Orbital Theory, Electron Configurations, Hybridisation
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
What is valency?
Atoms want a complete outer shell of electrons
For example, carbon has 4 electrons in the outer shell, so for a full outer shell, it needs 4 extra electrons. This is done by forming covalent bonds with other atoms and sharing the bond electrons. The valency of carbon is 4.
Valency of an atom = The number of bonds needed to complete the shell
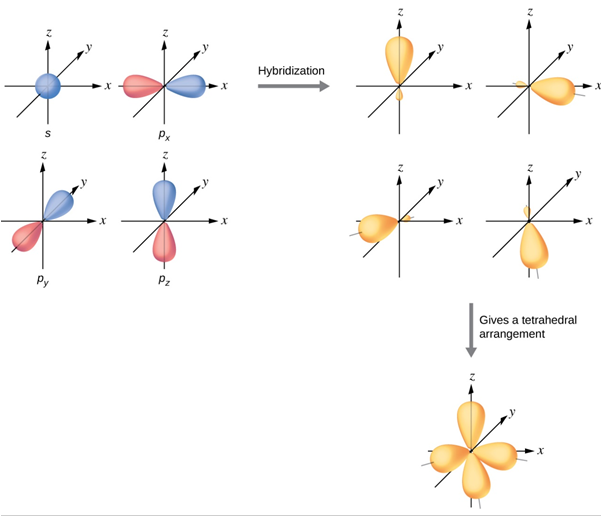
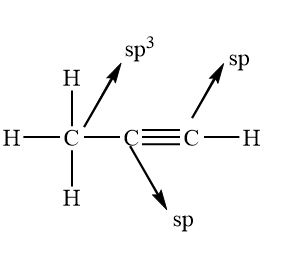
sp3
Tetrahedral
109.5
All bonds are sigma
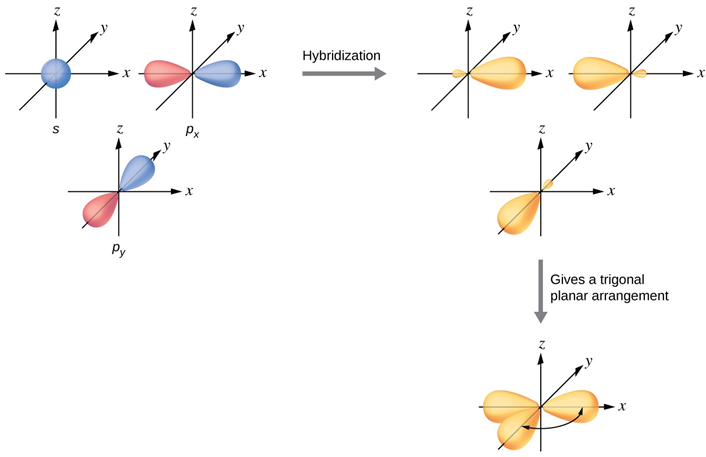
sp2
Trigonal planar
120
Bonds are single to the hydrogens, and a double bonds to carbons (sigma and pi combination)
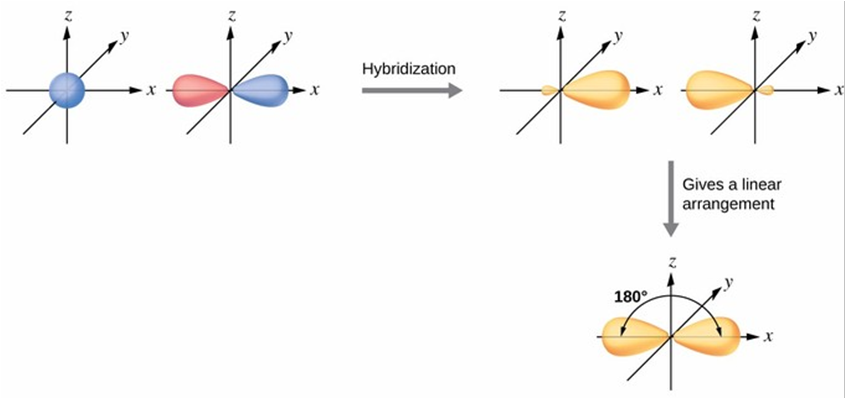
sp
Linear
180
Sigma bonds to the hydrogens and 2 pi bonds between the carbons along with a sigma bond, making a triple bond
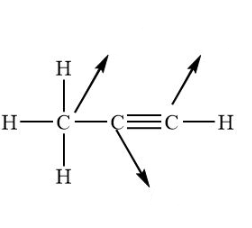
What’s the hybridisation in these molecules?
Sigma bonds
Strongest type of covalent chemical bond
Forms when atomic orbitals overlap in a head on arrangement (like two 2 s orbitals, one s orbital and one p orbital)
σ
How does sigma bonding affect the shape and flexibility of drug molecules?
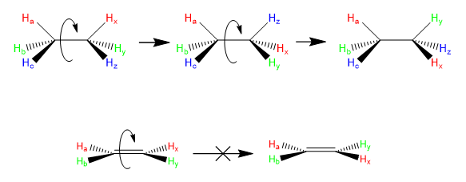
Sigma bonds = single bond from orbital overlap means free rotation
How does pi bonding affect the shape and flexibility of drug molecules?
Multiple bond from overlap of p-orbitals means fixed orientation
Double bonds in structures means there is…
restricted rotation