mass spectrometer and atomic mass
1/3
Earn XP
Description and Tags
10/2/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
purpose of mass spectrometer
a sample is heated, vapourized and ionized
travels down to the detector
calculated force of the magnets that bend the sample
based on the magnetic force you know the masses and you know the relative amount of the
isotope
Each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element
how to determine average atomic mass?
Calculate average of the mass of the isotopes
Multiply masses by percentage
add them up
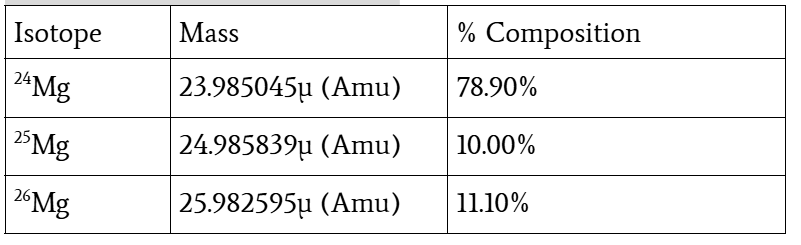
Determine the average atomic mass of a sample of magnesium based on the percent composition data in the picture
MAvg = (CMg-24 x MMg-24) + (CMg-25 x MMg-25) + (CMg-26 x MMg-26)
= (0.7890)(23.98504 amu) + (0.1000)(24.985839 amu) + (0.1110)(25.982595 amu)
= 24.306852 amu
= 24.31 amu