Chemistry Chapter 10 & 11
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
Kinetic Molecular Theory
Based on the idea that particles of matter are always in motion
Used to explain properties of solids, liquids, and gases in terms of the energy of particles and forces that act between them
Ideal Gas
hypothetical gas that perfectly fits all assumptions of KMT
KMT of gases assumptions
Gases consist of large numbers of tiny particles that are far apart relative to their size
Collisions between gas particles and between particles and container walls are elastic collisions (collisions where KE is conserved)
Gas particles are in continuous, rapid, random motion and therefore possess KE
There are no forces of attraction between gas particles
The temperature of gas is measure of average KE of particles
Most of the volume occupied by gas is…
empty space
KE equation
1/2mv²
All gases at the same temperature have the same…
average kinetic energy
How does speed of gas particles related to mass?
At the same temperature, lighter gas particles have higher average speeds than heavier gas particles
Ideal gas conditions
low pressure
high temperature
Gas Expansion
Gases do not have a definite shape or volume
Gases completely fill an enclosed container
Gas particles move rapidly in all directions without significant attraction between them
Gas Fluidity
Gas particles glide easily past one another because the attractive forces between them are insignificant
Gas Density
Gas density ≈ 1/1000 liquid or solid density of the same substance because gas particles are far apart
Gas Compressibility
Gas particles crowd closer together
Gas Diffusion
spontaneous mixing of particles of two substances caused by their random motion
Gas effusion
gas particles pass through tiny opening
molecules of low mass effuse faster
Gas Rate of effusio n
directly proportional to the velocity of their particles
Real gas
all real gases deviate from ideal gas behavior
at high pressures and low temperatures, gas is behaves like non-ideal gas
more polar gas molecules deviate more from ideal gas behavior
Liquid
form of matter that has a definite volume and takes the shape of its container
Attractive forces liquid vs gas
attractive forces between particles in a liquid are more effective than those between particles in a gas
what causes attraction between liquid particles
intermolecular forces
LD
dip-dip
hydrogen bonding
London Dispersion forces
weak forces that result from temporary shifts in density of electrons in electron clouds
dipole-dipole forces
attractions between oppositely charged regions of polar molecules
hydrogen bonding
occur between molecules containing a hydrogen atom bonded to a small, highly electronegative atom (N,O,F) with at least one lone electron pair
Fluid
substance that can flow and take the shape of its container
Liquid Density
typically 100x denser than gas at normal atmospheric pressure
Liquid Compressibility
relatively incompressible because particles are more closely packed together
Liquid Diffusion
constant, random motion of particles causes diffusion
gradually diffuse throughout other liquid
slower than gas because liquid particles have more attractive forces that slow movement and particles are closer together
diffusion happens quicker when liquid temperature is increased
Surface tension
force that tends to pull adjacent parts of a liquid’s surface together, decreasing surface area to the smallest possible size
relationship between attractive forces and surface tension
higher force of attraction between particles of a liquid, higher the surface tension
capillary action
attraction of surface of a liquid to surface of a solid
tends to pull liquid molecules upward along surface and against pull of gravity
causes meniscus
meniscus
Concave liquid surface in a test tube or graduated cylinder due to capillary action
vaporization
liquid or solid changes to gas
evaporation
particles escape the surface of a nonboiling liquid and enter gas state
occurs because particles of liquid have different KE
freezing
liquid to solid by removing energy (heat)
liquid → solid + energy
properties of solids
particles are more closely packed
strong attractive intermolecular forces
particles vibrate in fixed positions
maintain definite shape
volume changes slightly due to change in temp or pressure
crystalline solids
consist of crystals, a substance in which particles are arranged in orderly, geometric, repeating patterns
crystals arranged in 3D arrangement called crystal structure
amorphous solid
solid where particles are arranged randomly
melting
physical change of solid to liquid by addition of energy (heat)
solid + energy → liquid
melting point
temperature at which solid because liquid
at this temp, KE of particles overcome attractive forces holding them together
amorphous solid melting point
no definite melting point bc of random arrangement
supercooled liquids
substance that retain certain liquid properties even at temperatures at which they appear to be solid
solid density
most dense bc particles are very closely packed
solid compressibility
solids are incompressible
solid diffusion
million times slower than liquid
lattice
arrangement of particles in crystal represented by coordinate system
unit cell
smallest portion of crystal lattice that shows 3D pattern of entire lattice
ionic crystals
consist of pos and neg ions arranged in regular pattern
generally group 1 or 2 metals and group 16 or 17 nonmetals or polyatomic ions
hard, brittle, high melting points, good insulators
covalent network
each atom is covalently bonded
covalent bonding extends throughout network
hard, brittle, high melting point (highest of the solids), nonconductors or semiconductors
metallic
consist of metal cations surrounded by sea of electrons
high electric conductivity bc of sea of electrons
covalent molecular
covalently bonded molecules held together by intermolecular forces
low melting points, easily vaporized, soft, insulators
nonpolar has lower melting point than polar
phase
any part of a system that has a uniform composition and properties
condensation
gas → liquid + energy
vapor
gas in contact with its liquid or solid phase
equilibrium
dynamic condition in which two opposing changes occur at equal rates in a closed system
liquid-vapor equilibrium system
rate of condensation equals rate of evaporation
liquid and vapor coexist in stable state
equilibrium vapor pressure
pressure exerted by vapor in equilibrium with its corresponding liquid at a given temperature
increases with increasing temperature
every liquid has specific equilibrium vapor pressure at given temp
volatile liquids
liquids that readily evaporate
weak attractive forces
nonvolatile liquids
do not evaporate readily
strong attractive forces
boiling
liquid to vapor within liquid and liquid surface
boiling point
temp at which equilibrium vapor pressure equals atmospheric pressure
lower atmospheric pressure = lower boiling point
Molar enthalpy of vaporization
The amount of energy needed to vaporize one mole of a substance
measure of attraction between liquid particles
at melting and freezing points, solid and liquid are in…
equilibrium
ex: at normal atm, the temperature of a system containing ice and liquid water will remain 0 degrees as long as both ice and water are present. only after ice is melted will the additional energy increase system temp
molar enthalpy of fusion
amount of energy required to melt one mold of solid at solid’s melting point
magnitude depends on attractive forces between particles
sublimation
solid + energy → gas
when at low temp and pressure conditions, solid exists in equilibrium with gas bc liquid cannot exist
deposition
gas → solid + energy
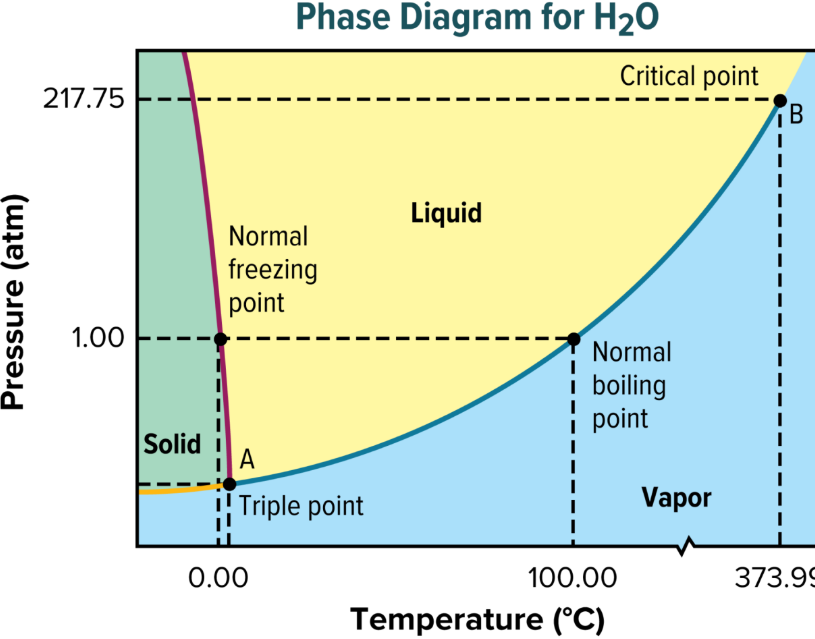
phase diagram
graph of pressure vs. temp
shows conditions under which phases of substance exist
triple point
indicates temp and pressure at which solid, liquid, and gas coexist at equilibrium
critical point
critical temp and critical pressure
critical temp
temp above which substance cannot exist in liquid state
above this temp, water cannot be liquified
critical pressure
lowest pressure at which substance can exist as liquid at critical temp
exothermic
releases heat/energy
freezing, condensation, deposition
endothermic
process that requires heat/energy
melting, vaporization, sublimation
Celsius → Kelvin
K = C + 273
atm → mm Hg
1 atm = 760 mm Hg
atm → torr
1 atm = 760 torr
atm → kPa
1 atm = 101.3 kPa
What is Standard Temperature and Pressure equal to at 0°C
1 atm
Molar volume
one mole of any gas at STP has a volume of 22.4 L
molar volume = 22.4 L at STP
4 variables that determine gas behavior
temperature, pressure, volume, number of particles
Pressure and number of gas molecules are … related
directly (more molecules are colliding within given space)
Where does gas naturally flow?
gas naturally flows from areas of high pressure to areas of low pressure until pressure becomes equal
What creates pressure?
Changing the size of the container. In smaller containers, molecules have less room to move, therefore they hit the sides of the container more often.
What does temperature of gas increase?
increase in kinetic energy
Relationship between temperature + pressure and volume
increase in kinetic energy causes gas molecules to hit walls of container even harder, resulting in increased pressure or increased volume
Dalton’s Law of Partial Pressure
Equal amounts of gas at the same temperature and volume have equal pressure. Total pressure inside a container is equal to partial pressure of each gas.
Pt = P1+P2+P3+…
Boyle’s Law
At constant temperature, pressure and volume are inversely related
volume decreases, pressure increases
P1V1 = P2V2
Charle’s Law
volume of gas is directly proportional to Kelvin temperature if pressure is constant
temperature increases, volume increases
V1/T1 = V2/T2
Gay Lussac’s Law`
At constant volume, as temperature increases, pressure also increases (directly related)
Temperature increase means molecules collide more with walls of container to cause increased pressure
P1/T1 = P2/T2
Combined Gas Law
only applies when number of molecules stay constant but volume, temperature, and pressure change
P1V1/T1 = P2V2/T2
Ideal Gas Law Equation
PV = nRT
P - pressure
V - volume
n - number of moles
R - ideal gas constant
T - Kelvin temperature
Molar mass equation
M = mRT/PV
density equation
D = MP/RT
ideal gas constant (R)
R = 0.0821 (L*atm)/(mol*K)
R = 62.4 (L*mm Hg)/(K*mol)
R = 8.314 (L*kPa)/(mol*K)
Avogadro’s Law
an equal volume of gas at constant temperature and pressure will have the same number of molecules
V = kn
Graham’s Law of Effusion/Diffusion
for two different gases, A and B, at the same temperature:
½MAVA2 = ½MBVB2
MAVA2 / MBVB2 = MBVB2 / MAVA2
Rate of effusion of A/B = square root of MB/MA
What do coefficients in chemical equations for gases represent
molar amounts of substance and relative volumes in reaction