Exo/Endothermic reactions and Bond energies
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Exothermic reaction
A reaction that releases energy to surroundings
Example of an exothermic reaction
Combustion
Endothermic reaction
A reaction that takes in energy from its surroundings
Example of an endothermic reaction
Melting with a Bunsen burner
Activation energy
The minimum amount of energy the reactant particles need to collide with each other and react
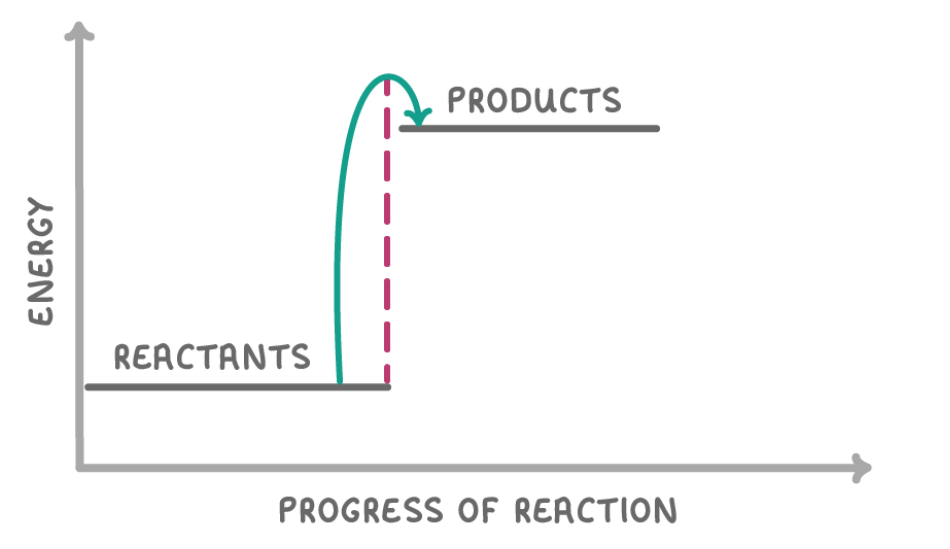
What type of reaction is this?
Endothermic reaction
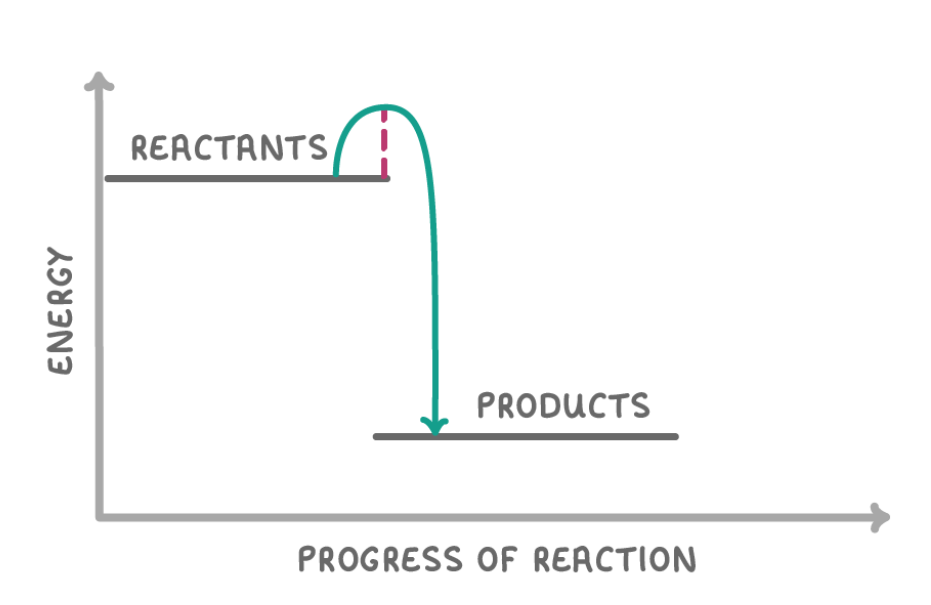
What type of reaction is this?
Exothermic reaction
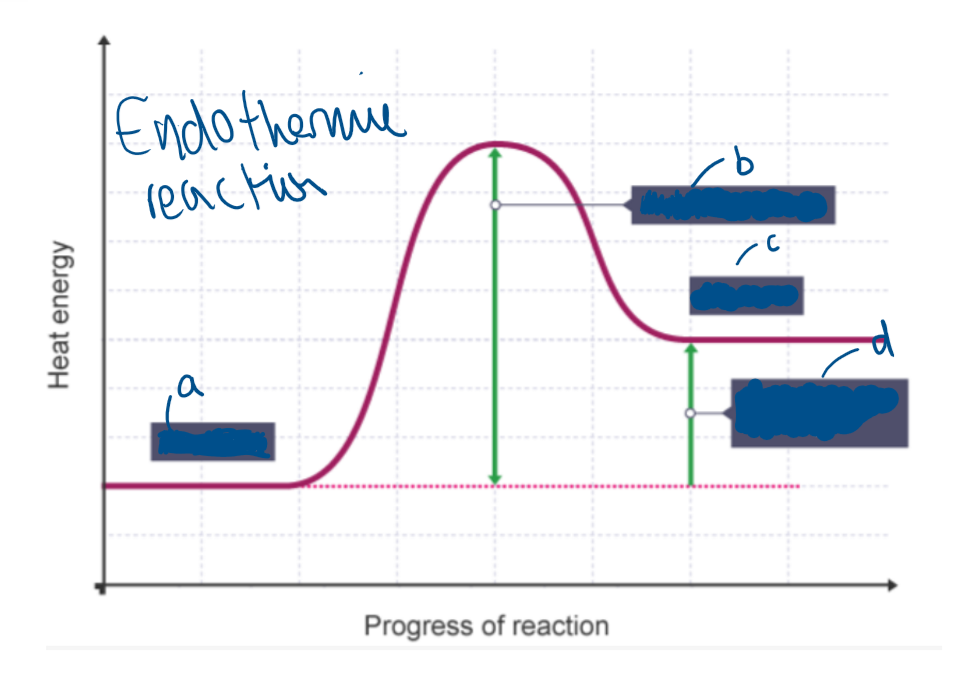
Label A
Reactants
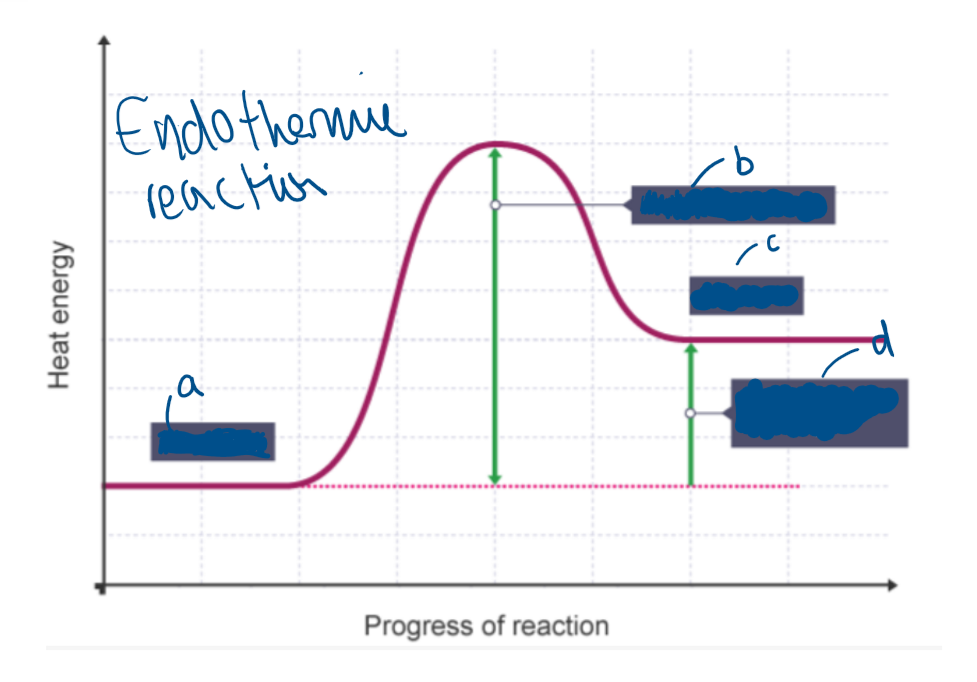
Label B
Activation energy
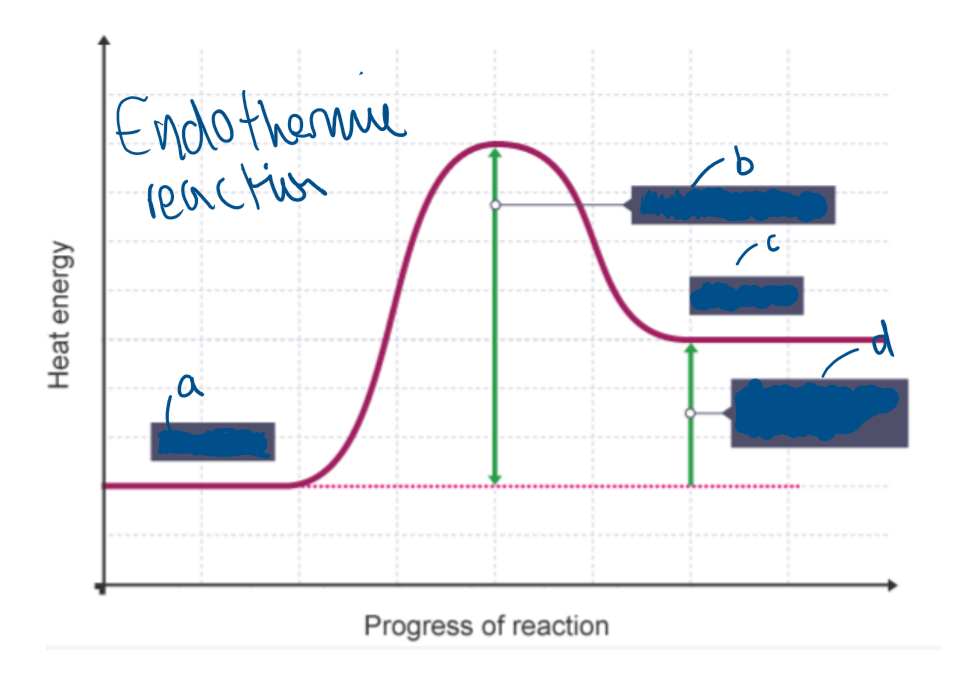
Label C
Products
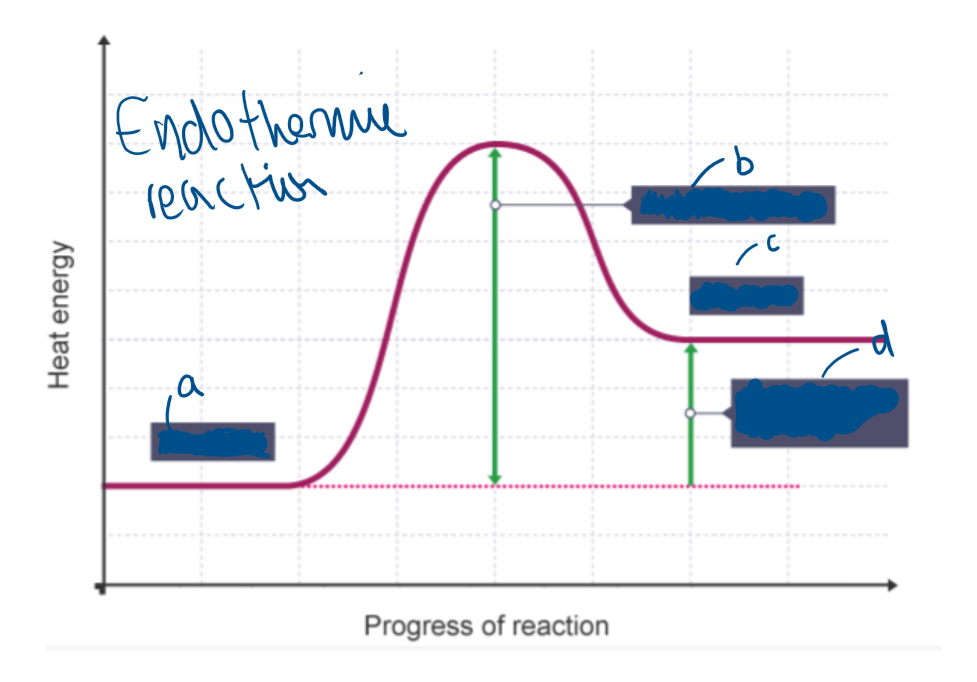
Label D
Overall change in energy
Bond energies definition
the amount of energy required to break one mole of a particular covalent bond
How much 1 mole is
Avogadro’s constant: 6.02×10²³
What bond breaking is in terms of energy
It is an endothermic process
What bond making is in terms of energy
It is an exothermic process
How to determine whether a reaction is exothermic or endothermic
Compare the total amount of energy released when the bonds form, to the total amount of energy required to break the bonds
How to calculate the overall energy change of a reaction
energy required to break bonds (reactants) - energy released by forming bonds (products)
What a negative energy change means
That it has lost energy so the reaction is exothermic
What does a positive energy change mean
It had gained energy, so the reaction is endothermic