Nucleic Acids + Proteins
1/61
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
Nucleic Acids
information molecules that encode instructions for the synthesis of proteins and are large linear polymers of nucleotides
Types of nucleic acids
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)
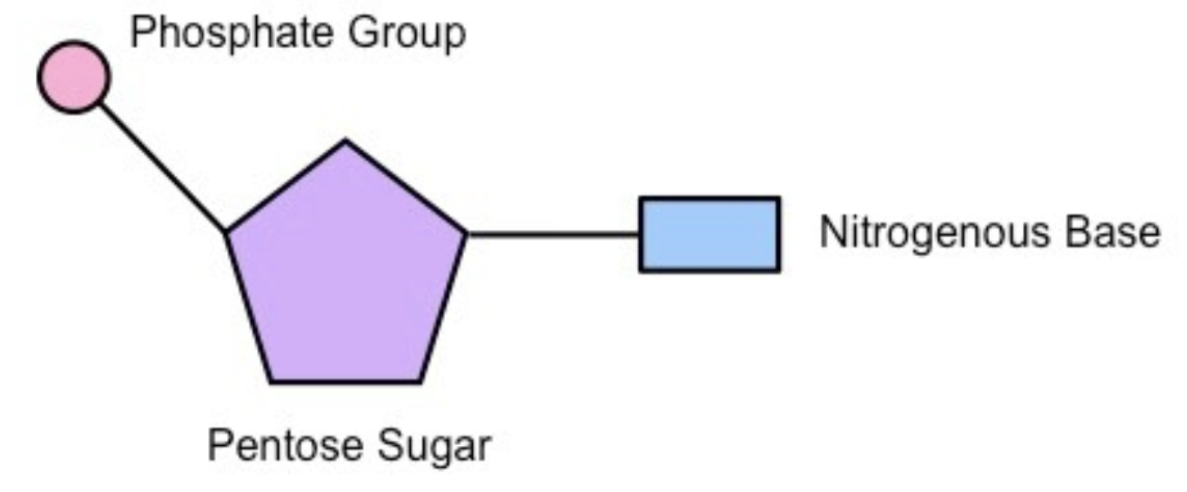
Nucleotides
monomers of nucleic acids (DNA and RNA), which differ depending on which nucleic acid it is — phosphate group, pentose sugar, and nitrogenous base
Genetic Code
a universal triplet code that is degenerate
Monomers
small, simple molecules that bind chemically to other molecules
Polymers
large, complex molecules made up of repeating smaller units (monomers)
Proteins
a type of bio-macromolecule made of amino acid chains folded into a 3D shape
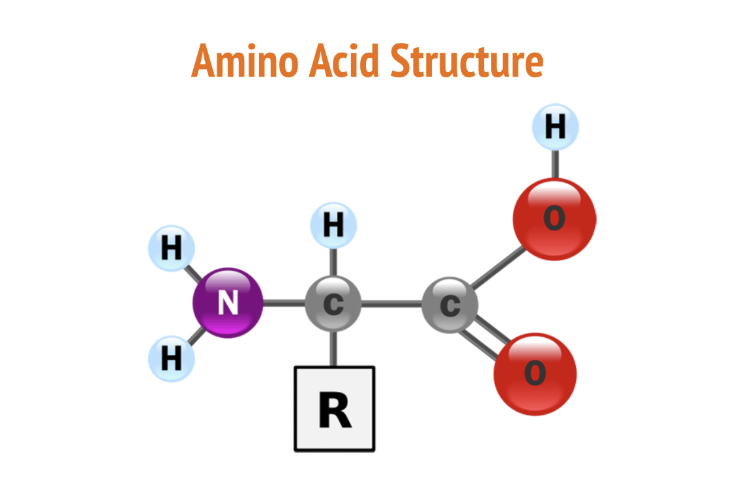
Amino acids
building blocks of proteins
20 different types of amino acids make all the proteins in the body
Are joined together to form chains (polypeptides) which form proteins
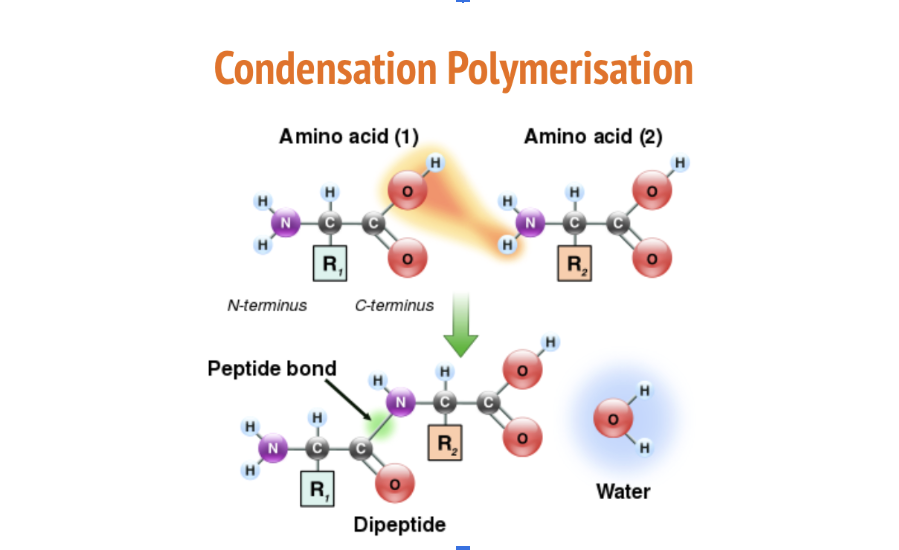
Condensation Polymerisation
the reaction that joins amino acids to form a polypeptide
The carboxyl group of one amino acid is joined to another, forming a peptide bond
Releases water (condensation) + requires an input of energy (anabolic)
4 Structures of Protein
Primary, secondary, tertiary, and quaternary
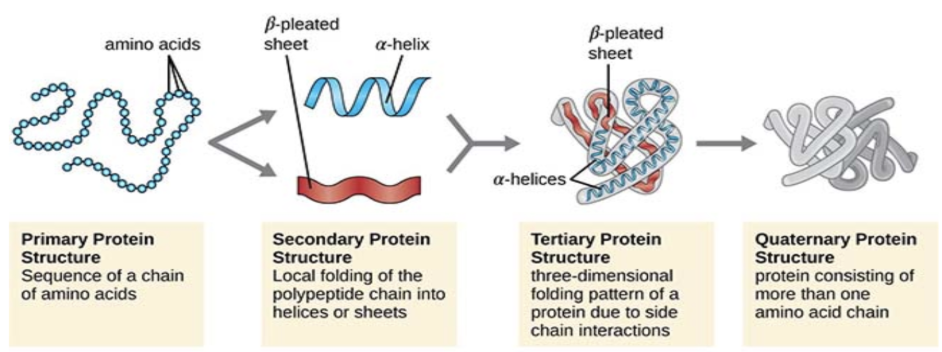
Primary structure of protein
sequence of a chain of amino acids
Secondary structure of protein
local folding of the polypeptide chain into a-helices (helix) or b-pleated sheets, sections in between are called random loops
Tertiary structure of protein
3D folding pattern of a protein due to side chain interactions
Most proteins become functional
Due to the electric charge of R group → attraction/repulsion between sections of polypeptide chain, making it fold into 3D shape
Change of protein can indicate biological INACTIVITY
Quaternary structure of protein
proteins made of two or more polypeptides joined together
Not all proteins will have a quaternary structure — many are functional at the tertiary level
What can happen to Protein Structure?
if there is a change to the specific shape of a protein due to incorrect addition of an amino acid OR environmental change, the enzyme will not function properly
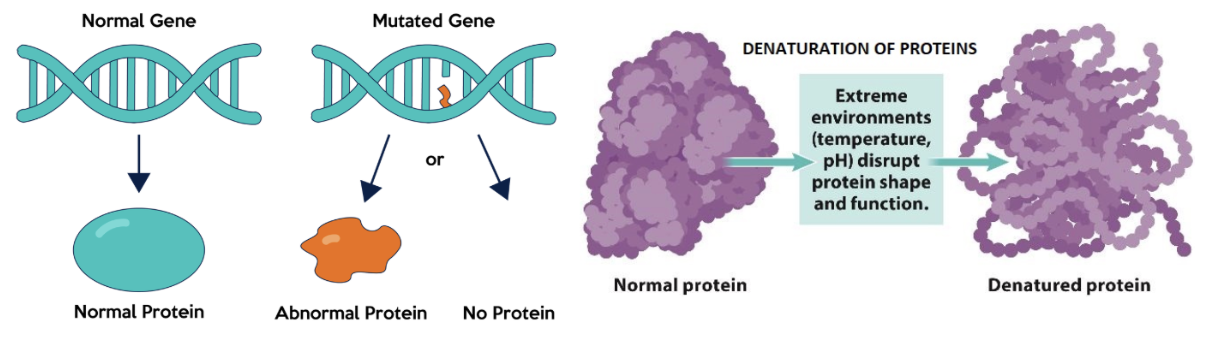
Proteome
the whole set of proteins produced by an organism or cell
Proteome of a cell is different to its genome — not all cells make the same proteins, yet they all contain the same genes
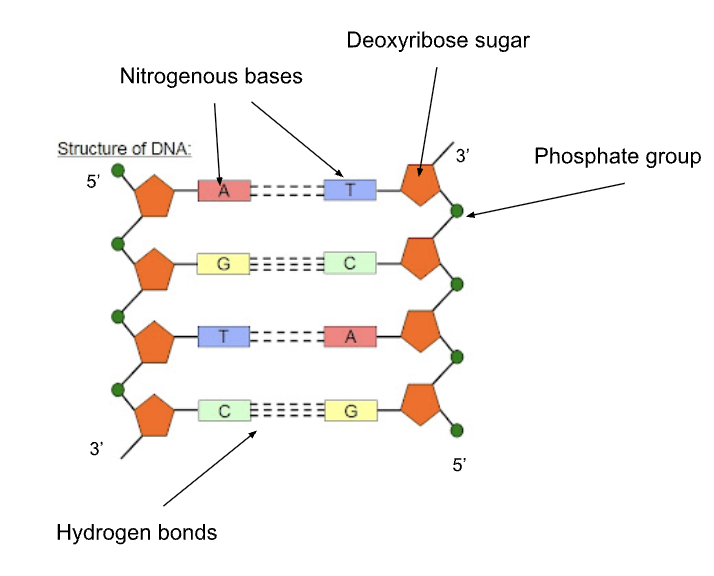
Structure of DNA
made up on smaller ones called nucleotides — made up of 3 parts:
a phosphate group, pentose sugar (deoxy/ribose), a nitrogenous base (Adenine, Guanine, Cytosine, Thymine/Uracil)
3 main forms of RNA
messenger RNA (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA)
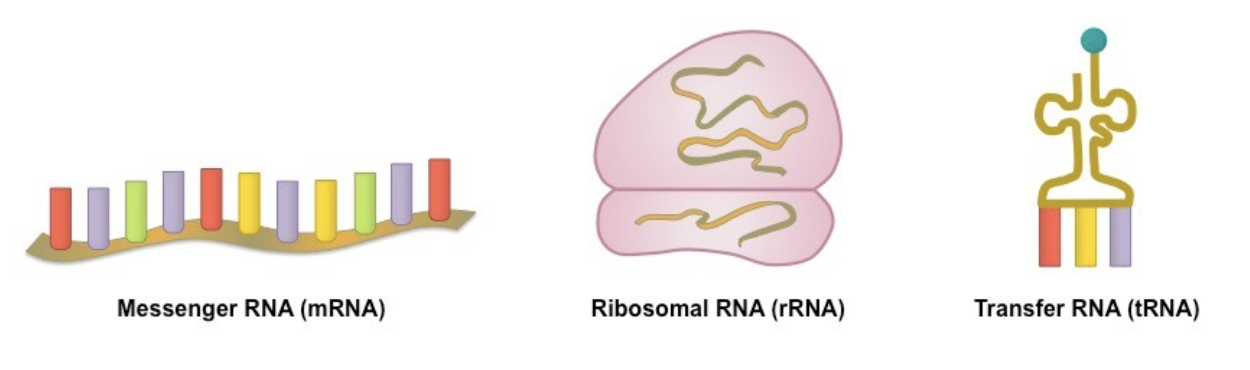
messenger RNA (mRNA)
messenger strand made in nucleus that carries the genetic information for protein synthesis from nucleus to ribosomes
specifies the order of amino acids in the polypeptide chain
ribosomal RNA (rRNA)
strand of RNA which is synthesised in the nucleus + binds to proteins within the cell to form ribosomes
transfer RNA (tRNA)
attached to specific amino acids + transports amino acids to the ribosome during protein synthesis
transcription
process (in the NUCLEUS) by which genetic information in a specific segment of DNA (a gene) is copied into a complementary mRNA strand by RNA polymerase
FIRST step of Transcription
Initiation
RNA polymerase binds to the promoter (near the start of the gene)
The DNA double helix unwinds, exposing the template strand
SECOND step of Transcription
Elongation
RNA polymerase moves along the DNA template strand. synthesising a complementary RNA strand by adding RNA nucleotides (A U C G) in the 5’ to 3’ direction
RNA strand is complementary to the DNA template strand + identical to DNA coding strand except RNA uses uracil instead of thymine
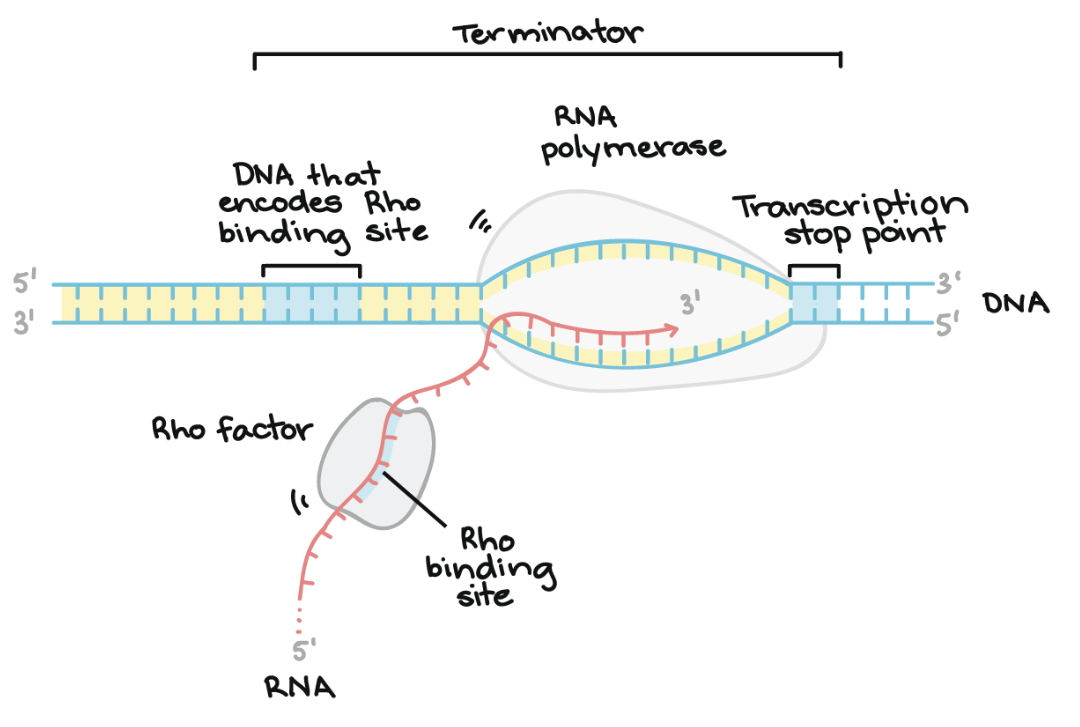
THIRD step of Transcription
Termination
Transcription ends when RNA polymerase reaches a termination sequence in the DNA
The newly synthesised RNA (pre-mRNA in eukaryotes) is released and RNA polymerase detaches from the DNA
Importance of Transcription
First step of the central dogma of molecular biology (DNA → RNA → Protein)
Allows cells to selectively express genes + produce the proteins needed for specific functions
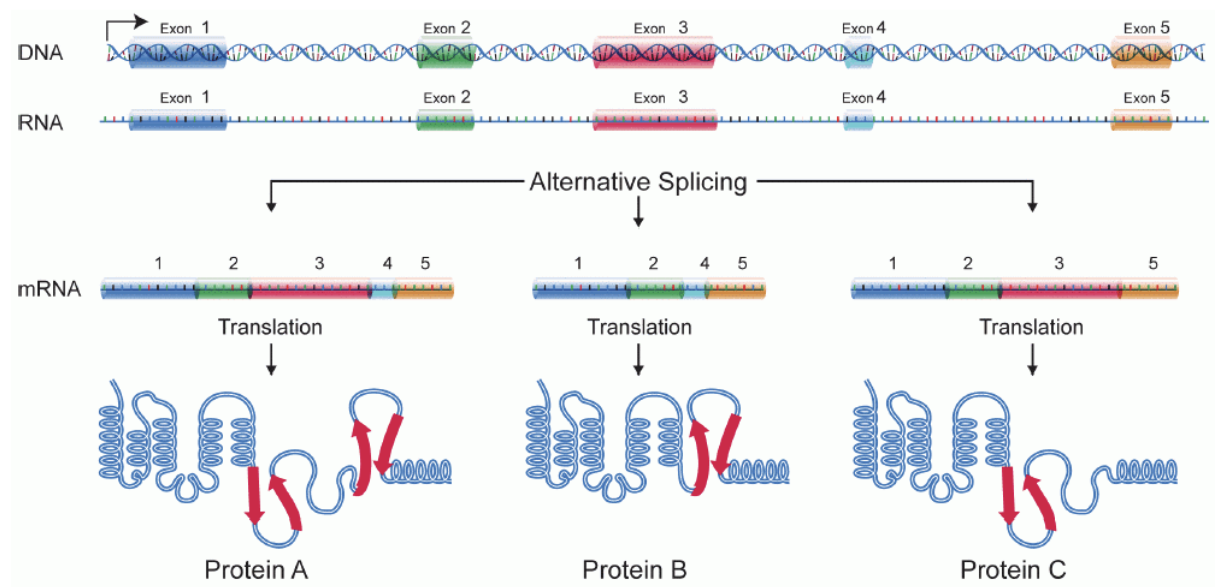
Alternative Splicing
a process (in the nucleus) where a single pre-mRNA can be cut and joined in different ways to produce multiple mRNA variants — allowing one gene to make different proteins
FIRST step of Alternative Splicing
1. As the pre-mRNA is transcribed, a 5’ cap is added to the beginning of the RNA molecule
The cap is a modified guanine nucleotide (methylated) attached to the 5’ side
Function: Protects mRNA from degradation + helps ribosome bind during translation
SECOND step of Alternative Splicing
2. Near the end of transcription, a poly-A tail is added to the 3’ end of the pre-mRNA
The tail is a long chain of adenine nucleotides (AAAAA…)
Function: Stabilises the mRNA, protects it from degradation + helps it exit the nucleus
THIRD step of Alternative Splicing
3. The spliceosome (a molecular machine) removes introns (non-coding regions) and joins exons (coding region) together to form mature mRNA
Spliceosome can include or skip certain exons, creating different mRNA variants from the same pre-mRNA
Importance of Alternative Splicing
Increases diversity of proteins without needing more genes
Allows cells to produce different proteins for different tissues or stages of development
Translation
a process (in RIBOSOMES) by which the genetic information carried by mRNA is decoded to produce a specific protein
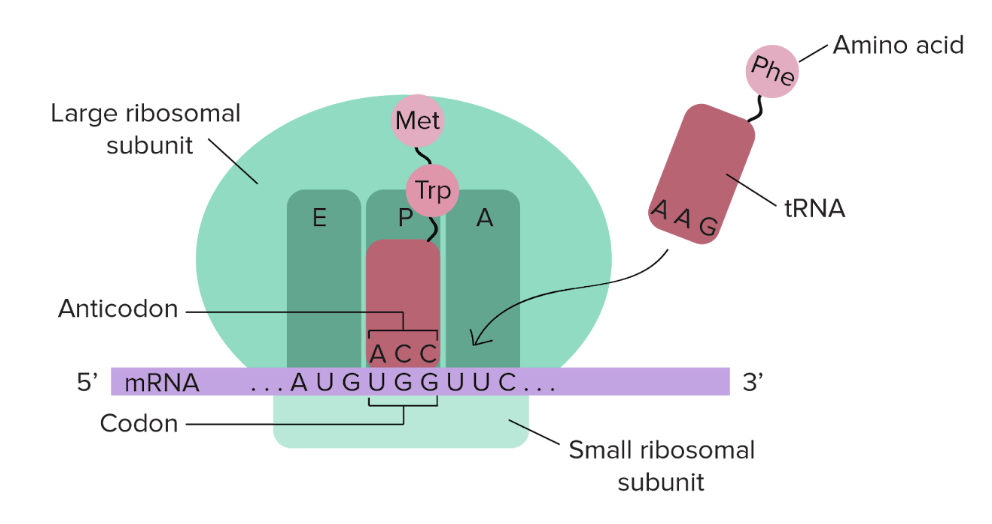
FIRST step of Translation
Initiation
The small ribosomal subunit binds to the mRNA at the start codon (AUG, which codes for MET)
The initiator tRNA, carrying MET, binds to the start codon
The larger ribosomal subunit joins, forming a complete ribosome
SECOND step of Translation
Elongation
The ribosome moves along the mRNA, reading each codon one by one
tRNA molecules, each carrying a specific amino acid, bind to the corresponding codons on the mRNA
The ribosome catalyses the formation of peptide bonds between amino acids, building a polypeptide chain
THIRD step of Translation
Termination
Ends when the ribosome reaches a stop codon (UAA, UAG, or UGA) on the mRNA
The polypeptide chain is released and the ribosome disassembles
Importance of Translation
Converts the genetic code into functional proteins, which perform most of the work in cells
Essential for gene expression and proper functioning of cells and organisms
Gene Regulation
the process of turning genes “on” and “off”
Allows cells to save energy by not producing unnecessary proteins + to specialise
Two mechanisms of gene regulation
Repression and attenuation
Both occur within E.coli bacteria in a section of DNA — “trp operon”
Two types of genes
Regulatory genes — DNA sequences that code for proteins that control the expression of other genes
Structural genes — DNA sequences that code for proteins that are not regulatory proteins
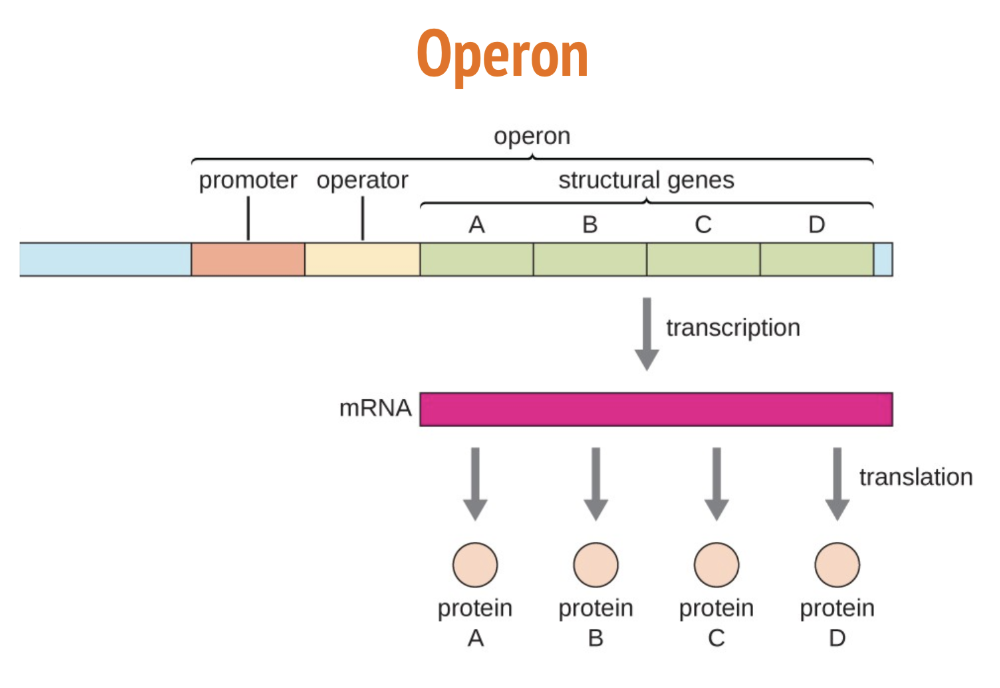
Components of the Operon — gene expression in prokaryotes
Operon — a set of adjacent genes and nearby regulatory sequences that can affect transcription of the genes
Promoter — section of DNA where RNA polymerase binds + transcription beings
Operator — section of DNA where proteins that control transcription bind (repressor proteins)
Trp operon
a group of genes that code for enzymes that make the amino acid “trp” and regulatory sequences that control their expression
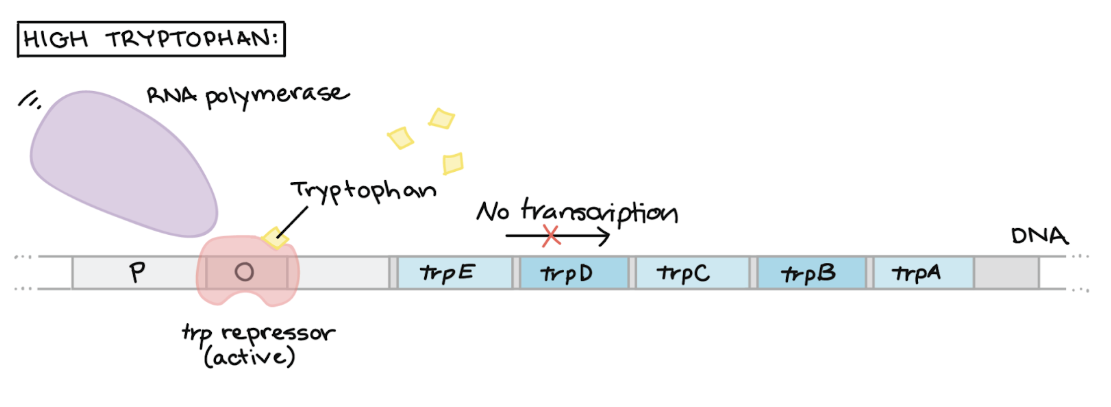
Repression of Trp Operon — HIGH Trp levels
Trp acts as a corepressor and binds to the Trp repressor protein
Active repressor-corepressor complex binds to the operator region
Blocks RNA polymerase from binding to promoter, preventing transcription of structural genes
RESULT → Trp synthesis enzymes ARE NOT producted
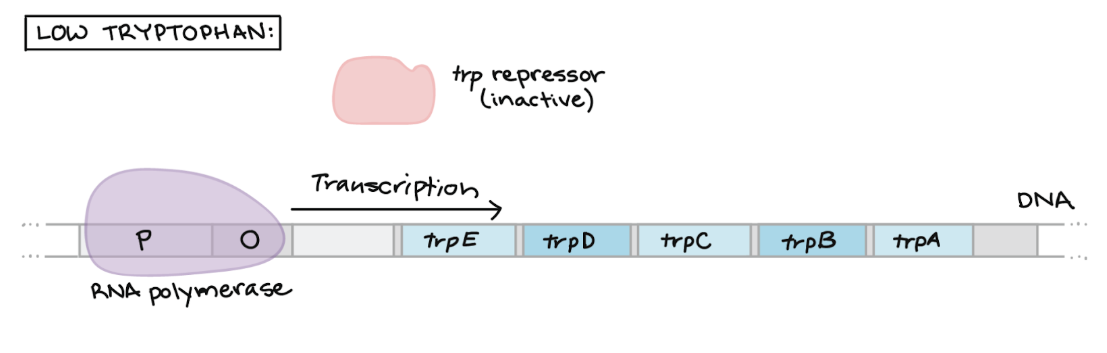
Repression of Trp Operon — LOW Trp levels
Trp repressor protein cannot bind to operator without tryptophan
RNA polymerase can bind to promoter + transcribe structural genes
RESULT → Trp synthesis enzymes ARE produced
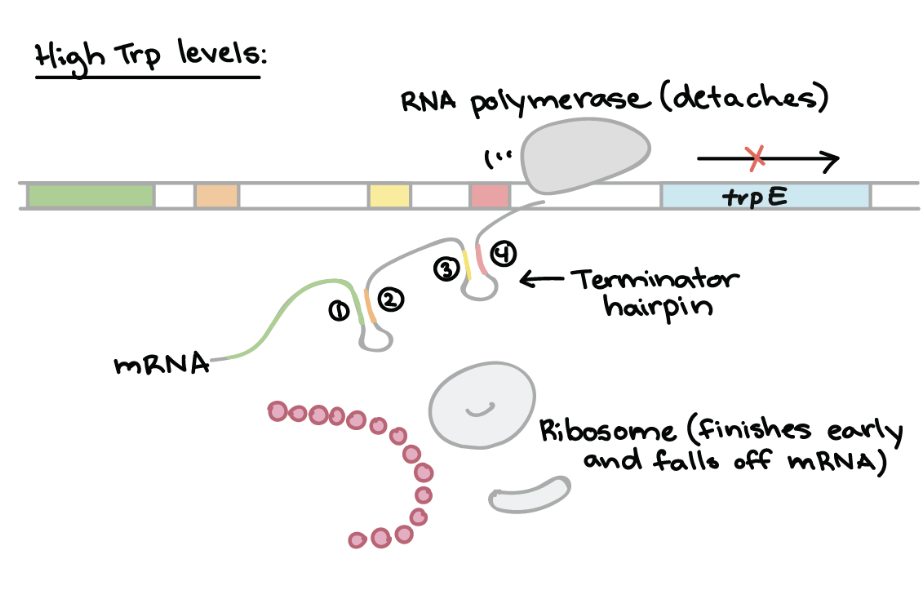
Attenuation of Trp Operon — HIGH Trp levels
Ribosomes quickly translate the leader peptide sequence, which includes two tryptophan codons
Forms a terminator hairpin in the mRNA, halting transcription prematurely
RESULT → Transcription of structural genes is STOPPED EARLY
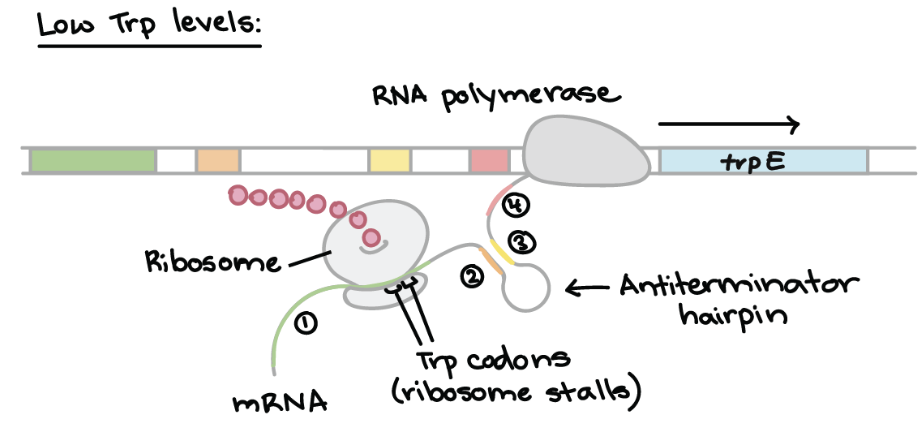
Attenuation of Trp Operon — LOW Trp levels
Ribosomes stall at the tryptophan codons in the leader sequence
Forms an anti-terminator hairpin in the mRNA, enabling RNA polymerase to continue transcription
RESULT → Structural genes are fully transcribed and tryptophan synthesis enzymes ARE produced
Importance of Gene Regulation
Ensures that tryptophan is synthesised only when needed, saving energy + resources
Demonstrates how bacteria can efficiently regulate gene expression in response to environmental conditions
Exocytosis
a cellular process that involves the transport of materials OUT of the cell
a form of bulk transport
essential for the secretion of hormones, enzymes and waste products + incorporation of proteins and lipids into the cell membrane
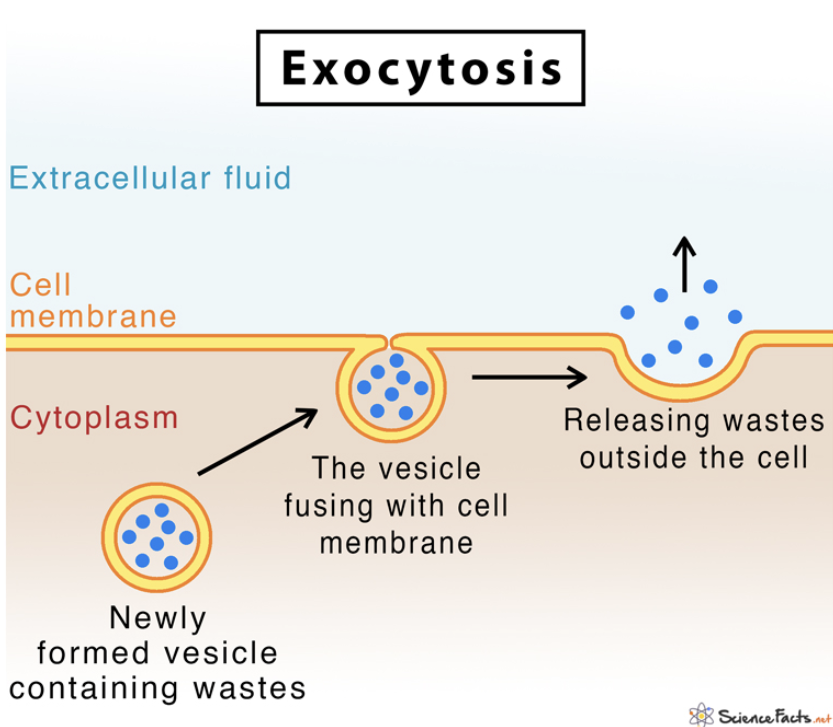
Exocytosis — Process
Vesicles containing the material to be exported move toward the cell membrane
The vesicle membrane fuses with the cell membrane
The contents of the vesicle are released outside the cell
Exocytosis — Energy Requirement
an active process, requiring energy in the form of ATP (Adenosine Triphosphate)
Exocytosis — Role in the Cell
Allows cells to secrete large molecules (proteins/hormones) that cannot pass the cell membrane via diffusion or other passive transport
Helps maintain the cell membrane by adding new lipids + proteins
Endocytosis
a cellular process that allows cells to take in large molecules by engulfing them
a form of bulk transport
imports materials into the cell
essential for nutrient uptake, cell signalling, and immune responses
Endocytosis — Process
The cell membrane folds inwards, forming a pocket around the material to be imported
The pocket pinches off, creating a vesicle inside the cell that contains the engulfed material
The vesicle then transports the material to the appropriate location within the cell
Endocytosis — Energy Requirement
an active process, requiring energy in the form of ATP (Adenosine Triphosphate)
Types of Endocytosis
Phagocytosis — engulfs large solid particles (bacteria/dead cells)
Pinocytosis — takes in extracellular fluid containing dissolved substances (absorption of nutrients)
Receptor-Mediated — specific molecules bind to receptors on cell membrane, triggering formation of vesicle
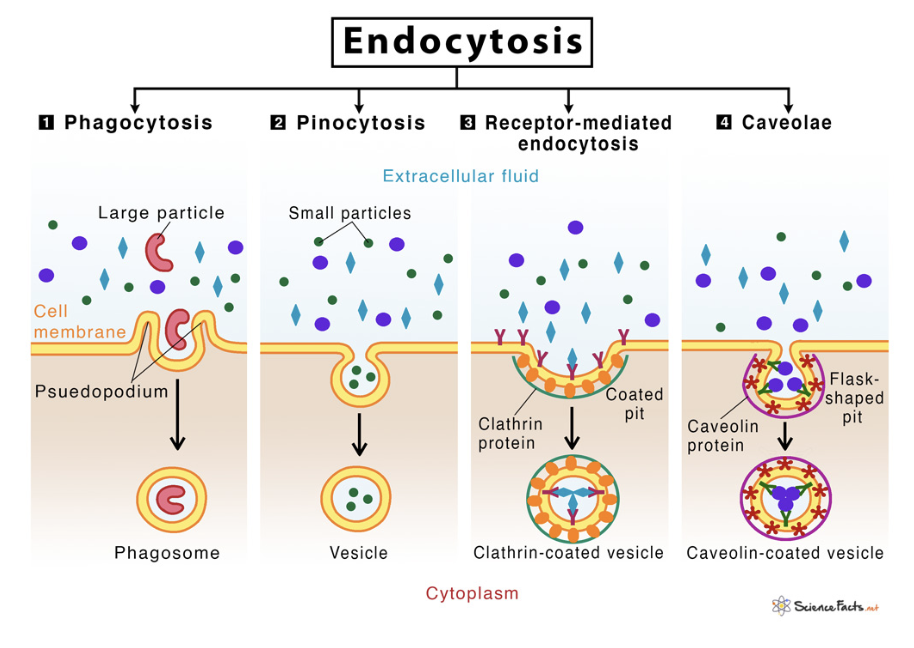
Endocytosis — Role in Cell
Allows cells to take in essential nutrients (lipids and proteins)
Plays a role in immune defence by enabling cells to engulf pathogens
Helps regulate cell signalling by internalising receptor proteins
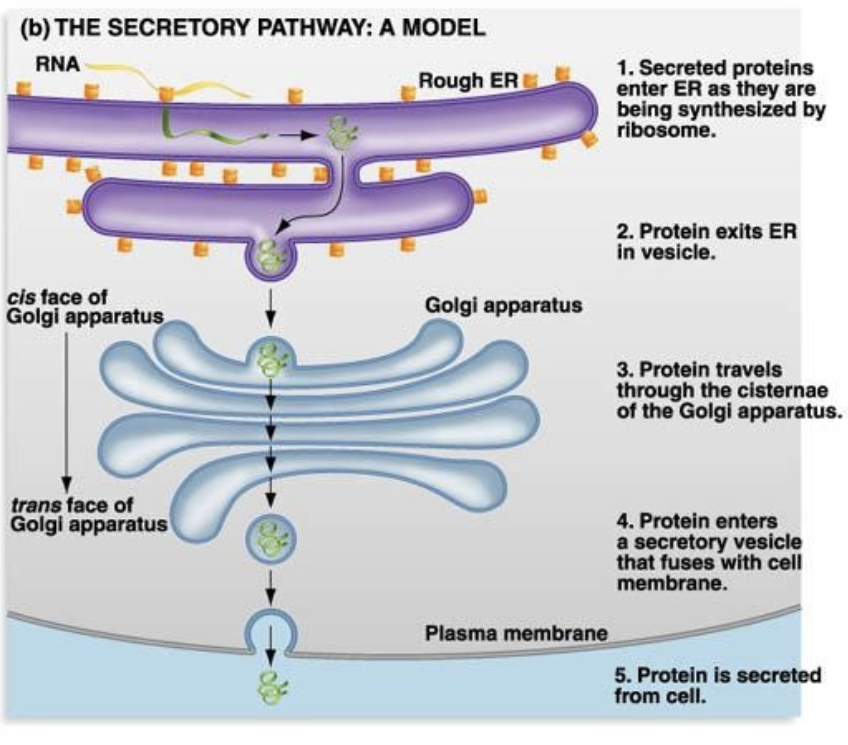
Secretory Pathway
a cellular process involving the synthesis, modification and transport of proteins and lipids for secretion from the cell or incorporation into the cell membrane
Includes endoplasmic reticulum + Golgi apparatus + vesicles
Secretory Pathway — Process
1. Synthesis — Proteins are made by ribosomes on the rough ER and pushed inside its lumen
2. Modification — Proteins are folded and modified (e.g glycosylation) in the rough ER
3. Transport — Proteins are packaged into vesicles + sent to Golgi apparatus
4. Sorting + Packaging — In the Golgi, proteins are further modified, sorted + packed into secretory vesicles
5. Release — Vesicles fuse with cell membrane, releasing proteins outside the cell (exocytosis) OR inserting them into the membrane
Properties of the Genetic Code
Triplet Code
Universality
Redundancy/degeneracy
Non-ambiguous
Triplet Code
each sequence of three nucleotide bases in DNA (or RNA, called a codon) specifies a particular amino acid or a signal to start or stop protein synthesis
Universality
the same sequence of three-base codons specifies the same amino acid in almost all living organisms
Redundancy/Degeneracy
each amino acids is encoded by more than one mRNA codon
Non-Ambiguous
each specific will always code for only one particular amino acid or a start/stop signal