Chapter 13: Temperature, Kinetic Theory, and the Gas Laws
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
absolute zero
the lowest possible temperature; the temperature at which all molecular motion ceases
Avogadro’s number
the number of molecules or atoms in one mole of a substance (units are particles/mole)
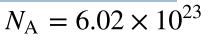
Boltzmann Constant
a physical constant that relates energy to temperature
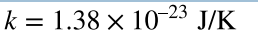
Celsius Scale
temperature scale in which the freezing point of water is 0C and the boiling point of water is 100C
coefficient of linear expansion (a)
the change in length, per unit length, per 1C change in temperature; a constant used in the calculation of linear expansion; the coefficient of linear expansion depends on the material and to some degree on the temperature of the material
coefficient of volume expansion (B)
the change in volume, per unit volume, per 1C change in temperature
critical point/temperature
the temperature above which a liquid cannot exist
critical pressure
the minimum pressure needed for a liquid to exist at the critical temperature
Dalton’s Law of Partial Pressures
the physical law that states that the total pressure of a gas is the sum of partial pressures of the component gases
degree Celsius
unit on the Celsius temperature scale
degree Fahrenheit
unit on the Fahrenheit temperature scale
dew point
the temperature at which relative humidity is 100%; the temperature at which water starts to condense out of the air
Fahrenheit scale
temperature scale in which the freezing point of water is 32F and the boiling point of water is 212F
ideal gas law
the physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas
Kelvin Scale
temperature scale in which 0 K is the lowest possible temperature, representing absolute zero
mole
the quantity of a substance whose mass (in grams) is equal to its molecular mass
partial pressure
the pressure a gas would create if it occupied the total volume of space available
percent relative humidity
the ratio of vapor density to saturation vapor density
phase diagram
a graph of pressure vs. temperature of a particular substance, showing at which pressures and temperatures the three phases of the substance occur
PV diagram
a graph of pressure vs. volume
relative humidity
the amount of water in the air relative to the maximum amount the air can hold
saturation
the condition of 100% relative humidity
sublimation
the phase change from solid to gas
temperature
the quantity measured by a thermometer
thermal energy
the average translational kinetic energy of a molecule
thermal equilibrium
the condition in which heat no longer flows between two objects that are in contact; the two objects have the same temperature
thermal expansion
the change in size or volume of an object with change in temperature
thermal stress
stress caused by thermal expansion or contraction
triple point
the pressure and temperature at which a substance exists in equilibrium as a solid, liquid, and gas
vapor
a gas at a temperature below the boiling temperature
vapor pressure
a gas at a temperature below the boiling temperature
zeroth law of thermodynamics
law that states that if two objects are in thermal equilibrium, and a third object is in thermal equilibrium with one of those objects, it is also in thermal equilibrium with the other object