fuels and heats - ethyne experiment
0.0(0)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
1
New cards
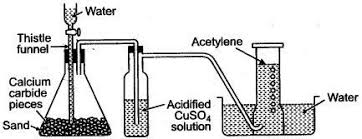
diagram for preparation of ethyne
CaC2 + 2H2O → Ca(OH2) + C2H2
2
New cards
observation
calcium carbide is a grey-black solid
fizzing takes place in the buchner flask
bubbles of gas start to collect in the glass jar
3
New cards
procedure
test tubes are collected
first test tube is left because it has too much air
4
New cards
tests on ethyne: physical properties
colourless gas with sweet smell
5
New cards
combustion
jar is in a fume cupboard
lighted taper - ethyne burns with a luminous smoky flame and soot is formed
6
New cards
addition of bromine
addition of bromine water
colour of bromine water changes from red to colourless
7
New cards
adition of potassium permangante
added to the test tube
potassium permanganate turns from purple to colourless