Immunology - Lecture 18 - T cell priming by dendritic cells
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What is the significance of dendritic cells in T cell priming, and who contributed to the understanding of immune system connections?
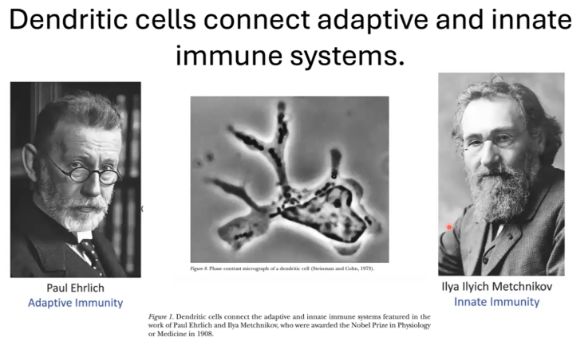
Dendritic cells are key in linking the innate and adaptive immune systems by priming T cells.
Ralph Steinman identified dendritic cells as essential for this connection.
The 1908 Nobel Prize in Medicine was awarded to:
Paul Ehrlich for research on antibodies and adaptive immunity.
Ilya Ilyich Metchnikov for work on phagocytes and innate immunity.
Both made foundational contributions to immunology.
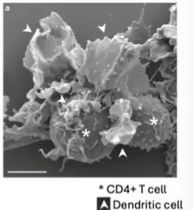
What was the significance of discovering dendritic cells in understanding immune responses?
When T and B cells were placed with antigen in a dish, no immune response occurred until an "accessory cell" from the spleen was added.
This accessory cell was identified as the dendritic cell.
Unlike macrophages, dendritic cells have a unique morphology and are essential in linking the innate and adaptive immune systems.
Dendritic cells act as "professional antigen-presenting cells", specifically activating T cells.
This activation allows T cells and B cells to interact and coordinate an immune response.
The discovery transformed immunology by revealing how the immune system learns about antigens and connects adaptive and innate immunity.
What are dendritic cells and what is their role in the immune system?
Dendritic cells are short-lived immune cells serving as immune sentinels.
Constantly sample tissues to capture and process antigens, monitoring for pathogens and homeostatic changes.
Exist in two functional states:
Immature/resting: focused on antigen capture.
Mature/activated: responsible for T cell activation.
Dendritic cells are both functionally and ontogenetically heterogeneous.
In their mature state, they deliver antigens and essential signals for T cell activation.
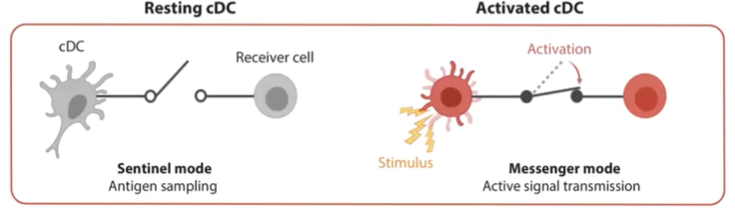
What is the role of a resting conventional dendritic cell (cDC)?
In their resting state, conventional dendritic cells (cDCs) operate in "sentinel mode."
Primarily focused on information sensing and antigen sampling.
Extend dendrites to cover a large surface area for continuous monitoring.
Constantly gather information but do not initiate an immune response in this state.
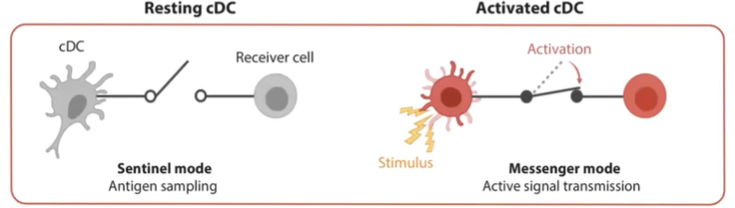
What changes occur in a conventional dendritic cell (cDC) when it becomes activated?
Upon detecting a pathogen or danger signal (e.g., a dying cell), cDCs become activated.
Stop antigen sampling and reduce phagocytic activity.
Begin migrating to the lymph node.
Primary mission shifts to transmitting information to initiate an immune response.
What are the primary and additional functions of dendritic cells in the immune system?
Dendritic cells (DCs) start in tissues, capturing antigens.
Travel to lymph nodes to primarily activate T cells and aid in generating new T cell types.
Main role: T cell activation, but they also influence other tissue cells (e.g., fibroblasts and neurons), shaping the tissue environment.
Form synapses with NK cells and interact with other innate immune cells through cytokine secretion.
Central to coordinating immune responses across various cell types.

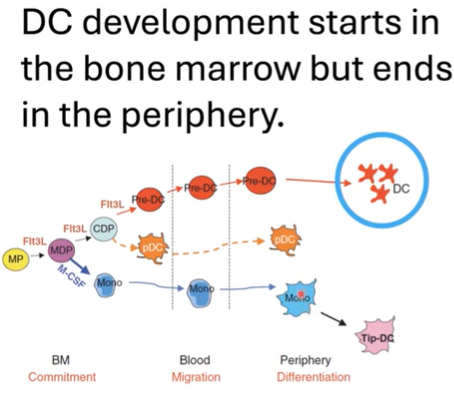
How does dendritic cell (DC) development progress, and what key stages and factors are involved?
Dendritic cell (DC) development begins in the bone marrow and completes in peripheral tissues.
Starts with hematopoietic progenitors that produce myeloid cells committed to the DC lineage.
Pre-dendritic cells differentiate into subsets in the bone marrow:
Conventional/classical DCs (cDCs).
Plasmacytoid DCs (pDCs): specialize in interferon production for viral infections.
Monocyte-derived DCs also belong to the DC lineage.
Flt3L is a crucial ligand required for DC development in both bone marrow and peripheral tissues.
In tissues, immature DCs are in a resting state, focused on antigen sampling.
Upon maturation, DCs activate, shifting function to antigen presentation for T cell activation.
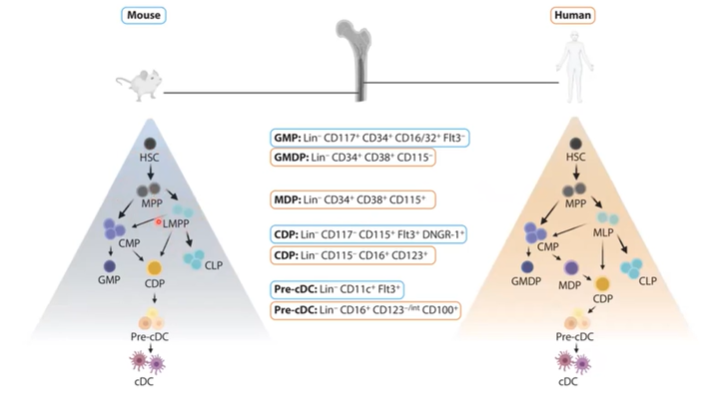
How do mouse and human dendritic cell (DC) development programs compare, and why are mice used as models?
Mouse and human dendritic cells (DCs) follow a similar developmental program.
Both progress through stages from hematopoietic stem cells (HSCs) to pre-DCs and mature cDCs.
Mice are often used as models due to this developmental similarity.
Human DCs show greater plasticity in differentiation pathways, adding complexity to human studies.
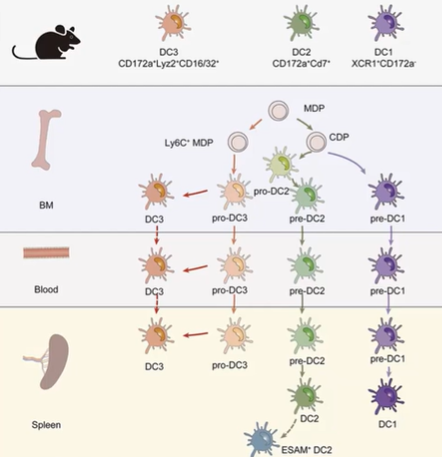
What are the main classical dendritic cell (cDC) subsets in humans and mice, and how do they function in T cell activation?
The main subsets of classical dendritic cells (cDCs) are cDC1, cDC2, and cDC3.
Initially thought:
cDC1 mainly cross-presents antigens to CD8+ T cells.
cDC2 primarily presents to CD4+ T cells.
However, all cDC types can present antigens to both CD4+ and CD8+ T cells, demonstrating functional overlap.
In both humans and mice:
cDC1 is well-characterized and distinct.
cDC2 and cDC3 are interrelated and challenging to differentiate during inflammation (e.g., during infections).
What are the steps and functional outcomes of dendritic cell (DC) maturation?
Dendritic cells switch from a resting to an activated state through a maturation process.
Traditionally believed:
Immature DCs induce tolerance.
Mature DCs drive protective T cell immunity.
Current understanding:
Mature DCs can lead to two functional outcomes:
Immunogenic: Activating T cells for immunity.
Homeostatic: Promoting tolerance.
Both outcomes follow the same maturation pathway.
What are the key changes in a dendritic cell (DC) during maturation?
DC maturation involves a transition from a sensing mode to an information-relaying mode.
Early immature (resting) cDC1 cells:
High expression of XCR1 (chemokine receptor).
High expression of MHC II.
Moderate expression of TLRs (Toll-like receptors).
Upon sensing a maturation signal (e.g., a pathogen or dying cell), DCs rapidly mature:
Pathogen signals lead to an immunogenic state.
Dying cell signals lead to a homeostatic state.
During full maturation:
TLR and XCR1 expression is downregulated, reducing sensing ability.
CCR7 is upregulated to facilitate entry into the lymph node.
MHC II and cytokine expression is increased for effective T cell activation and antigen presentation.
What are the three phases of T cell priming by dendritic cells, and what occurs at each phase?
T cell priming by dendritic cells (DCs) occurs in three phases at the T cell synapse:
Transient Serial Encounters (First 8 hours):
T cells decrease motility.
Upregulate activation markers.
Stable Contacts (Next 12 hours):
T cells form stable contacts with DCs.
Begin secreting cytokines such as IL-2 and IFN-gamma.
Rapid Migration and Short DC Contacts (>24 hours):
T cells start rapid proliferation.
Establish brief interactions with DCs.
Discovery Protocol:
Antigen-pulsed dendritic cells were injected into recipient footpads 18 hours prior to adoptive T cell transfer, allowing observation of these priming phases.
What occurs during the first phase of T cell priming by dendritic cells?
Phase 1 - Transient Serial Encounters (First 8 hours):
T cells decrease motility and upregulate activation markers.
Naive T cells sample multiple dendritic cells (DCs) through transient interactions, primarily mediated by adhesion molecules.
These brief encounters enable T cells to "scan" DCs for antigen specificity.
Once a T cell identifies a DC with the correct antigen, it progresses to the next phase of priming.
What occurs during the second phase of T cell priming by dendritic cells?
Phase 2 - Stable Contacts (Next 12 hours):
The T cell and dendritic cell form a stable interaction known as the T cell synapse, where the T cell stops moving.
During this stable interaction, the T cell begins producing cytokines, starting with IL-2, which supports T cell survival and proliferation.
The T cell upregulates the high-affinity IL-2 receptor and begins producing other cytokines, such as IFN-gamma.
After this phase, the T cell is primed and ready to proliferate, having received all necessary signals from the dendritic cell.
What happens during the third phase of T cell priming by dendritic cells?
Phase 3 - Rapid Migration and Short DC Contacts:
T cells enter a rapid proliferative program, dividing up to 3 times per day.
During this phase, T cells establish brief, transient contacts with dendritic cells as they proliferate quickly.
This expansion increases the population of activated T cells ready to respond to the antigen.
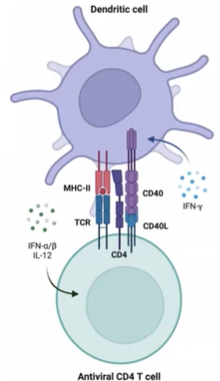
What are the primary factors that govern T cell priming, and how does the TCR-MHC interaction contribute to antigen specificity?
T cell priming is governed by:
TCR activation: The interaction between TCR and MHC-peptide provides antigen specificity, essential for synapse formation.
Costimulation: Additional signals needed for full activation.
Cytokines: Help shape T cell response.
TCR and MHC interaction is the activation signal from the dendritic cell to the T cell.
Coreceptors CD4 or CD8 lower the threshold of required TCR/MHC interactions.
This synapse formation polarizes the T cell, changing its morphology to focus on the DC for effective communication.
What are the key components of the central supramolecular adhesion complex (C-SMAC) in the immunological synapse, and how do they influence T cell differentiation?
The C-SMAC (central supramolecular adhesion complex) forms at the T cell and dendritic cell synapse and includes:
TCR/Antigen-MHC: Provides antigen specificity.
Coreceptors (CD4/CD8): Lower activation threshold.
Costimulatory receptors:
CD28
CD40
ICOS
Cytokines: Influence the differentiation path of the T cell, determining the specific T cell subtype that will develop.
These components collectively impact T cell activation and differentiation.
How do cytokines from dendritic cells determine T cell effector subsets, and what are the resulting immune responses?
Cytokines from dendritic cells guide the differentiation of T cell effector subsets:
IL-12 from dendritic cells promotes Th1 differentiation:
Leads to the production of Interferon-gamma by Th1 cells.
Drives a cytotoxic, IgG-rich response that effectively clears bacteria with minimal infection risk.
IL-4 promotes Th2 differentiation:
Results in the production of cytokines such as IL-5, IL-4, and IL-13.
Th2 responses are associated with allergic reactions and produce antibodies that do not effectively clear certain bacterial infections.
This can lead to persistent infections, tissue damage, and severe complications.
These cytokine-driven pathways shape the immune response and significantly impact infection outcomes.
What happens if a naive T cell does not receive all three signals (TCR activation, costimulation, and cytokines) for activation?
If a naive T cell does not receive all three signals, it can undergo:
Anergy (a state of unresponsiveness)
Death by deletion
Tolerance to the antigen
Inability to form long-lived memory cells
These outcomes prevent effective immune responses against the antigen.
How do CD4 T helper cell subsets and their functions depend on cytokines?
CD4 T helper cells differentiate into specific subsets based on initial cytokine signals from dendritic cells.
This "first order" of cytokines determines the transcription factors activated in T cells.
The activated transcription factors lead to specific "second order" cytokine production.
This process results in functional programs tailored for different immune responses.
What does a Tfh (T follicular helper) cell do?
Cytokine: IL-6
Function: Supports strong B cell responses and antibody production; crucial for germinal center formation through upregulation of Bcl6.
What does a Th2 cell do?
Cytokine: IL-4
Function: Initiates type 2 immunity to fight parasites and plays a role in allergy; driven by GATA3.
What does a Th1 cell do?
Cytokine: IL-12
Function: Promotes type 1 immunity, essential for clearing intracellular pathogens, by upregulating T-bet and producing interferon-gamma.
What does a Th17 cell do?
Cytokines: IL-6 and TGF-beta
Function: Promotes responses against extracellular pathogens like fungi, mediated by RORγT upregulation.
What does a Treg (Regulatory T) cell do?
Cytokines: IL-2 and TGF-beta
Function: Suppresses immune responses, maintaining tolerance through IL-10 and TGF-beta production, driven by Foxp3.
How do dendritic cells enable CD8 T cells to recognize exogenous antigens on MHC Class I?
Through cross-presentation, dendritic cells phagocytose exogenous antigens and redirect them into the cytosol.
This process deviates from the typical MHC Class II pathway.
The processed antigen is then presented on MHC Class I, facilitating recognition by CD8 T cells.
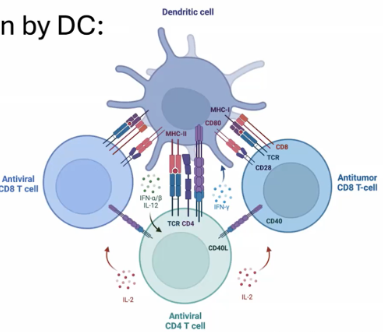
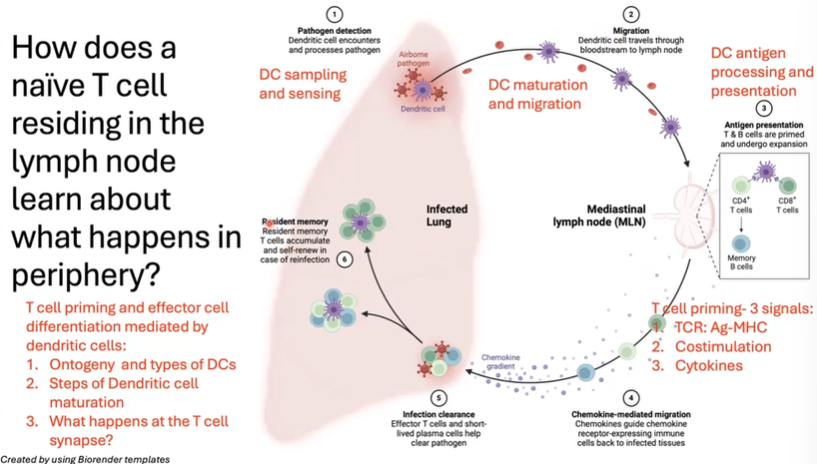
What steps allow a naive T cell in the lymph node to respond to an infection in the periphery?
The process begins with dendritic cell (DC) sampling and sensing in the periphery.
Upon detecting pathogens or danger signals, the DC matures and migrates to the lymph node.
In the lymph node, it presents antigens to both CD4 and CD8 T cells. Activation requires three signals:
TCR/MHC interaction
Costimulation
Cytokine signaling
Once primed, the T cell proliferates and follows a chemokine gradient to the infected tissue.
The end result is the establishment of resident memory T cells at the infection site and in secondary lymphoid organs like the spleen and lymph nodes.