Chapter 14: Antimicrobial drugs
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
Early 1900s- Paul Ehrlich and his assistant Sahachiro Hata
Found compound 606- killed Treponema pallidum sold under the name of Salvarsan
1928 Alexander Fleming
Discovered penicillin, the first natural antibiotic
1930s Klarer, Mietzsch, and Domagk
Discovered prontosil-killed streptococcal and staphylococcal infections
-the active breakdown product of prontosil is sulfanilamide
-sulfanilamide was the first synthetic antimicrobial created
Early 1940s Dorthy Hodgkin
Determined the structure of penicillin using x-rays
-scientists could then modify it to produce semisynthetic penicillins
1940s Selman Waksman’s research team
discovered several antimicrobials produced by soil microorganisms
chemotherapeutic agent
drug is any chemical agent used in medical practice
antibiotic agent
usually considered to be a chemical substance made by a microorganism that can inhibit the growth or kill microorganisms
antimicrobic or antimicrobial agent
a chemical substance similar to an antibiotic, but may be synthetic
Antibiotic
usually one bacterial target; e.g. a key bacterial enzyme is blocked
Antimicrobial
a broad term but often can mean multiple targets; e.g. membranes and DNA
Selective toxicity
harms microbes but not damaging the host
Chemotherapeutic index
maximum tolerable dose per Kg of body weight which cures the disease
Spectrum of Activity
▪ No single chemotherapeutic agent affects all microbes.
▪ Antimicrobial drugs are classified based by the type of organism they affect (ex: antibacterial, antifungal, etc).
▪ Even within a group, one agent may have a narrow spectrum of activity, whereas another may affect many species.
Spectrum of antimicrobial activity
▪ Narrow spectrum - targets only specific subsets of bacterial pathogens
▪ Broad spectrum – targets a wide variety of bacterial pathogens – including Gram-positive and Gram-negative species.
Development of Superinfections
normal microbiota keeps opportunistic pathogens in check
broad-spectrum antibiotics kill nonresistant cells
drug-resistant pathogens proliferate and can cause superinfection
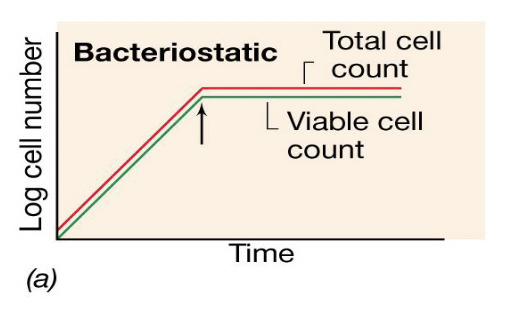
Bacteriostatic antibiotic activity graph
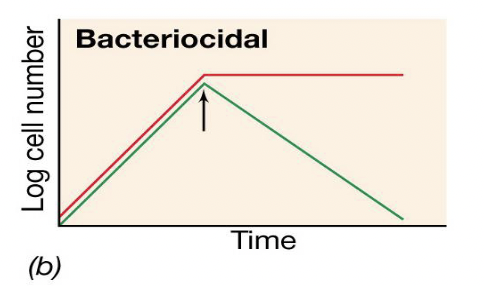
Bactericidal antibiotic activity graph
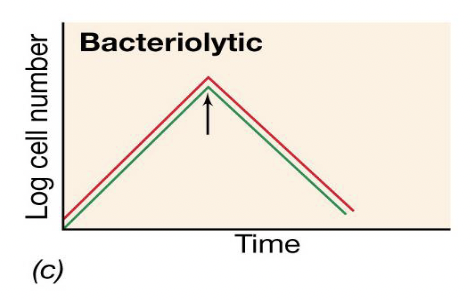
Bacteriolytic antibiotic activity graph
minimal inhibitory concentration (MIC)
the lowest concentration of the drug that will prevent the growth of an organism
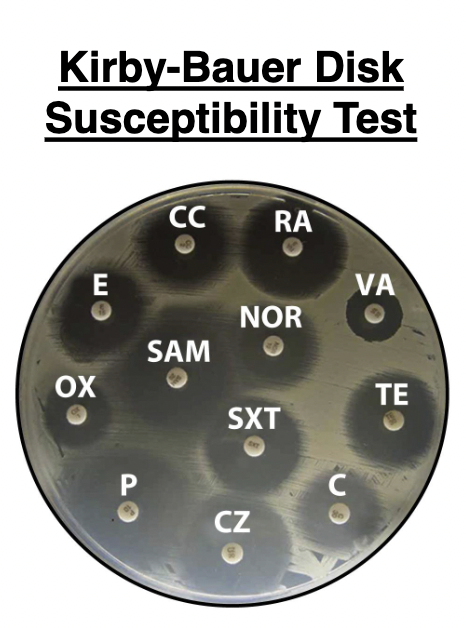
Kirby-Bauer Disk Susceptibility Test
Series of round filter paper disks impregnated with different antibiotics. A dispenser delivers up to 12 disks simultaneously to the surface of an agar plate covered by a bacterial lawn
The standard medium used is Mueller-Hinton agar. During incubation, the drugs diffuse away from the disks into the surrounding agar and the diameter of the zone of inhibition can be measured to determine drug susceptibility.
E-test
commercially prepared strip that produces a gradient of antibiotic concentration (μg/ml) when placed on an agar plate. The MIC corresponds to the point where bacterial growth crosses the numbered strip
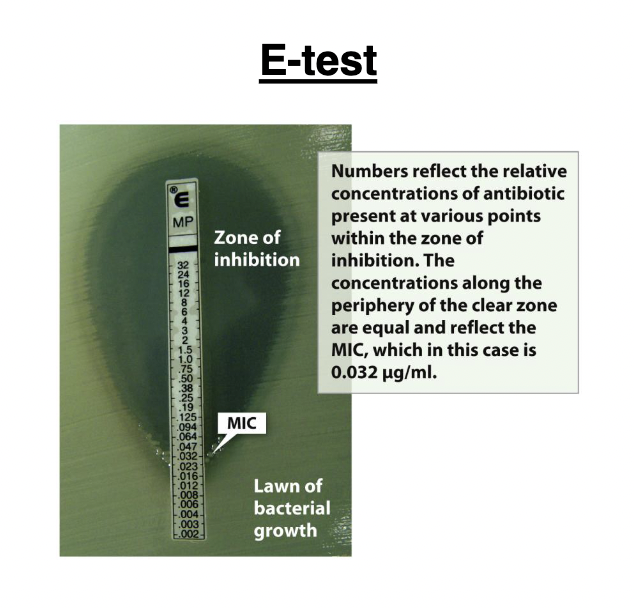
minimum bactericidal concentration (MBC)
determined by using a tube dilution test and removing
the antibiotic
**IIf cells grow in the fresh medium without antibiotic, the drug is bacteriostatic; if cells do not grow, the drug is bactericidal.
Attributes to an ideal antimicrobial
1. Solubility in Body fluids.
2. Selective toxicity
3. Toxicity not easily altered
4. Non-allergenic
5. Stability
6. Resistance by microorganisms not easily
acquired
7. Long shelf-life
8. Reasonable cost
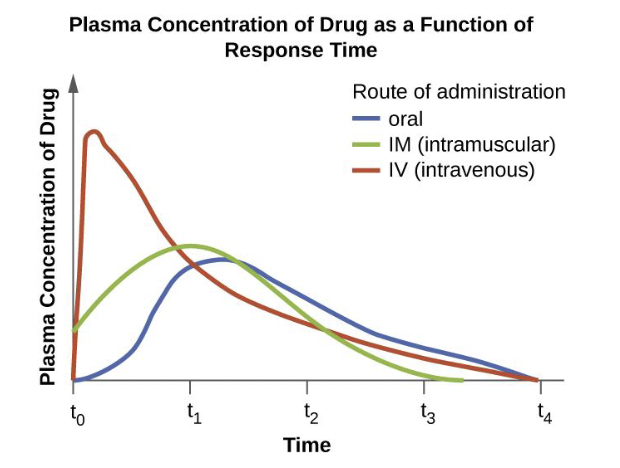
Dosage
amount of medication given during a certain time interval
▪ In children, dosage is based upon the patient’s mass
▪ In adults a standard dosage is used, independent of mass
Half- life of the antibiotic
Rate at which 50% of a drug is eliminated from the plasma
therapeutic dose
minimum dose per kg of body weight that stops pathogen growth
toxic dose
maximum dose tolerated by the patient
chemotherapeutic index
The ratio of the toxic dose to therapeutic dose; the higher the chemotherapeutic
index, the safer the drug
Synergistic drugs
may work poorly when they are given individually but very well when combined (combined effect is greater than additive effect). example: aminoglycoside + vancomycin
antagonistic drugs
mechanisms of action of the drugs interfere with each other and diminish their effectiveness.
example: penicillin + macrolides
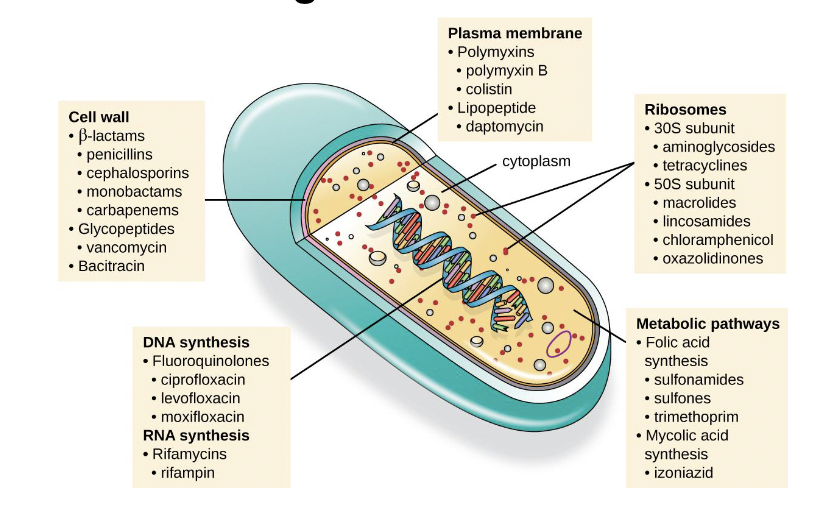
Mechanisms of Antibiotics
• cell wall synthesis
• cell membrane integrity
• DNA synthesis
• RNA synthesis
• protein synthesis
• metabolism
Penicillins
•affect cell wall
The enzymes that attach the disaccharide units to preexisting peptidoglycan and produce peptide cross links are collectively called penicillin-binding proteins (PBPs).
• Without an intact cell wall, the growing cell eventually bursts due to osmotic effects.
• It is a bactericidal drug
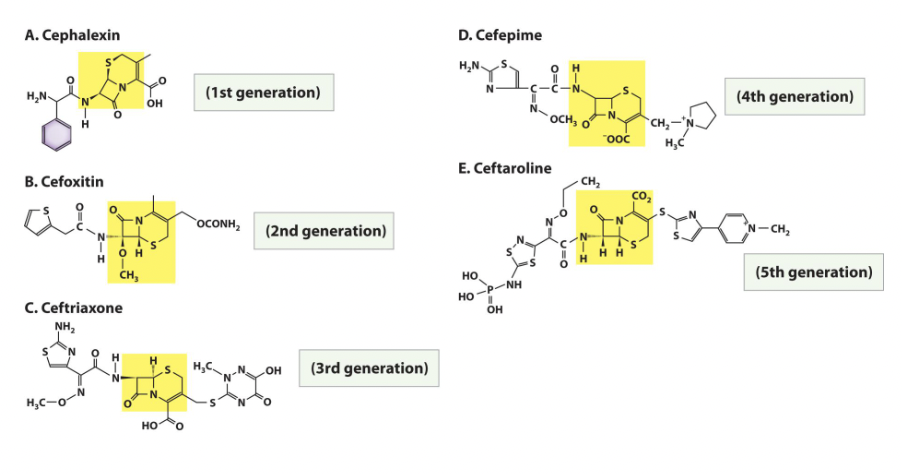
Cephalosporins
• Beta-lactam antibiotic (affecting cell wall) originally discovered in nature but modified in the laboratory, a type of semisynthetic drug.
• Chemists have modified the basic structure of cephalosporin in ways that improve the drug’s effectiveness against penicillin-resistant pathogens.
• Each modification is a new “generation” of cephalosporins. There are currently 5 generations of this antibiotic.
Inhibitors of cell wall synthesis
Polypeptide antibiotics
Bacitracin
-Topical application
- Against gram-positives
Vancomycin
-Glycopeptide
-Important “last line” against antibiotic-resistant
-S. aureus
Antimycobacterial antibiotics
Isoniazid (INH)
- Inhibits mycolic acid synthesis
Ethambutol
-Inhibits incorporation of mycolic acid
Microbial Resistance to cell wall inhibiting antibiotics
Ex. penicillin with beta-lactamase
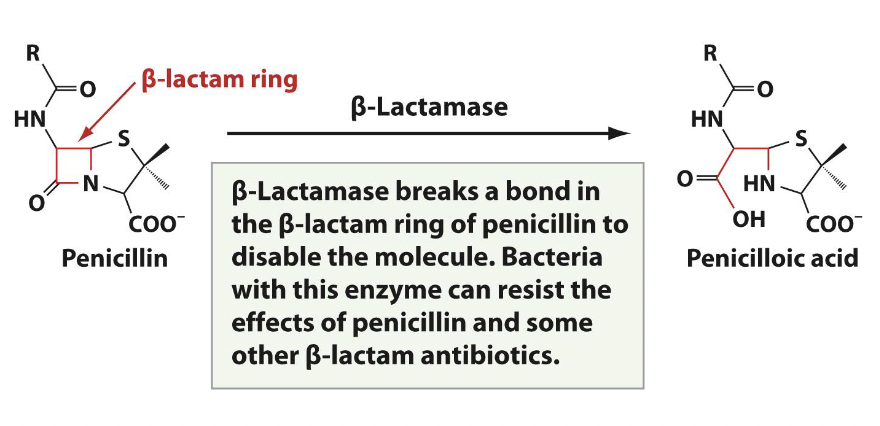
Antibiotics that target the bacterial membrane
polymyxin (polypeptide antibiotics that has positive charge and attaches to gram negative bacteria, narrow spectrum)
tyrocidin (used for skin infections)
platansimycin (produced by streptomycin, broad spectrum)
gramicidin (cyclic peptide)
Polymyxin, Tyrocidin and Platansimycin
• Act as detergents and disrupt the structure of the cell membrane by binding to the phospholipids.
• Mode of action: Interacts with lipopolysaccharide in the outer membrane of gram- negative bacteria, killing the cell through the eventual disruption of the outer membrane and cytoplasmic membrane
• Highly toxic
Gramicidin
cyclic peptide
mode of action: Inserts into the
cytoplasmic membrane of gram- positive bacteria, disrupting the membrane and killing the cellmakes membrane porous and forms cation channel which hydrogen, sodium and potassium ions can flow through
disrupts ion gradient
Antibiotics that affect DNA Synthesis and Integrity
metronidazole
sulfonamides
quinolones
Metronidazole
activated after being metabolized by microbial protein cofactors ferredoxin found in anaerobic and microaerophilic bacteria such as Bacterioides and Fusobacterium.
• Aerobic microbes are resistant because they do not possess the electron transport proteins capable of reducing metronidazoleused orally
used for trichomonas vaginalis
narrow spectrum
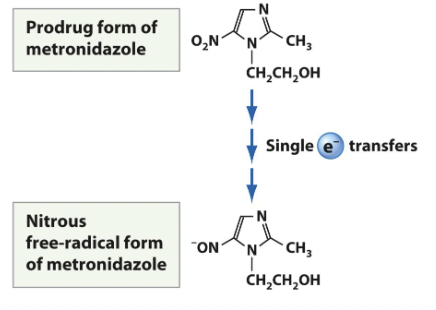
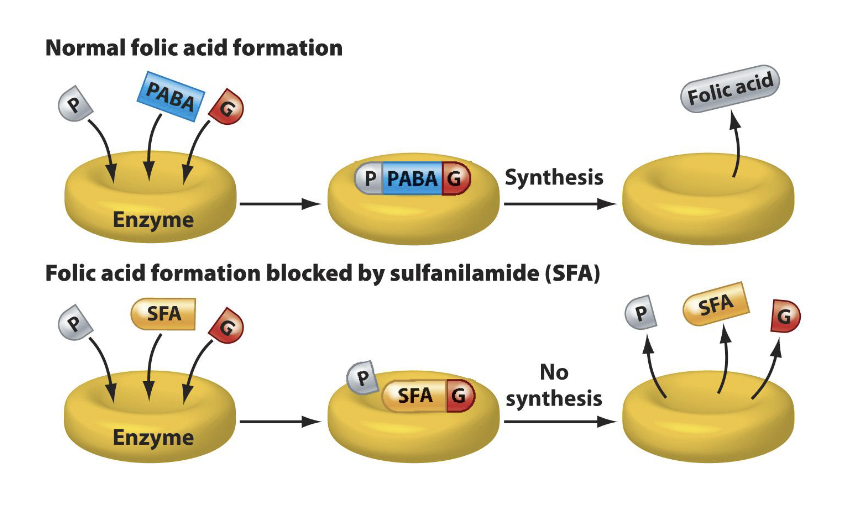
Sulfonamides
Sulfonamide (sulfa) drugs act to inhibit the synthesis of nucleic acids by preventing the synthesis of folic acid, an important cofactor in the synthesis of nucleic acid precursors.
All organisms use folic acid to synthesize nucleic acids. Bacteria make folic acid from the combination of PABA, glutamic acid, and pteridine.
Mammals do not synthesize folic acid and must get it from the diet or microbes
Quinolones
DNA gyrase bound to and inactivated by a quinolone will block progression of a DNA replication fork
• Because bacterial DNA gyrases are structurally distinct from their mammalian counterparts, quinolone antibiotics will not affect mammalian DNA replication
Rifampin
RNA synthesis inhibitor
best-known member of the rifamycin family of antibiotics that selectively binds to bacterial RNA polymerase and prevents transcription.
• Rifampin is also used to treat tuberculosis and meningococcal meningitis
Two classes of antibiotics that Inhibit Protein Synthesis
The major classes of protein synthesis inhibitors target the 30S or 50S subunits of cytoplasmic ribosomes.
Drugs that affect the 30S ribosomal
subunit
aminoglycosides
tetracyclines
glycylcyclines
aminoglycosides
Cause misreading of mRNA and inhibit peptidyl- tRNA translocation
Ex. streptomycin, gentamicin, tobramycin
tetracyclines
Bind to the 30S subunit and prevent tRNAs
carrying amino acids from entering the A
site
Ex. doxycycline
glycylcyclines
Bind to 30S subunit and inhibit the entry of
aminoacyl-tRNA into the A site; able to function in tetracycline resistant cells.
Ex. tigecycline
Drugs that affect the 50S ribosomal
subunit
chloramphenicol
macrolides
lincosamides
oxazolidinones
streptogramins
chloramphenicol
Prevents peptide bond formation by inhibiting peptidyltransferase in the 50S subunit
macrolides
Bind to 50S subunit and inhibit translocation of tRNA from the A site to the P site.
ex.) erythromycin, azithromycin, clarithromycin
lincosamides
Bind to peptidyltransferase and prevents peptide bond formation from the ribosome
Ex. clindamycin
oxazolidinones
Bind to 50S subunit and prevent assembly of the 70S ribosome
streptogramins
Bind to 50S subunit and block tRNA entry into the A site while blocking exit of a growing protein from the ribosome.
ex.) quinupristin, dalfopristin
mechanisms of drug resistance
1. Drug modification or Inactivation- By enzymes
2. Blocked penetration- Altering porins in the outer membrane
3. Efflux pumps- Altering porins in the outer membrane
4. Target modification- Mechanism allows a formerly inhibited reaction to occur Mechanisms of drug resistance
5. Target overproduction- microbe overproduces the target enzyme such that there is a sufficient amount of antimicrobial- free enzyme to carry out the proper enzymatic reaction.
6. Enzymatic bypass- microbe develops a bypass that circumvents the need for the functional target enzyme
7. Target mimicry- production of proteins that bind and sequester drugs, preventing the drugs from binding to their target.
How to fight drug resistance
A.) Dummy target compounds that inactivate resistance enzymes have been developed.
ex: Clavulanic acid binds and ties up beta-lactamases secreted from penicillin resistant bacteria.
B.) Individuals can take the following actions to help:
• frequent hand washing
• vaccinations
• avoiding use of antibiotics for viral infections
• refusing leftover antibiotics
• take full course of antibiotics prescribed
Two components in the influenza virus
hemagglutinin
neuraminidase
Hemagglutin
binds to the host membrane receptors for entry by phagocytosis
Neuraminidase
cleaves sialic acid to allow virus particles to escape from infected cells
Amantadine
antiviral agent that prevents the virus from uncoating and exiting by changing the pH of the phagolysosome
Oseltamivir (Tamiflu) and zanamivir (Relenza)
neuraminidase inhibitors prevent the virus particles from leaving the cell
Nucleoside and Nonnucleoside Reverse Transcriptase Inhibitors
protease inhibitors
entry inhibitors
HIV treatment regimens
Protease inhibitors
target the HIV protease enzyme (Nelfinavir (Viracept) and lopinavir (Kaletra))pt) and lopinavir (Kaletra)
Entry inhibitors
block virus envelope protein gp120 from binding to the host receptor CCR5 (CCR5 inhibitors (maraviroc), as a result, the virus never attaches)
HIV treatment regimens
HAART (highly active antiretroviral therapy) involves administering combinations of three or more antiretroviral drugs
Available antifungal agents
polyenes
azoles
allylamines
echinocandins
griseofulvin
flucytosine
polyenes
(nystatin, amphotericin B) - Disrupts
membrane integrity
azoles
(imidazoles, triazoles) - Interferes with ergosterol synthesis
allylamines
(terbinafine, lamisil) - Interferes with
ergosterol synthesis
echinocandins
(caspofungin) - Blocks fungal cell wall
synthesis
Griseofulvin
Blocks cell division
Flucytosine
Inhibits DNA synthesis
Antiprotozoan agents
**most are fairly toxic
-metronidazole
-quinine
-chloroquinine, primaquine
Metronidazole
causes DNA breakage; Used to treat
giardisis and Trichomonas infections
Quinine
commonly used in the past, now used as a last resort to treat malaria
chloroquinine, primaquine (antimalarials)
interfere with protein synthesis, specially red-blood cells
Antihelminthic agents
niclosamide
praziquantel
Mebendazole and albendazole
Ivermectin
Niclosamide
Prevents ATP generation (tapeworms)
Praziquantel
Alters membrane permeability (Flatworms)
Mebendazole and albendazole
Interfere with nutrient absorption (Intestinal roundworms)
Ivermectin
Paralysis of helminths (Intestinal roundworms)