charged plate (1)
so they accelerate (1)
What happens in stage 3 of mass spectrometry? (1)
Ion drift- Ions pass through a hole in the negatively-charged plate and move into the flight tube (1)
What happens in stage 4 of mass spectrometry? (3)
Detection - after they have passed through the mass spectrometer,
1+ ions will hit the - charged detector plate (1)
where they gain an electron (1)
a current is produced as ions are discharged (1)
Explain why a M+1 peak can be produced (1)
the presence of heavier and rarer isotopes (1)
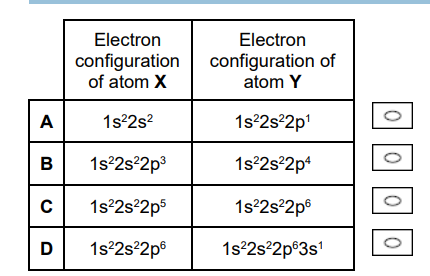
In which pair is the first ionisation energy of atom Y greater than that of atom X? (1)
C (1)
The first ionisation energy of atom Y (1s2 2s22p6, Neon) is greater than that of atom X (1s2 2s22p5, Fluorine) because Neon has a higher nuclear charge and a full, highly stable outer electron shell.
Which of these ions has the largest ionic radius? (1)
A (1)
For an isoelectronic series or elements in the same period, the lower the nuclear charge, the larger the radius.