Fractional distillation + Cracking + Reformation
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
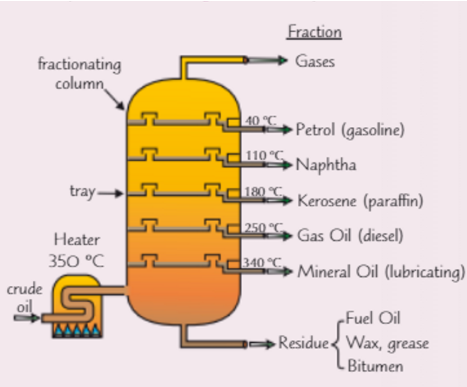
How does fractional distillation of crude oil work?
Mixture is vapourised at 350 celsius
Vapours rise, cool and condense
Short hydrocarbons will condense near the top of fractionating column where it is cooler (they have low boiling points)
Long hydrocarbons will condense at the bottom of fractionating column where it is warmer (they have high boiling points)
What is cracking?
Cracking- the breaking of a longer chain alkane to shorter hydrocarbons
What are two methods of cracking?
Thermal cracking
Catalytic cracking
What are the conditions needed for thermal cracking?

What does thermal cracking produce?
Alkenes (alkenes can be used to make polymers such as plastic)
What are the conditions needed for catalytic cracking?

What are products of catalytic cracking?
Aromatic hydrocarbons
Why is the zeolite catalyst used?
Zeolite catalyst lowers temperature and pressure needed for cracking to occur -> lowest cost and speeds up the process
What is knocking?
The explosion upon compression that straight hydrocarbons create.
How do we reduce knocking?
Adding branched and cyclic alkanes (increases engine efficiency)
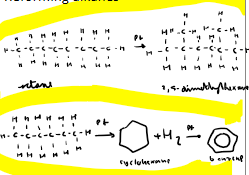
How can we make cyclic and branched alkanes?
With the use of a platinum catalyst in the process of reformation