Chemistry Chapter 4 : Bond energy and Bond length
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
Definition of bond energy
The energy required to break one mole of a particular covalent bond in the gaseous state.
Definition of bond length
Intermolecular distance between the nuclei of two covalently bonded atoms
Bond energy and Bond length
With the increase of atomic radius, bond length increases so bond strength decreases so less energy is required to break the bonds.
Thermal stability of hydrogen halides
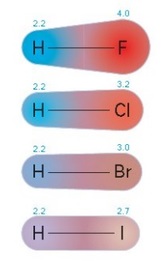
Thermal stability decreases down the group
Atomic radius of halogens increase
Bond length increase
Bond strength decreases
Less energy is required to break the bonds
Surface Tension
Tension is caused by the attraction of particles in the liquid surface
Water molecules on the surface attract sideways and downwards but not upwards
This pulls the water molecules into the liquid and tightens the surface creating surface tension