VSEPR Theory and Molecular geometry
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
VESPR definition
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules based on the repulsion between their electron pairs.
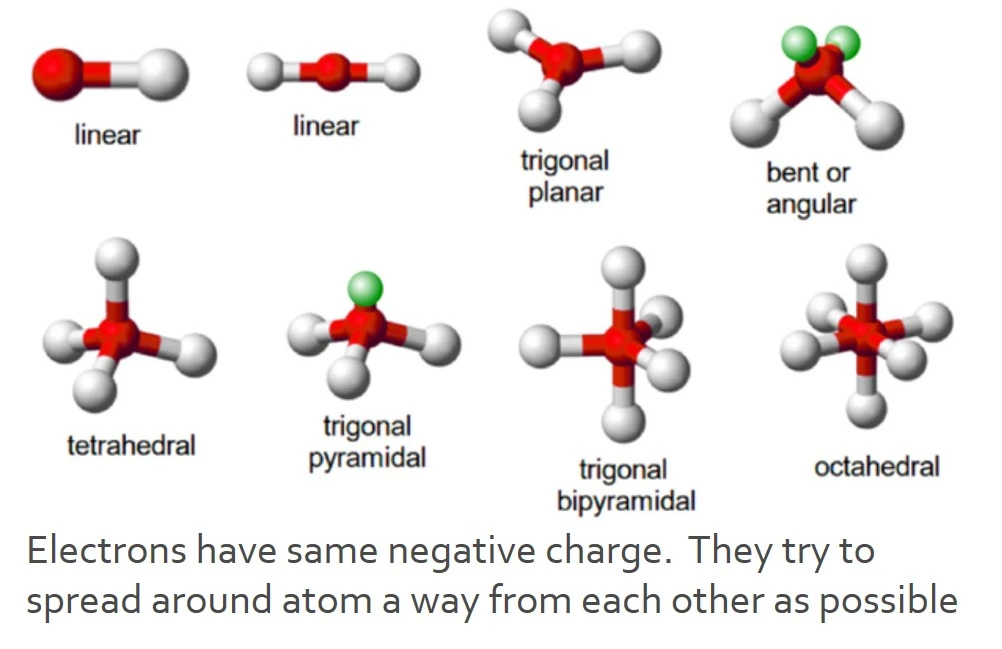
Tetrahedral
Atom with 4 sigma bonds
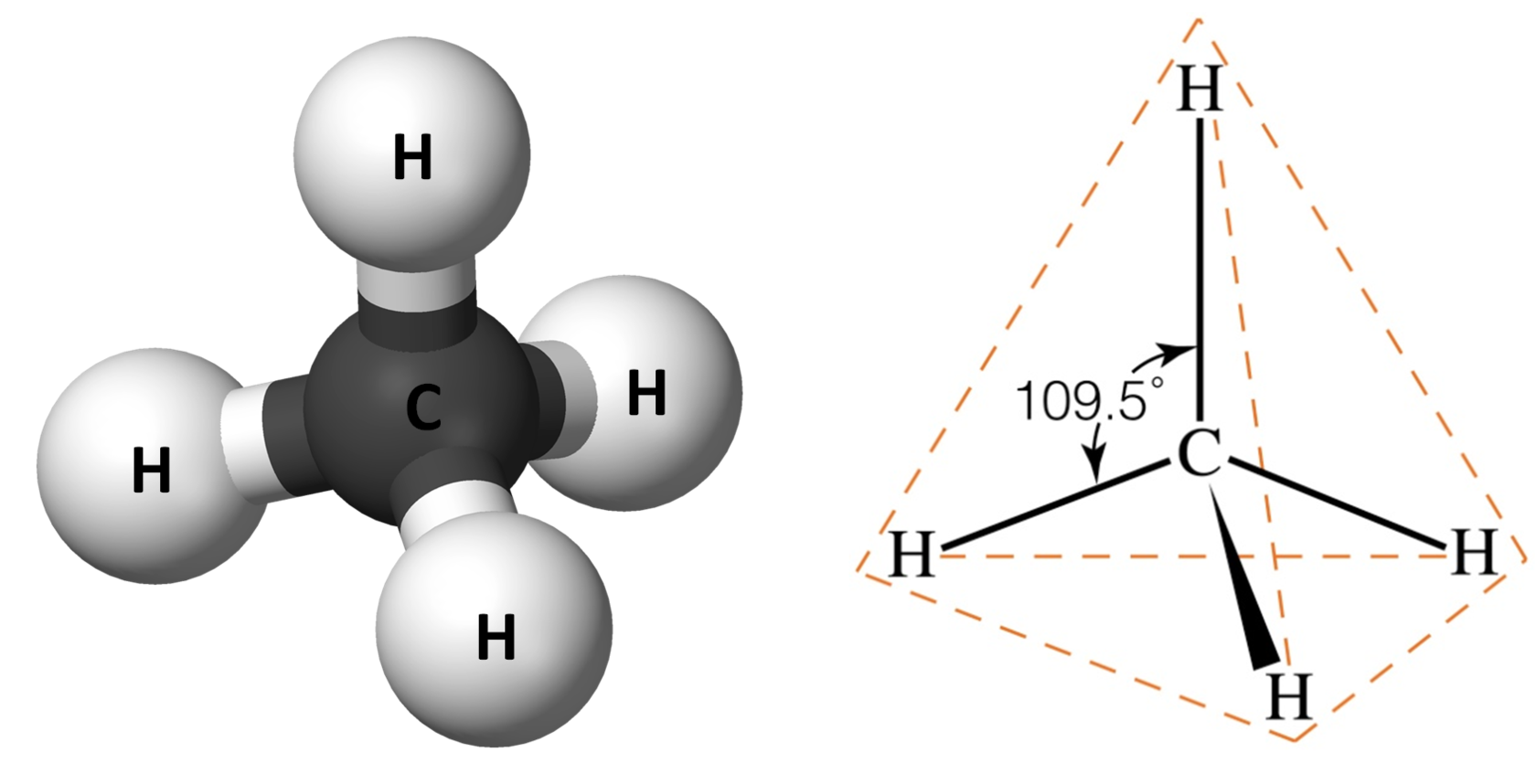
What is the bond angle for a tetrehedral?
109.5 degrees
Trigonal pyrmidal
3 sigma bonds and 1 unshared pair
-smaller because unshared electron pairs exert more repulsion than shared pairs
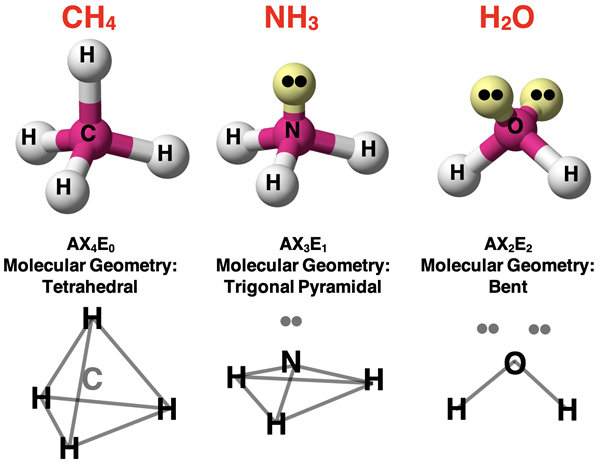
Bond angle for trigonal pyramidal
107 degrees
Bent structure
-2 sigma bonds
-2 unshared pairs
Bond angle of bent structures
104.5 degrees
Trigonal planar
3 sigma bonds
bond angle for trigonal planar
120 degrees
Angular
2 sigma bonds
1 unshared pair
-117 degrees
Linear bonds
2 sigma bonds
180 degrees
Trigonal bypyrmidal
-90 and 120 degrees
-5 sigma bonds
octahedral
-6 sigma bonds
-90 degrees