Chemistry - Unit 1 - Topic 1 - Time of Flight Mass Spectrometry
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
what is mass spectrometry used for? (elements)
to calculate the relative atomic mass of element
what is mass spectrometry used for? (compounds)
to identify unknown purified compounds by comparing the mass spectrum produced to another
what are the 6 stages of TOF mass spectrometry?
vaporisation
ionisation
electric field
ion drift
detection
data analysis and mass spectra production
describe the stage of vapourisation
samples of unknown substances are injected into a vacuum and vaporised
what are the two types of ionisation?
electrospray ionisation
electron impact ionisation
describe electrospray ionisation
the sample is dissolved in a polar solvent, which is pushed through the sample at high pressure
a high voltage is applied causing each sample to gain a H+ ion
the solvent is removed
this means that the Ars produced in the mass spectra will be 1 more than the true values
describe electron impact ionisation
electrons are fired at the sample
this causes one electron to be knocked off per atom, causing ions to be formed with +1 ions
give an equation for electron impact ionisation including state symbols
A (g) + e- → A+ (g) + 2e-
when would electron impact ionisation be used?
for compounds that have a low risk of fragmentation - low formula mass
when would electrospray ionisation be used?
for compounds with a high risk of fragmentation - high formula mass - as it rarely causes fragmentation
describe the stage of acceleration
an electric field is applied to the ions giving them all the same kinetic energy
this causes them to accelerate
heavier particles move more slowly than lighter particles
describe the stage of ion drift
ions enter a region with no electric field called the flight tube
ions are separated based on their different velocities (and thus masses)
smaller, fast ions travel through the flight tube more rapidly and arrive at the detector first
describe the stage of ion detection
ions collide with a negatively charged detection plate
they are given one electron by the plate, which causes a current to form
theoretically all ions of the same atomic mass will collide with the plate at the same time and therefore cause a large spike in current
describe the stage of data analysis
the flight time at which peaks form corresponds to the Ar
a mass spectrum is produced, which is a plot of relative abundance against mass to charge ratio (m/z)
current is proportional to relative abundance
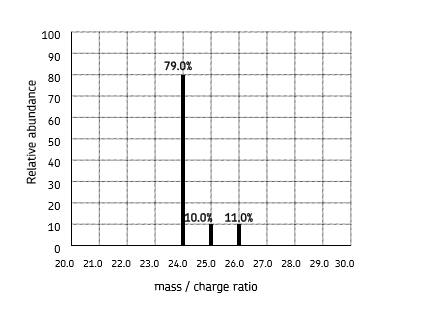
describe how to interpret a mass spectrum
number of peaks = number of isotopes or different particles (depending on what you are working out)
the relative atomic mass is the mean mass of the isotopes (combined mass of all isotopes(x abundance)/combined abundance)
m/z ratio is the same as Ar or Ir
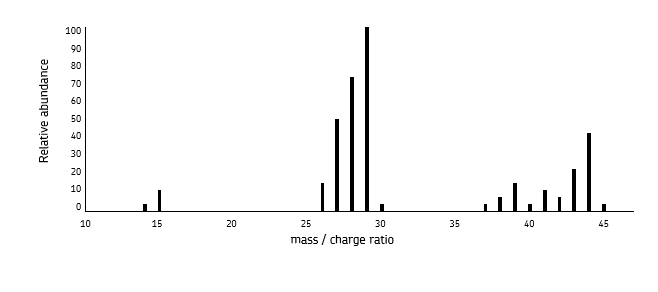
what must you do with Mrs, Ars and Irs in mass spectra from electrospray ionisation?
subtract 1 as a proton is added to each ion
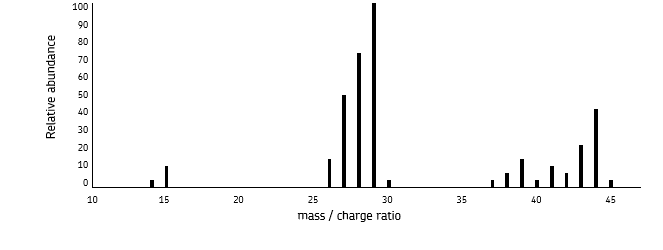
how would a mass spectrum for electron impact ionisation or a compound differ from a mass spectrum for electrospray ionisation?
the compound may fragment, causing many different peaks and groups of peaks
the largest peak is the base peak - most stable ion formed
the last major peak (here 46) is the the Mr of the compound
no fragmentation occurs with electrospray
describe how to interpret the mass spectra of molecules, using chlorine as an example
small molecules can form single ions with a positive charge, or can be broken up into atoms
so for chlorine, there are 5 possible atoms which should be detected - 35Cl+; 37Cl+; (35Cl-35Cl)+; (35Cl-37Cl)+; (37C-37C)+
so there will be peaks at 35 and 37 (ratio 3:1) as well as 70,72,74 (ratio 9:6:1 - multiplication of probability)