Chem test 3 - Light and Electrons
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
electromagnetic radiation speed
3.00 x 10^8 m/s - it all moves at the same speed
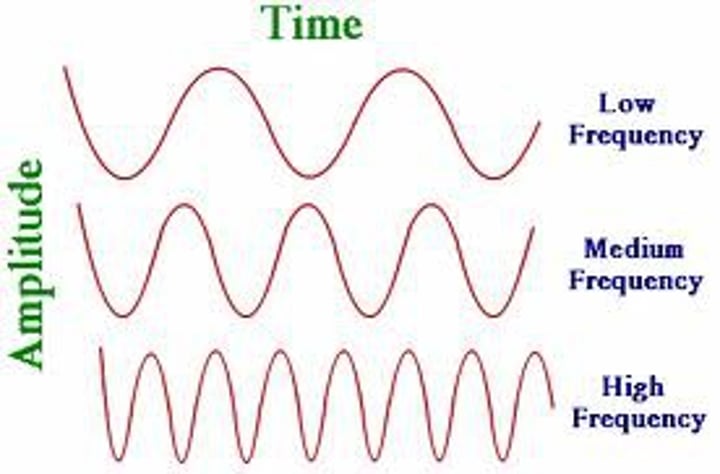
frequency
the number of complete wavelengths that pass a point in a given time
The energy of visible light
low energy - high energy
red - violet
The Photoelectric Effect
The emission of electrons from a material when high frequencies of light (orange-violet) shine on the surface of the material
dual nature of matter
matter can have properties of both a particle and a wave
- light can act + others
- the more massive the object, the smaller its wavelength
wave nature of electrons
- Tiny, fast-moving particles also behave as waves (this explains electron energy levels) proved by interference pattern vs two lines
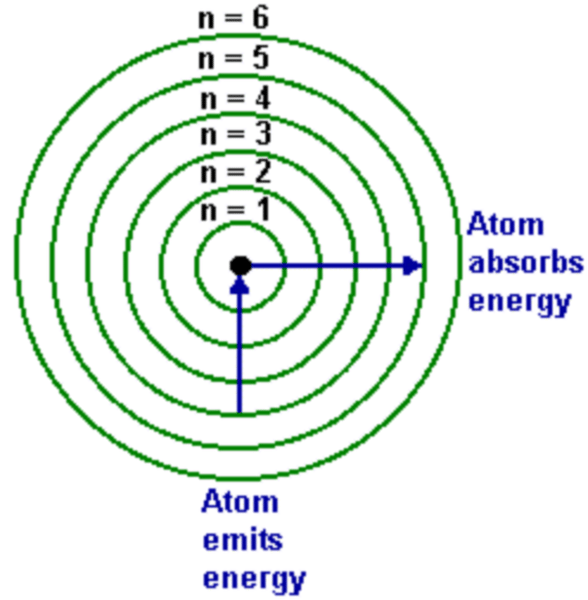
Bohr Energies model
- each level of energy gets closer to the one before it
- there are an infinite number of levels but they eventually get so close its hard to tell them apart
energy absorption
if an electron that's not charged gets struck by a photon with the right energy it jumps to a higher, possible state, and then falls releasing a photon with energy equivalent to the jump/fall made
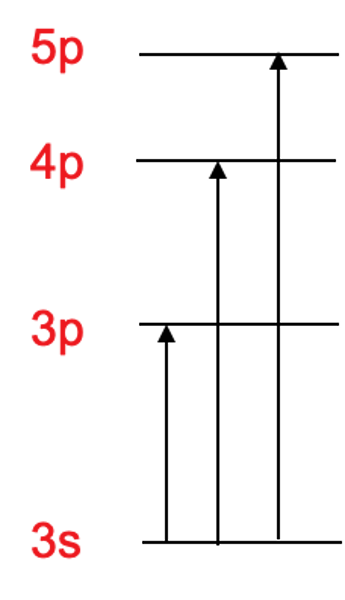
energy emission
after an atom is excited it returns down to its ground state, it emits a photon of energy in the form of electromagnetic radiation (light) that correlates with the size of energy/jump ex: big jump+energy = high energy radiation like UV
the smaller the energy...
the higher/longer the wavelength
light emission spectrum (bohr)
pattern of lines formed - each spectral line corresponds to a different electron transition from a higher to lower energy state - different atoms give certain colors based on electrons orbits - different charges gives different locations of electron orbits
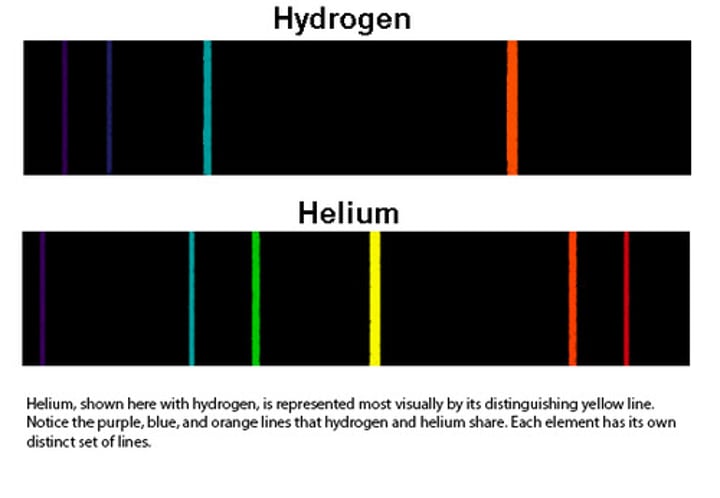
limitations of planetary model
electrons don't orbit like planets, they have regions in which they are likely to exist
Orbitals/region of probability
regions around the nucleus in which given electron or electron pair is 90% likely to be found - but can be anywhere/is everywhere
Superposition
electrons are mathematically everywhere until you search for it and its in one place (sports game has all possible outcomes until you watch the game play out)
valance electrons
the number of electrons in the outermost shell
the # of orbitals is _____ and # of electrons are
the rings/orbitals squared and the orbitals^2 x 2
exceptions to electron configuration
Cr
expected: [Ar] 4s^2 3d^4 actual: [Ar] 4s^1 3d^5
Cu
expected: [Ar] 4s^2 3d^9 actual: [Ar] 4s^1 3d^10
![<p>Cr<br>expected: [Ar] 4s^2 3d^4 actual: [Ar] 4s^1 3d^5<br>Cu<br>expected: [Ar] 4s^2 3d^9 actual: [Ar] 4s^1 3d^10</p>](https://knowt-user-attachments.s3.amazonaws.com/33519d2d-efed-461e-9350-112bea51bb11.image/png)
periods on periodic table
horizontal rows
families or groups on the periodic table
vertical columns (skipping d block)
The Octet Rule
Atoms gain or lose electrons to obtain 8/full outer shell/a charge of 0 + metals loose electrons & gasses gain electrons
alkali metals
- group 1 of the periodic table
- extremely reactive
- very soft
- reacts strongly with water
- have one valence electron (will often lose to form +1 ions)
alkaline earth metals
- group 2 on the periodic table
- very reactive but less then alkali metals (cannot be found in nature)
- soft, but less then alkali
- have two valence electrons (will often lose +2 ions)
transition metals
- groups 3-12 (d block) on periodic table
- less reactive then 1-2
- stronger, harder, high melting points than groups 1-2; what u think of metals
- most have 1-2 valence electrons (variety of charges +1-+3 bc d block)
Lanthanides and Actinides
- groups (f block)
- inner transitional metals
- lanthanides are reactive, silvery metals (row4)
- actinides are all radioactive (row5)
Halogens
- Group 17 of the periodic table
- very reactive non metals
- mostly found diatomic (together) in nature (F2)
- Have 7 valence electrons (will often gain 1 to form -1 ions)
noble gases
- Group 18 of the periodic table
- mostly unreactive
- full valence shells (do not form ions)
- all are gasses (unless veryyyyyy cold)
Properties of metals
- Malleable (can be bent and molded)
-Ductile (can be drawn into wires)
-Have luster (shiny)
-High melting points (mostly solids at room temperature, except mercury)
-Good at conducting heat and electricity
-High density
-Often react with acids
-Typically lose electrons to form positive ions
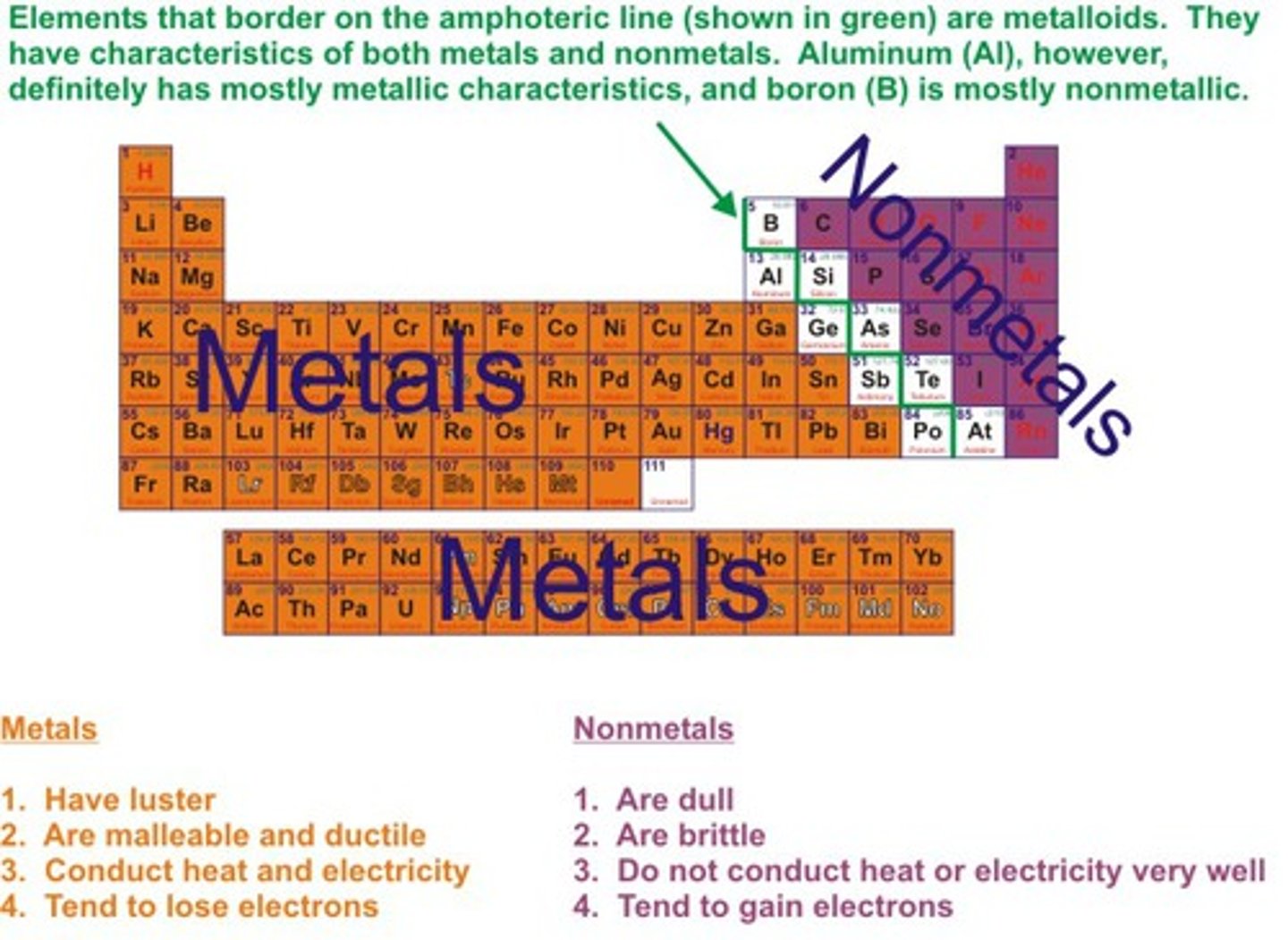
The metals in the periodic table are _______
left of staircase (not touching)
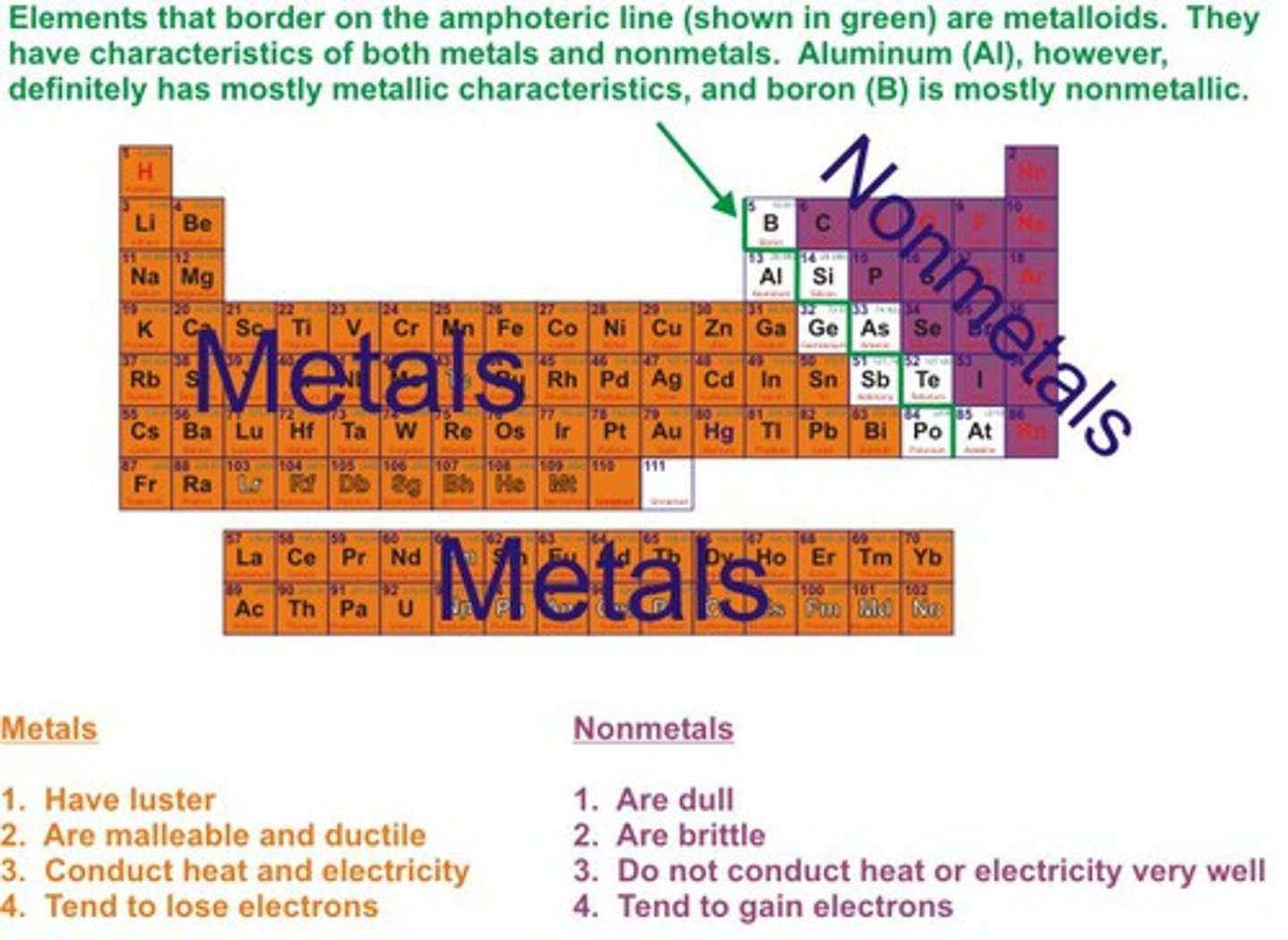
properties of nonmetals
-Brittle (shatter, rather than bend)
-Dull
-Low melting points (mostly gases at room temperature, though not all)
-Poor conductors of heat and electricity
-Low density
-Usually less reactive with acids
-Typically gain electrons to form negative ions
the nonmetals on the periodic table are ____
right + up of staircase (not touching)
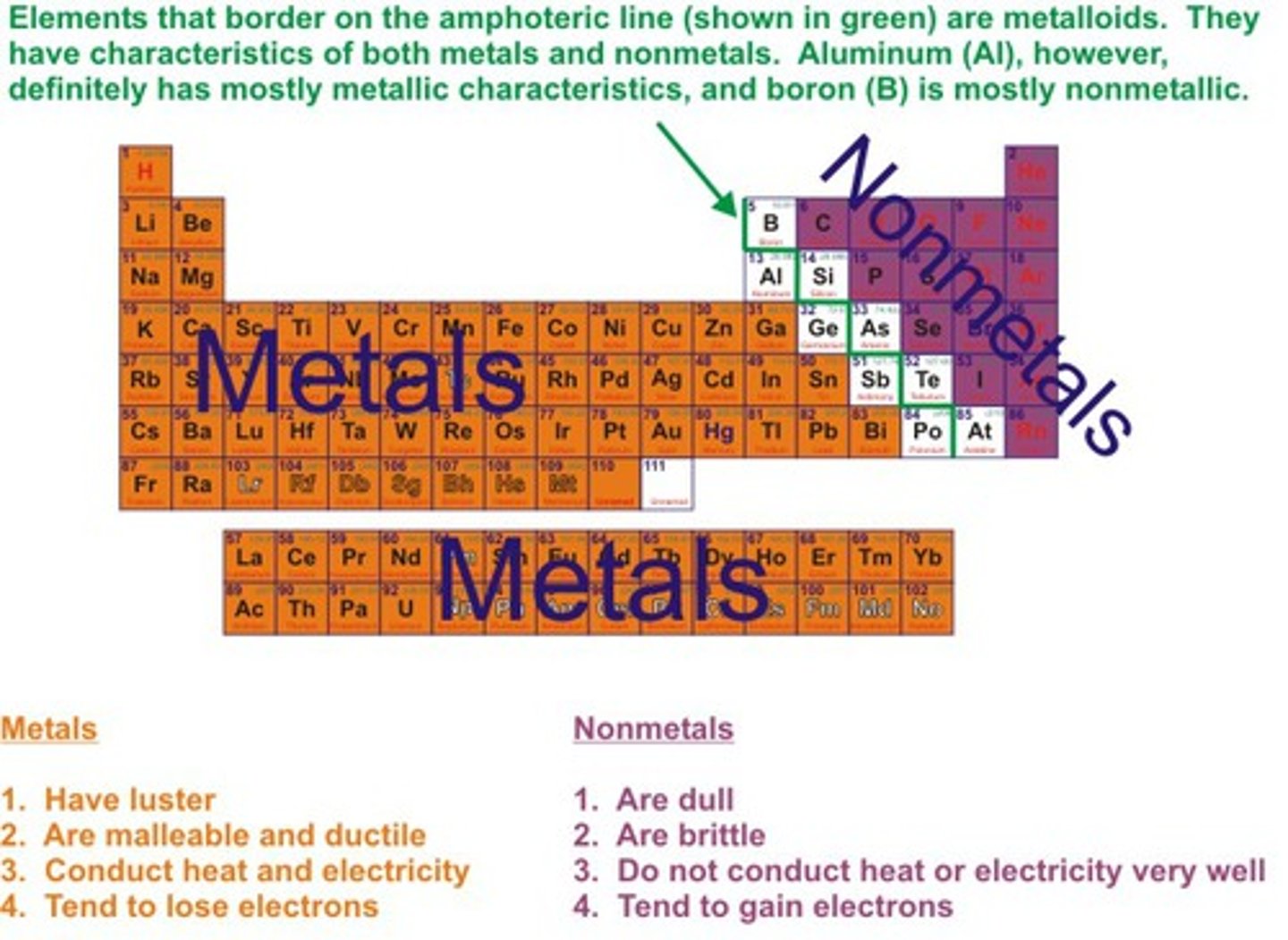
the semimetals/metalloid on the periodic table are _____
directly to the right of staircase
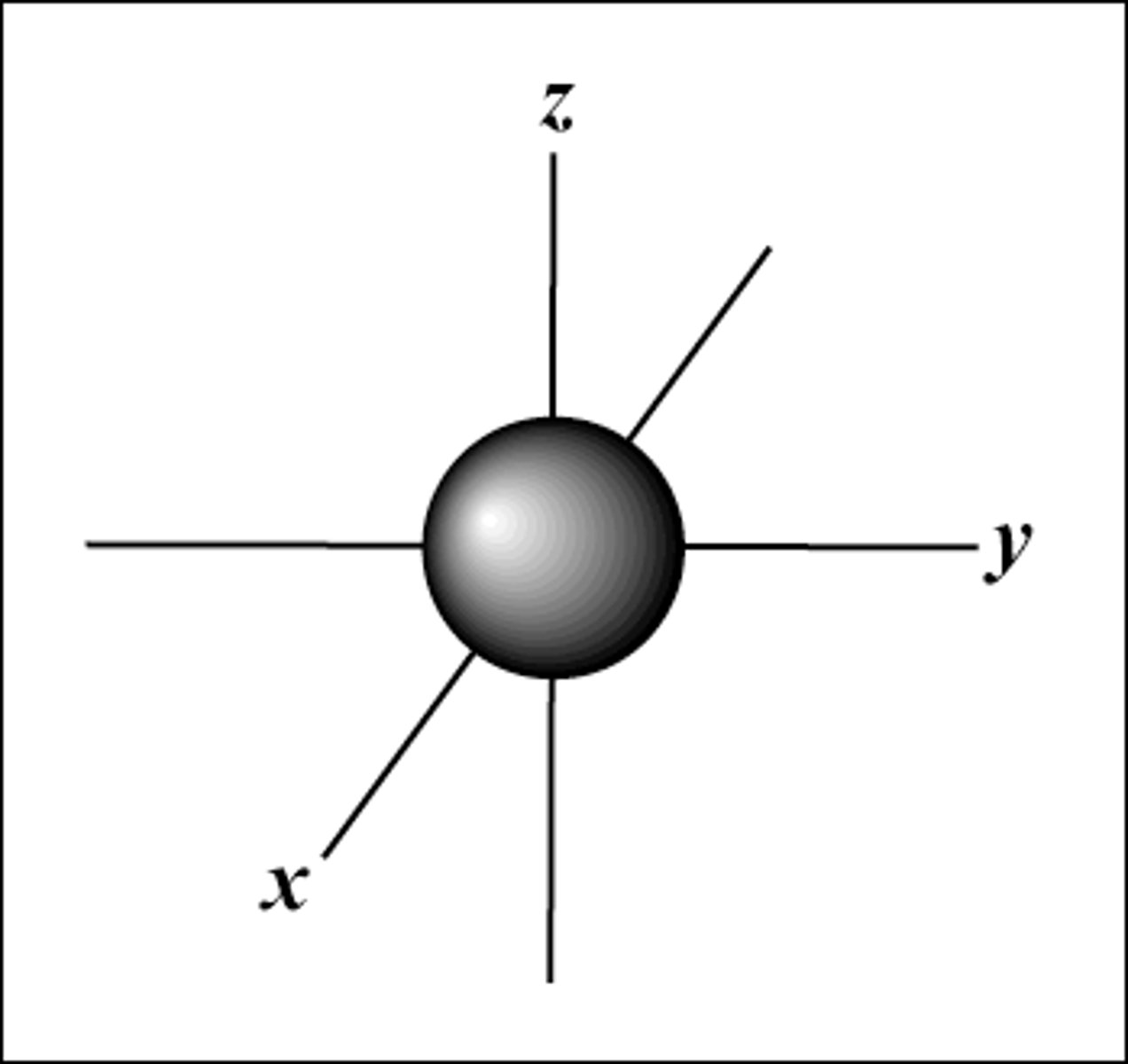
s orbital
- sphere shape
- group s
- holds 2 electrons
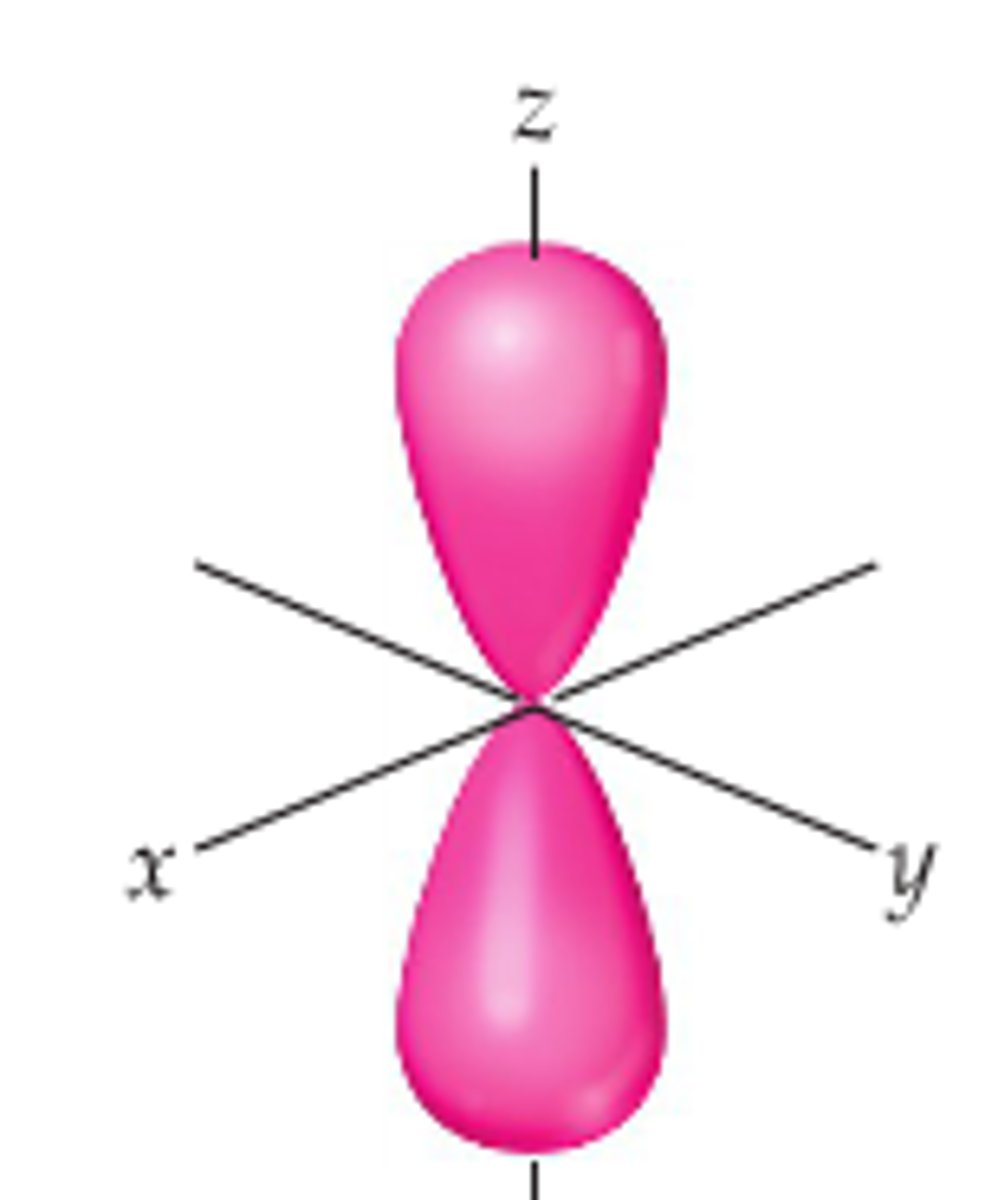
p orbital
- dumbbell shape
- group p
- holds 6 electrons
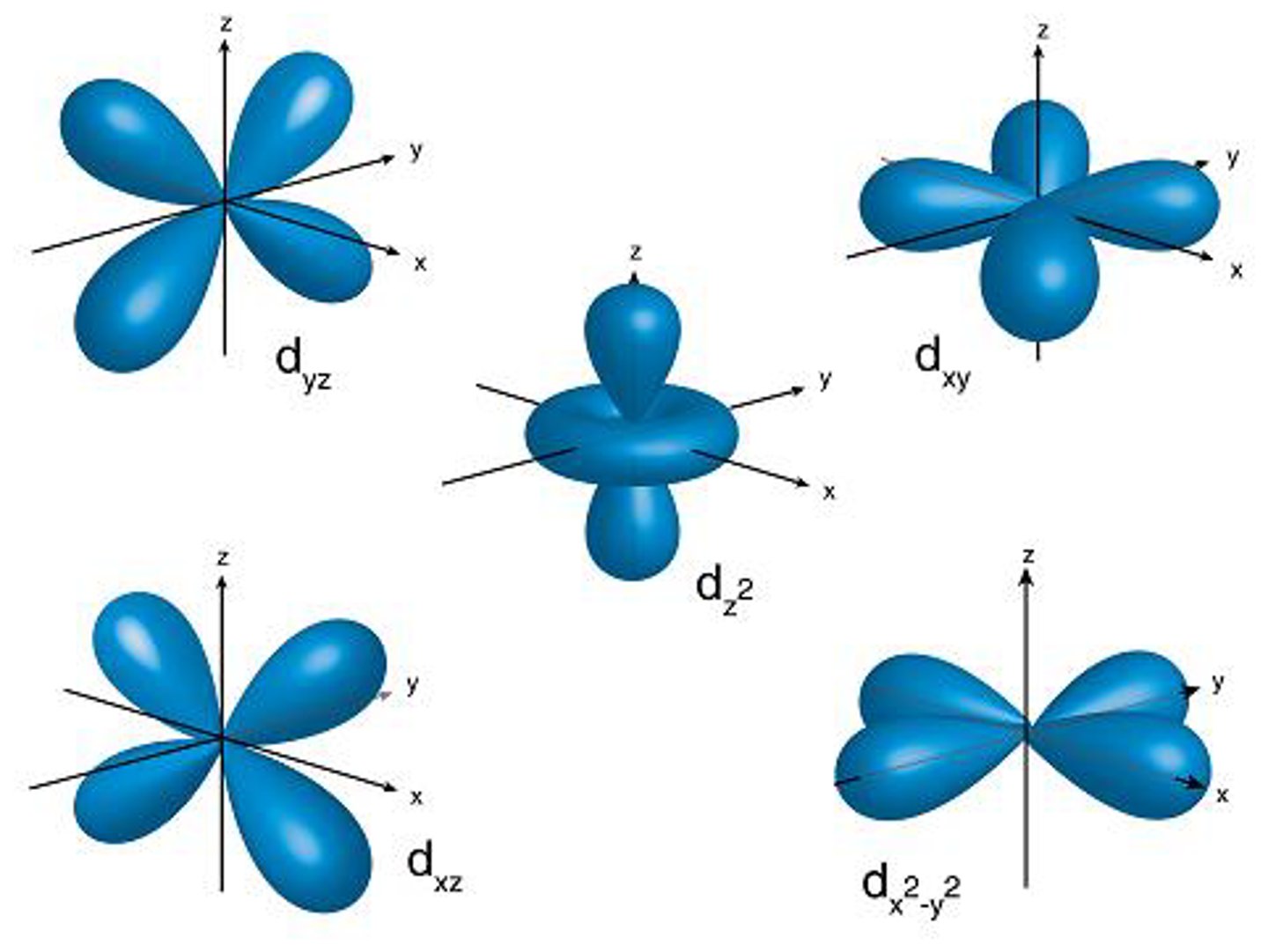
d orbital
- flower (dumbell + donut) shape
- group d
- holds 10 electrons
(its the center image in example photo)
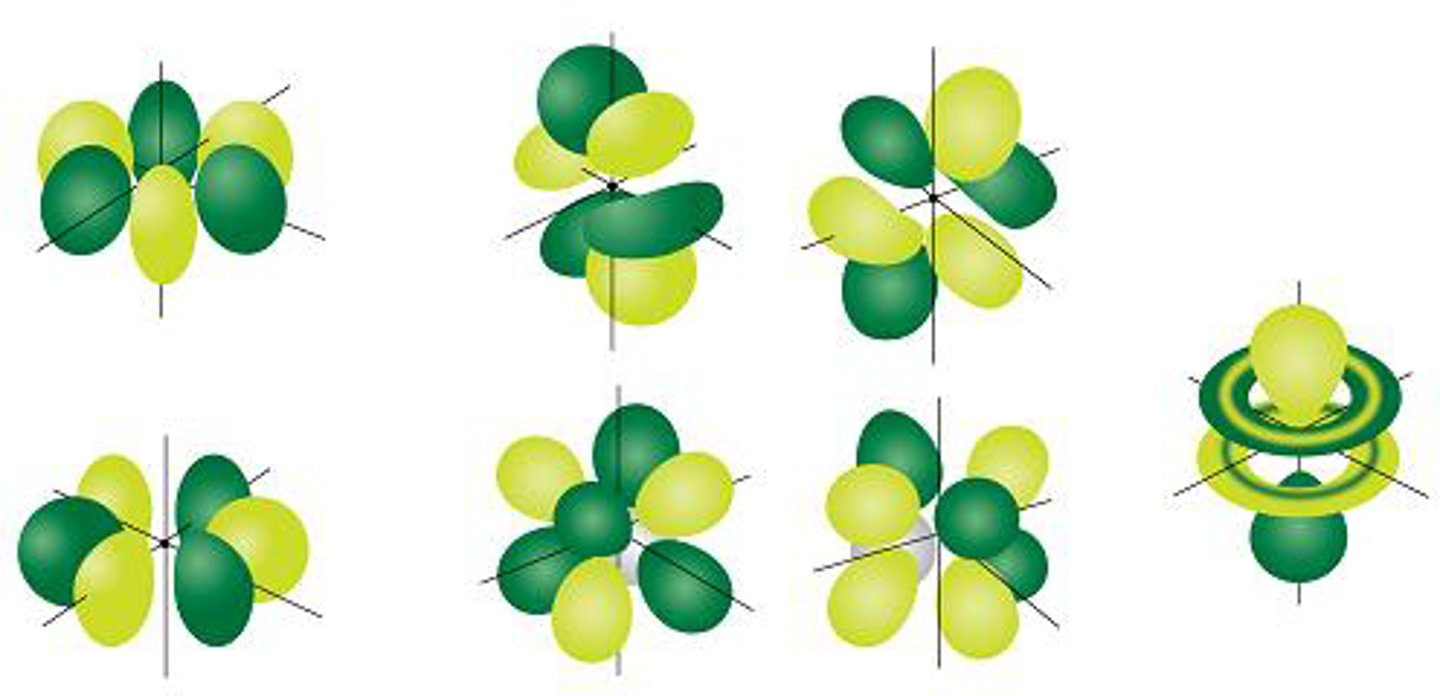
f orbital
- very complex shape
- group f
- holds 14 electrons
atomic radius
size of an atom/ its radius:
- one-half the distance between the nuclei of identical atoms that are bonded together
Trend:
- as u go down table, atoms get bigger
- as u go right the table, atoms get smaller (bc protons pull orbital in)
- more electrons = bigger atom bc more repulsion in cloud/between orbitals
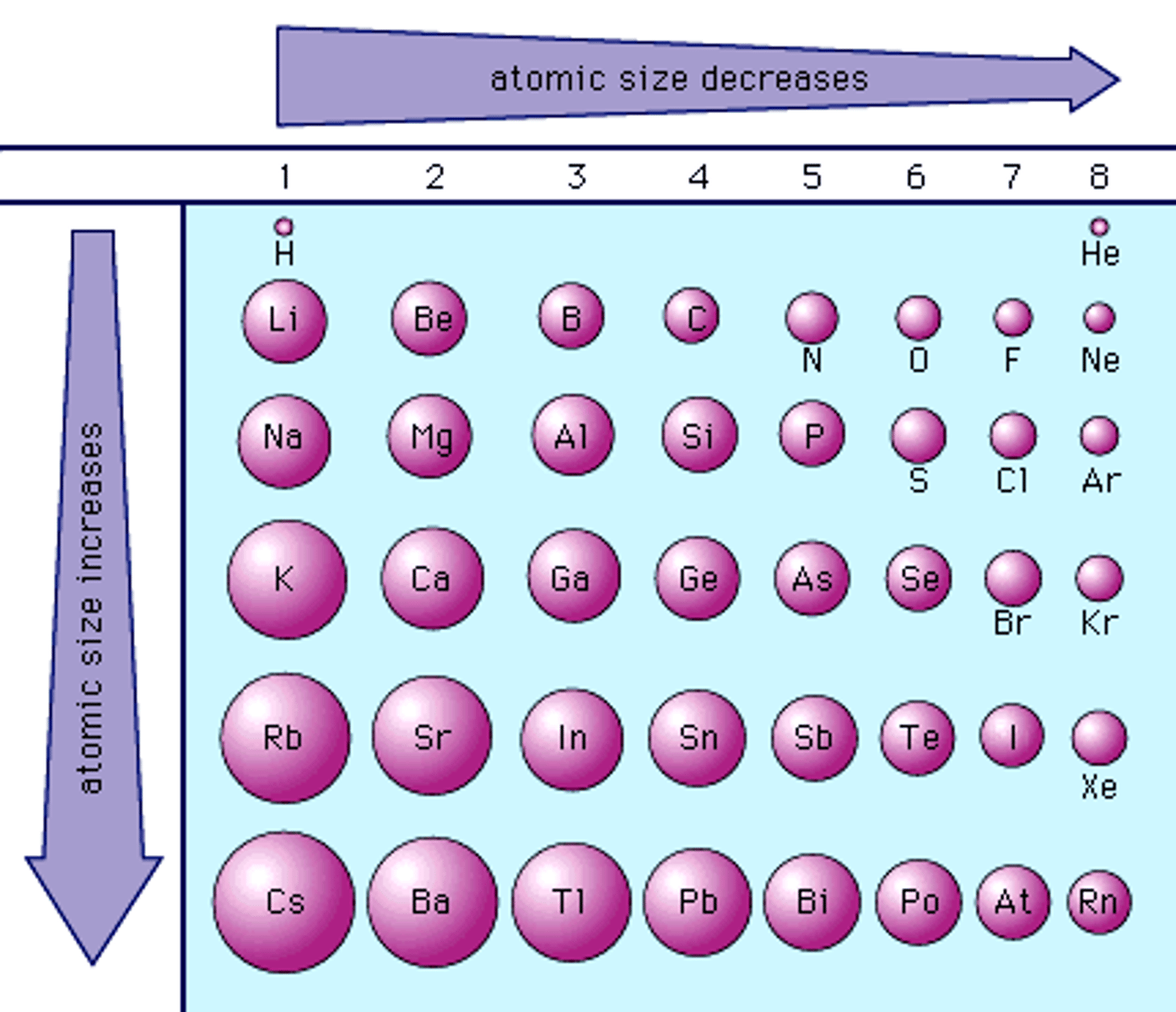
ionization energy
how much energy it takes to remove an electron from an atom
- ionize - turn an atom into ion
Trend:
- as u go down table/atoms get more shells its easier to remove electrons
- as u move right table/atoms get more protons/ its harder to remove bc higher IE
IE in metals and nonmetals
- metals don't want e = lower IE
- nonmetals want e = higher IE
Successive Ionization Energies
energies required to remove electrons beyond the first electron
- each successive electron is harder to pull of because there is more positive energy
- energy jumps occur when trying to pull off non-valence electron (off inner shell)
Electronegativity
how well an atom can attract an electron
Trend:
- as u go up/get less shells = higher electronegativity
- as u go right of table/get more protons = higher electronegativity
Reactivity
how easily an element will combine or start a chemical reaction with other substances
- metals usually react by losing electrons
trend: down to left/more shells, less p = more reactive
- nonmetals usually react by gaining electrons
trend: up to right/less shells, more p = more reactive
- generally you don't compare reactivity of metal to nonmetal
Zeff
effective nuclear charge= amount of pull felt by the outer electrons by the nucleus
Zeff explains...
atomic trends: as you move down a column atoms get larger despite the fact that doing so add protons (which usually makes atoms smaller) because Zeff spreads pull to all electrons
- atoms in first column have same Zeff, 1
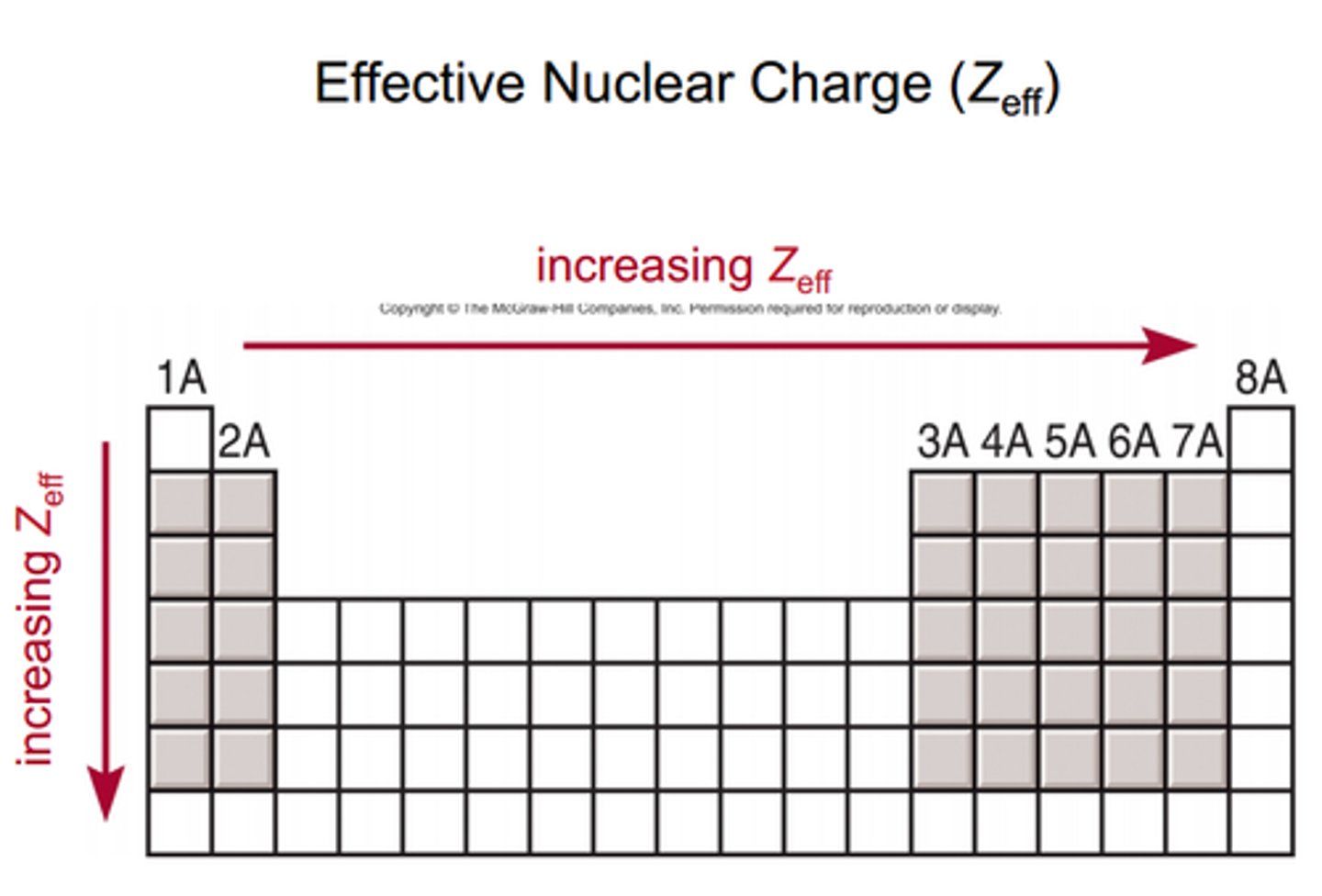
Z represents
the variable z represents the atomic number of an atom/ its nuclear charge/ # of protons
Zeff on valence electrons
Zeff = (# of protons)-(#of core elctrons)
electrons dont feel full power of nuclear charge
- the charge is lessened by electrons in inner shells
Zeff shortcut
for NEUTRAL atoms:
Zeff=valence electrons bc
protons=electrons
electrons=core e+valence e
Zeff for ions
- find w/ electron configuration
- find amount of valence electrons
- ex: zeff for NA+ = 8 bc 1s^2, 2s^2, 2p^6
Dimitri Mendeleev
created the 1st periodic table according to atomic mass
- there were some gaps and predicated there were elements we didnt know (questioned spot of I)
- in order for As to be next to P (the more similar atom) there had to be two elements between Zn and As
- Te and I should be swapped based on atomic mass but dimitri left them based on the elements they were most similar to in terms of properties, and questioned Te mass (which was right in end)
Henry Moseley
Arranged the periodic table by atomic number instead of mass number bc he discovered way to find the charge of atoms nucelus (atomic #)
- solved issue of atomic mass order with Te and I
- know where the gaps go bc whole number of atomic mass
Metals can be...
pure or as compounds often in solutions
when do metals/nonmetals react to each other
- a lone element attempts to replace another element in a compound (will replace if its more reactive)
- A+BC --> AB + C
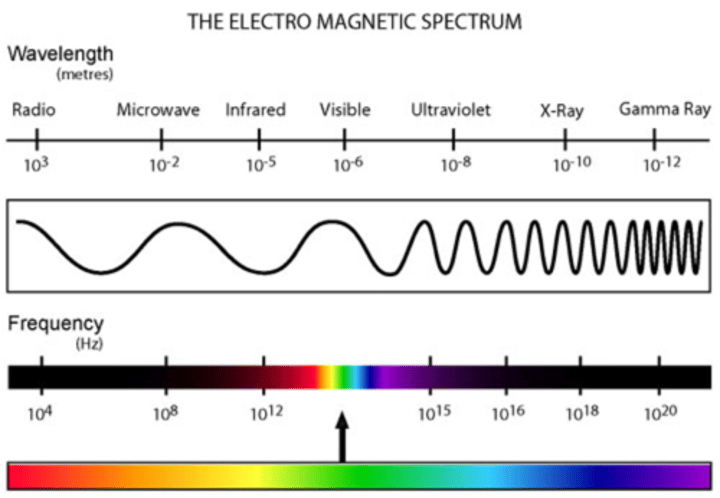
electromagentic spectrum
all the forms of electromagnetic radiation - and are energy that can w/o a medium
Isoelectronic
Having the same electronic configuration, although consisting of different elements