nucleophilic addition to carbonyls
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
what is an imine?
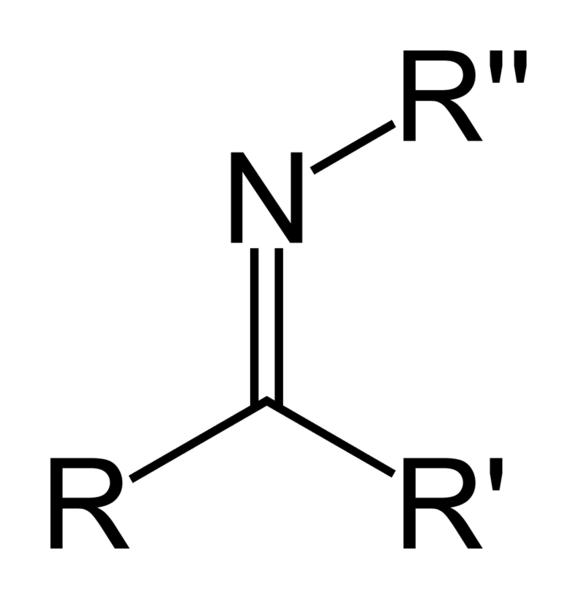
what is an oxime?

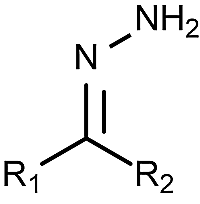
what is a hydrazone?
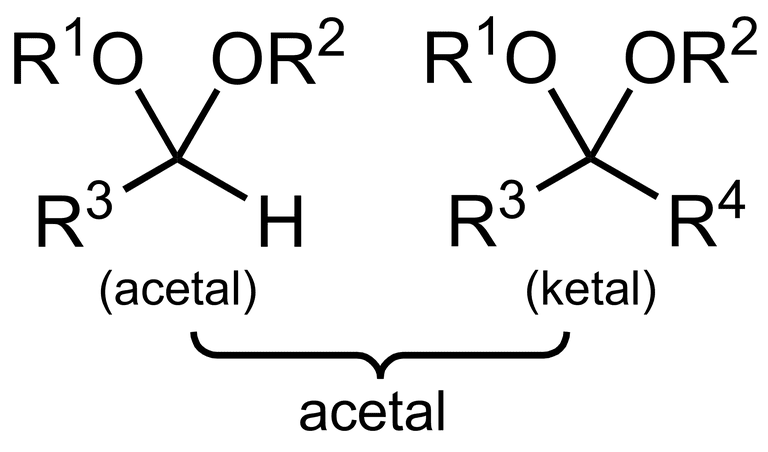
what is an acetal?
what is a nitrile?
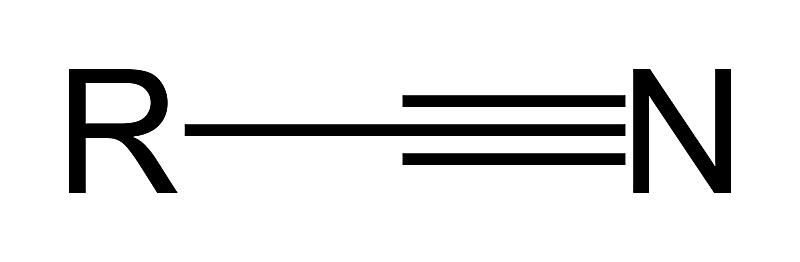
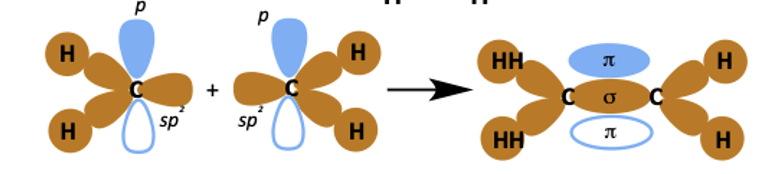
how do the sp2 and p orbitals overlap when alkenes form?
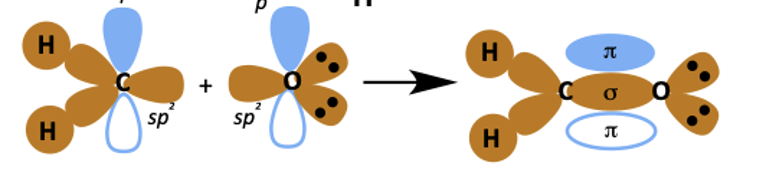
how do the sp2 and p orbitals overlap when carbonyls form?
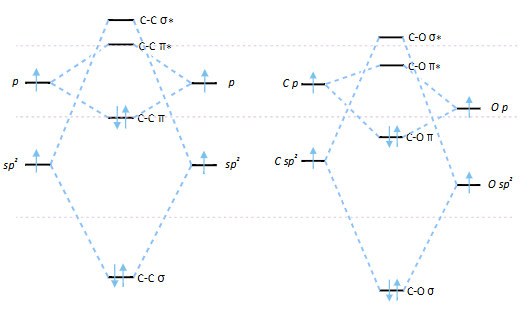
what is the difference between C=C MOs and C=O?
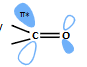
what is the carbonyl HOMO and LUMO?
why is carbonyl not nucleophilic? why is it electrophilic?
O orbitals lower in energy as more electronegative
MOs filled are lower in energy so not nucleophilic
O lone pairs are HOMOs
empty orbitals are lower energy - LUMO (π*) able to accept electrons electrophilic
C=C vs C=O MO diagram
are C=O bonds stronger or weaker than C-O?
twice as strong
why are carbonyl compounds still reactive to nucleophiles?
although C=O is strong, it is polarised
nucleophilic species are attracted to partially positive carbon - can attack the large lobe of low lying LUMO = π*
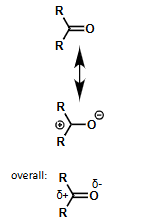
what are the resonance forms of carbonyl bond? what is it overall?
where is the greater orbital density in the carbonyl bond?
what is the shape of the π MO?
towards the O atom (electronegativity)
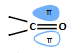
diagram of carbonyl HOMO
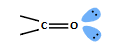
what is the LUMO diagram?
anti bonding MO has greater coefficient on C atom
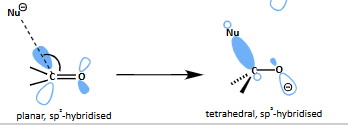
what is the mechanism for nucleophilic attack of C=O?
how does the shape change?
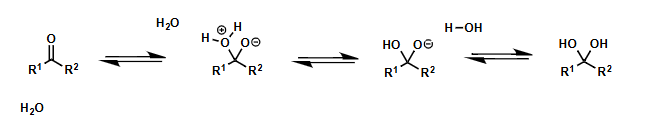
water as nucleophile reactions
what does it generate?
hydrates or 1,1-diols

which way does the equilibrium lie?
equilibrium lies to the side of the carbonyl compound (breaking strong C=O bond)

how does Keq change as substitution increases?
Keq decreases as substitution increases
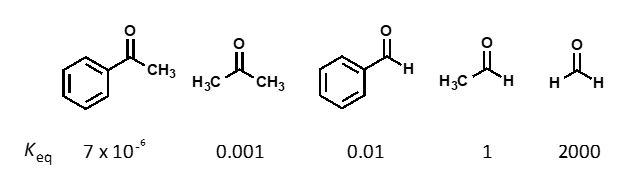
what are the causes of Keq trend?
sterics and electronics
how does sterics affect Keq?
larger atoms than H hinder the nucleophilic attack
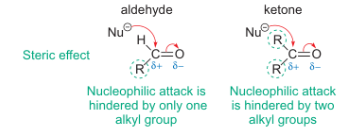
how do electronics affect Keq?
are alkyls or aryls more reactive?
stabilisation of carbonyl by hyperconjugation or conjugation lost on going to sp3
alkyls are more reactive as aryls are more stable
how does conjugation affect electrophilicity of carbonyl groups?
conjugation stabilises the C=O group
C has lower partial positive charge so is less electrophilic
how do inductive effects affect electrophilicity of carbonyl groups?
+I (electron donating) reduce electrophilicity
reduces size of partial positive charge on carbon
why do larger substituents disfavour the breaking of the C=O bond?
they would go to an sp3 system
reduces bond angle = greater repulsion
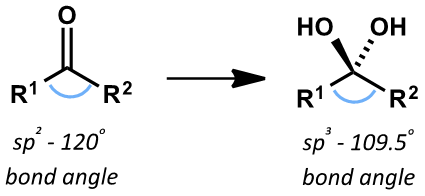
what is the formula and structure of acidified dichromate?
Cr2O72-
what do secondary alcohols oxidise to?
what is the reaction?
to ketones
using acidic dichromate
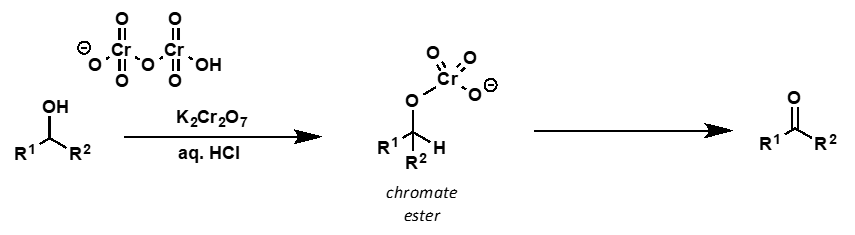
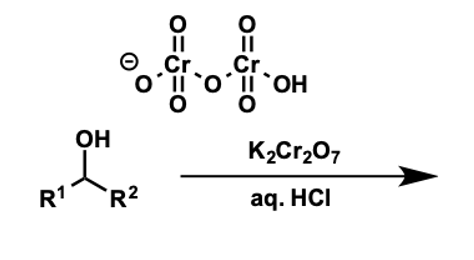
what is the mechanism?
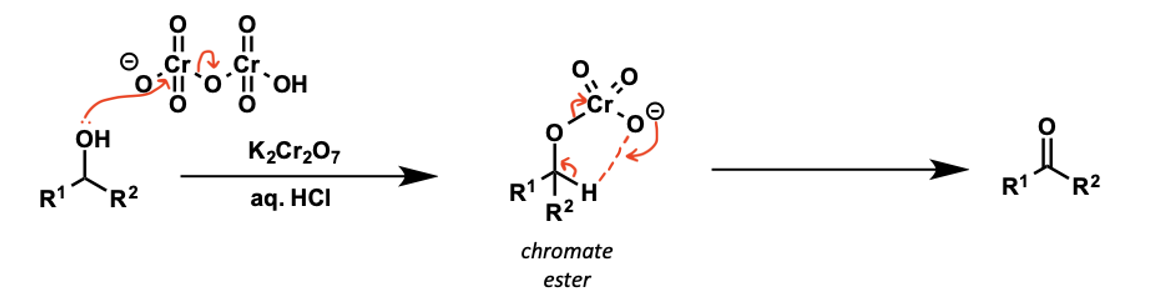
secondary alcohol mechanism with CrO3
what is the colour change?
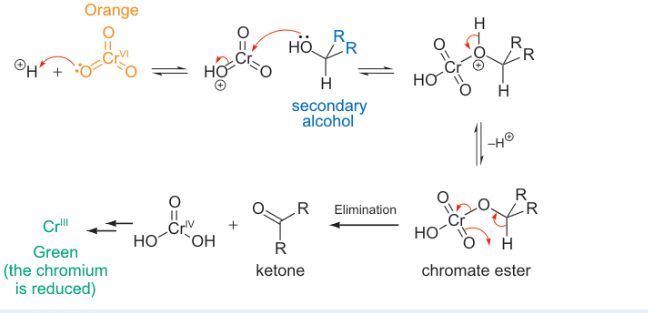
what do primary alcohols oxidise to?
aldehyde then carboxylic acid
primary alcohol oxidation reaction steps
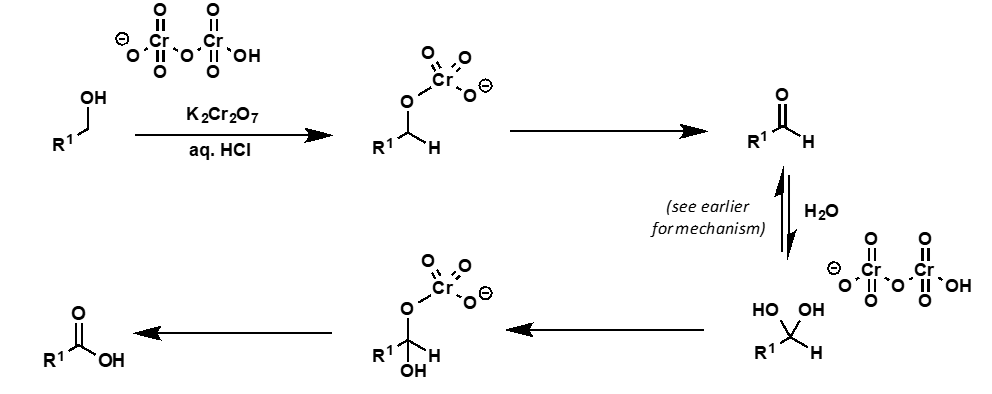
how to selectively oxidise a primary alcohol to aldehyde?
avoid water so hydrate and hence c. acid cannot form
use pyridinium dichromate
what is pyridinium dichromate?
form of dichromate anion which is soluble in organic solvents and
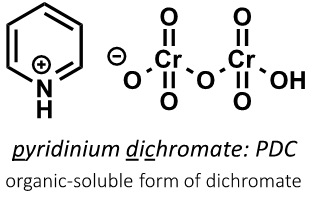
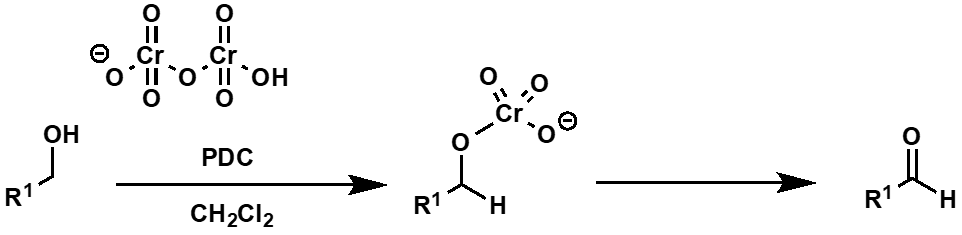
what is the reaction with a primary alcohol and PDC?
sodium borohydride reaction with aldehydes/ketones?
reduction
sodium borohydride reaction
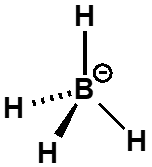
why is hydride anion not nucleophilic?
why is boron not nucleophilic?
what is reacting HOMO?
too hard and prefers to act as strong base
boron is not nucleophilic as all electrons are in B-H bonds
reacting HOMO is in B-H bond
what are conditions for sodium borohydride reductions?
alcoholic solvents e.g. MeOH or EtOH
what is an alternative reducing agent for aldehydes/ketones?
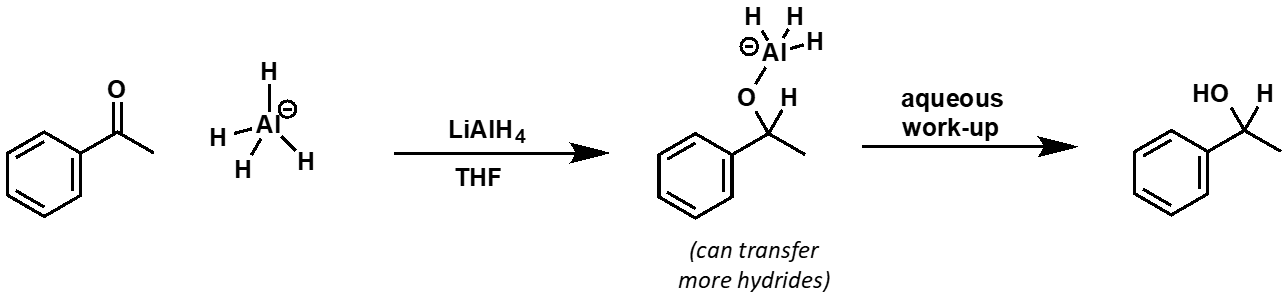
lithium aluminium hydride
lithium aluminium hydride reduction conditions
aprotic solvents - violent reaction with water/alcohols
lithium aluminium hydride reaction
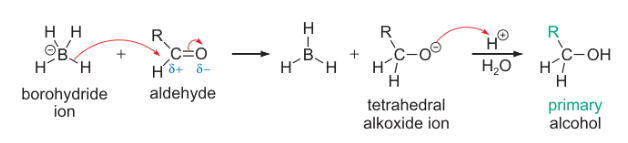
borohydride + aldehyde mechanism?
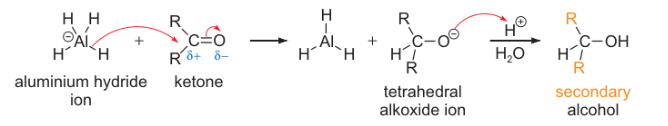
aluminium hydride + ketone mechanism?
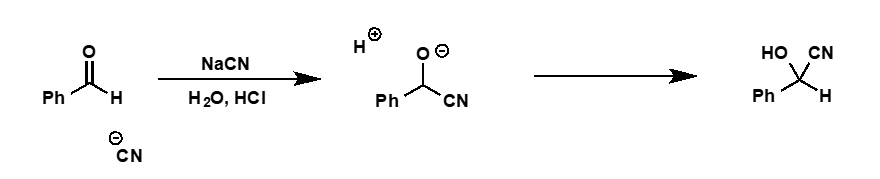
cyanide as nucleophile for carbonyls reaction
which side does equilibrium lie? why?
lies to the side under acidic conditions
how can nitrile group be converted to COOH?
aq. NaOH and heat
or aq. acid and heat
how can nitrile group be converted to amine?
LiAlH4
what are acetylide ions?

what is acetylide like as a reagent?
how is it an organometallic reagent?
not free anions
polar carbon metal bond
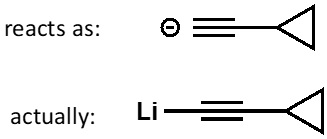
what is HOMO in acetylide?
how is bond polarised? diagram?
HOMO is high lying, weak carbon-metal sigma bond
metal is more electropositive so bond is polarised towards C

how are organometallics made?
conditions?
organic halides and elemental metal (Li or Mg)
air and moisture sensitive so must be in dry, aprotic solvents e.g. THF or Et2O
product of ketone/aldehyde reaction with organometallics?
alcohol
what are the general steps when organometallics react with carbonyls?
formation of alkoxide ion
reacts with H+ in separate stage
reaction of bromobenzene + Li followed by aldehyde

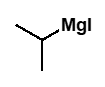
what are Grignard reagents?
organomagnesium halide reagents
R-Mg-X
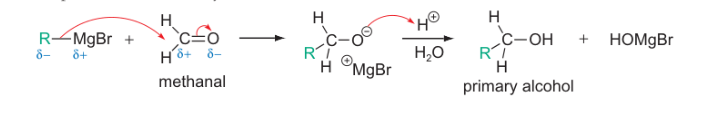
R-MgBr reacting with methanal reaction
how can secondary alcohols be made?
reduction of ketones
addition of organometallics to aldehydes
how can tertiary alcohols be made?
addition of organometallics to ketones
which group in an organometal is the nucleophile or base?
the organic group
carbon is partially negative as metal is more electropositive
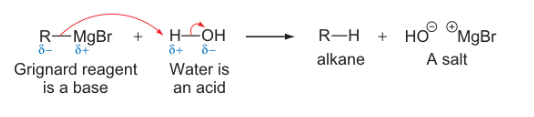
grignard reagent + water reaction?
what type of reaction is this? why is this possible?
acid-base
this is possible even though water is very weakly acidic as organometallics are such good bases (extremely reactive)
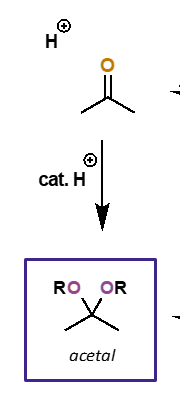
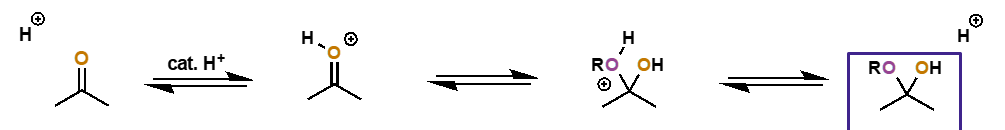
alcohol addition to carbonyls
catalysed?
what is the intermediate and what is the final product?
catalysed by acid
initially formed is hemiacetal adduct
final product is acetal
carbonyl + alcohol to hemiacetal mechanism
hemiacetal to acetal (acidic conditions with alcohol)
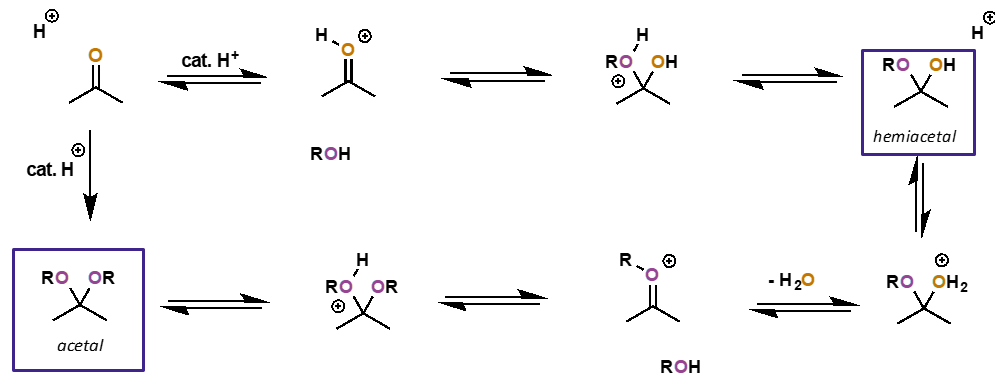
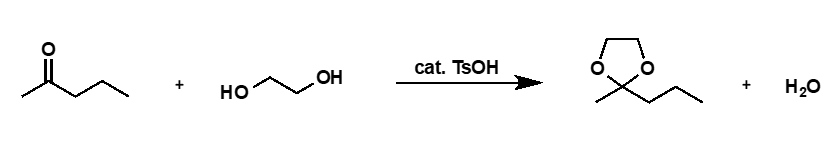
why are cyclic acetals more stable than acyclic ones?
less entropically disfavoured since two species on each side of equilibrium
how can an acetal be converted back to carbonyl?
why is this thermodynamically favourable?
reformation of strong C=O means energetically favourable
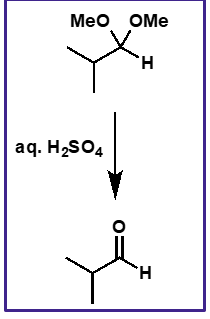
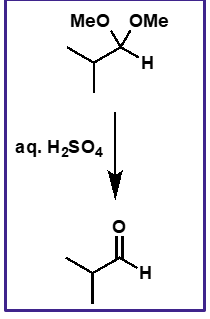
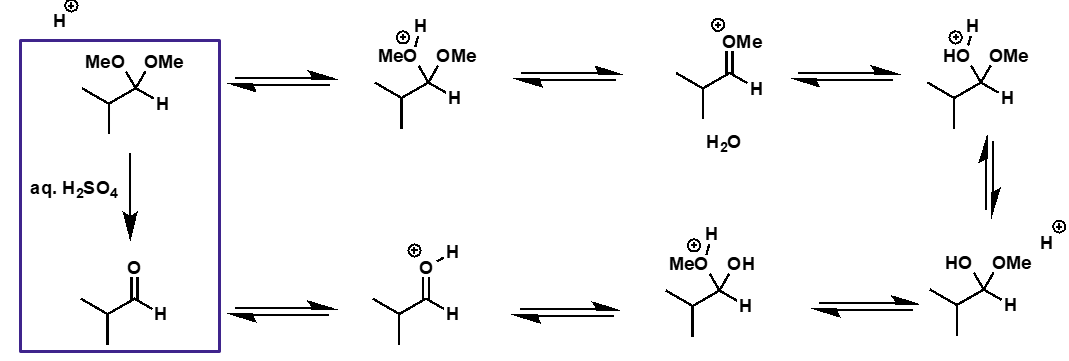
acetal to aldehyde mechanism
what is a hemiacetal?
when an alcohol and ether are attached to the same carbon
what are acetals sensitive and robust to?
sensitive to acids
robust to other reagents eg organometallics or bases
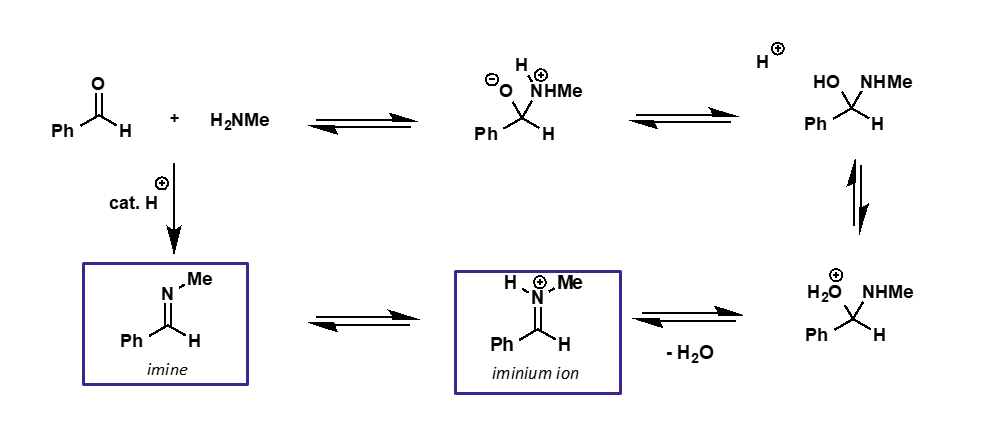
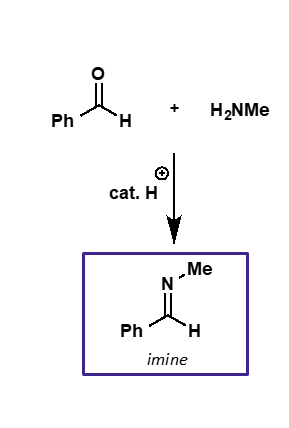
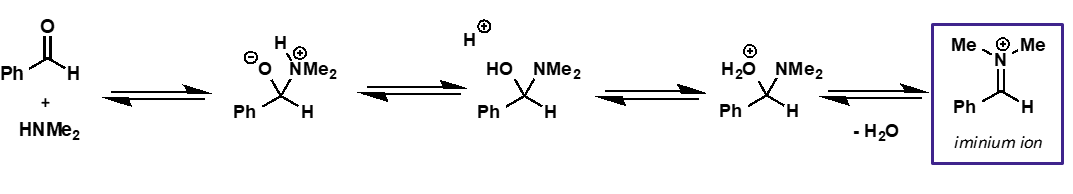
what is the intermediate and product of carbonyl + amine?
intermediate is iminium ion
product is imine
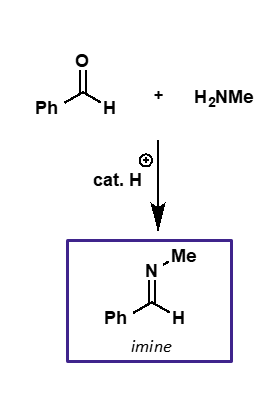
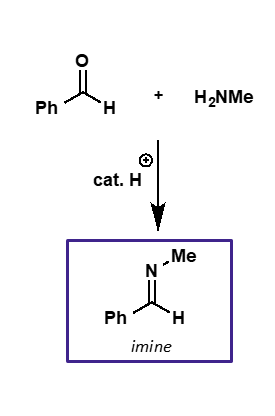
explain the catalysis needed for amine nucleophilic addition
initial addition doesn’t need to be catalysed as amine is a good nucleophile
hydroxide is a poor leaving group so mild acid catalysis needed
amine + carbonyl mechanism
which way does equilibrium lie?
why?
how is it shifted?
C=N weaker than C=O so equilibrium lies to carbonyl
shifted by azeotropic removal of water
what is the azeotropic removal of water?
toluene and water form a mixture which boils at a lower temperature than either component
water can therefore be removed by distilling azeotrope
what do imines hydrolyse to?
conditions?
back to carbonyl compound
mild acid catalysis

what is mechanism?
what is formed?
ketone formed

does a neutral imine species form when a secondary amine is the nucleophile?
no
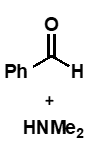
secondary amine + carbonyl reaction
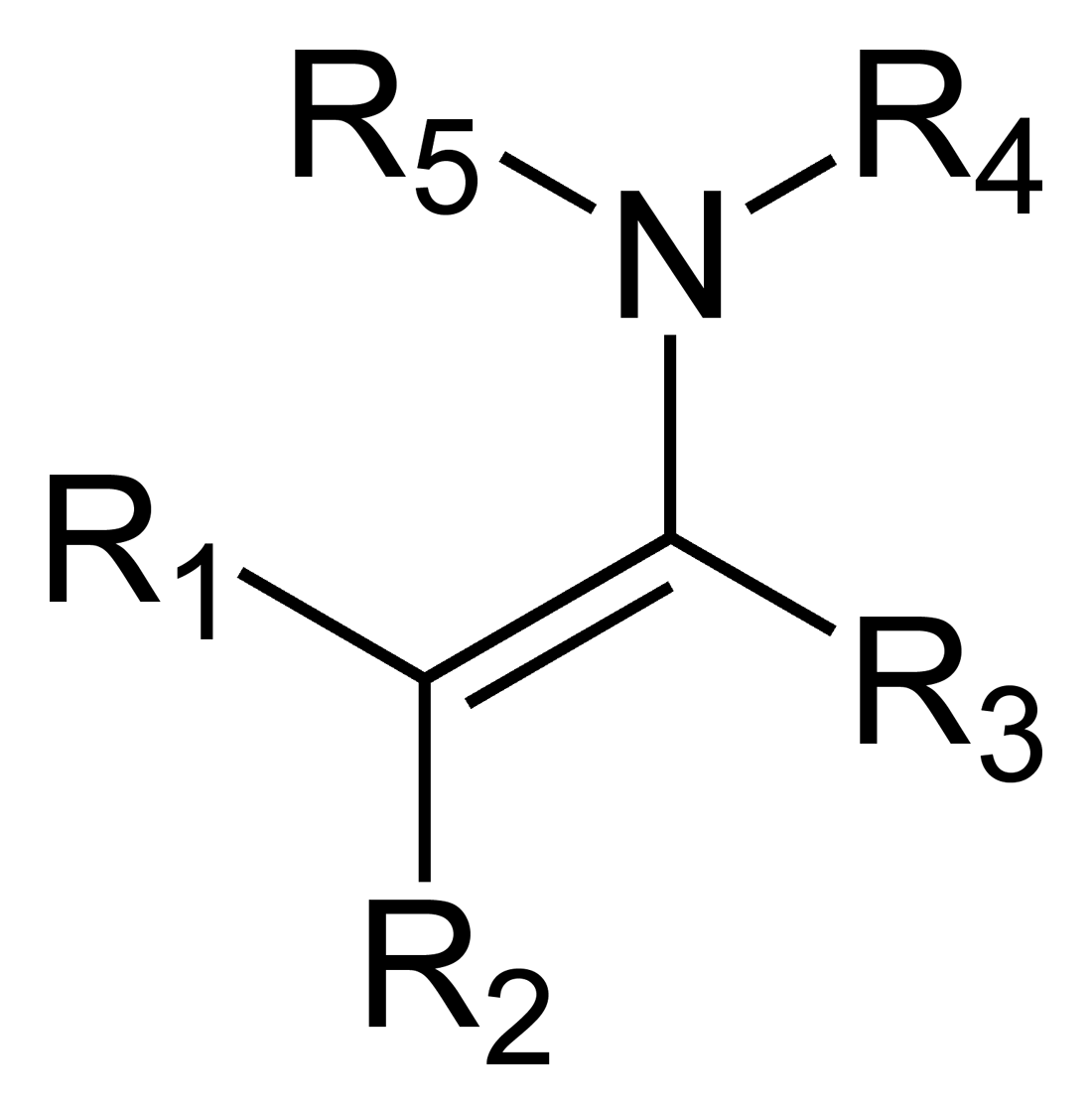
what is an enamine?
contains alkene and amine group
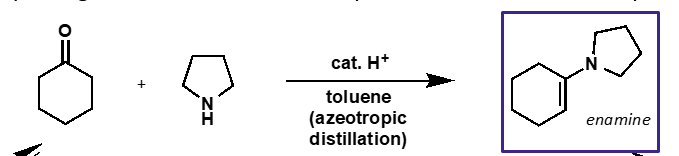
under what conditions is an enamine formed? why?
formed under dehydrating conditions
less stable than carbonyls
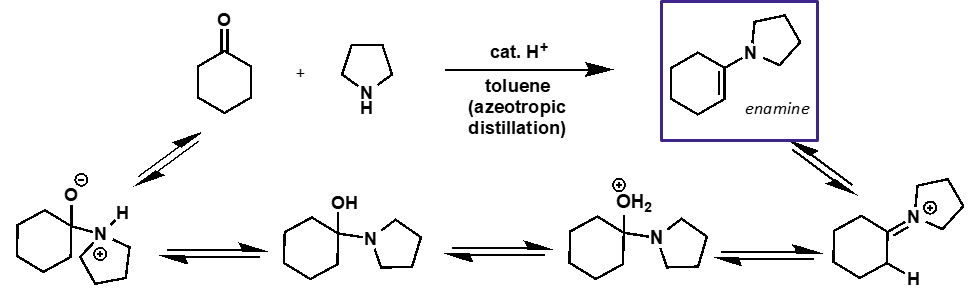
what is the mechanism
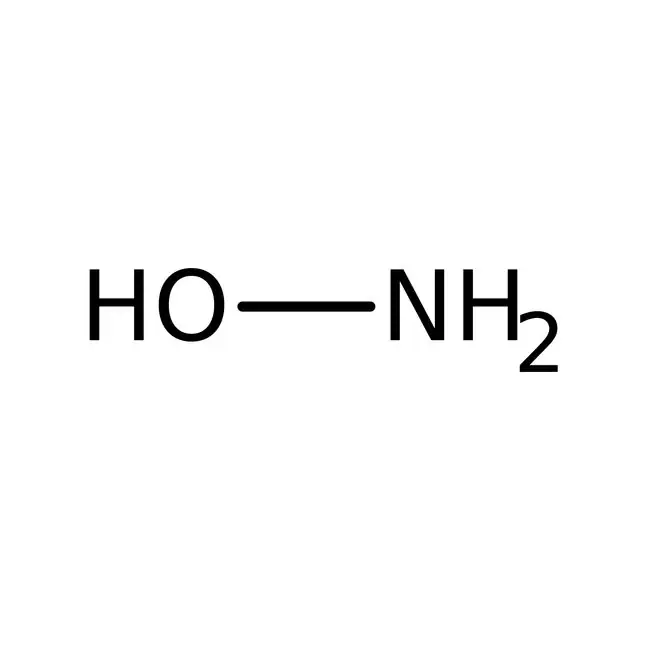
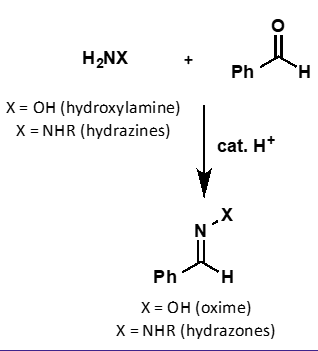
what is hydroxylamine?
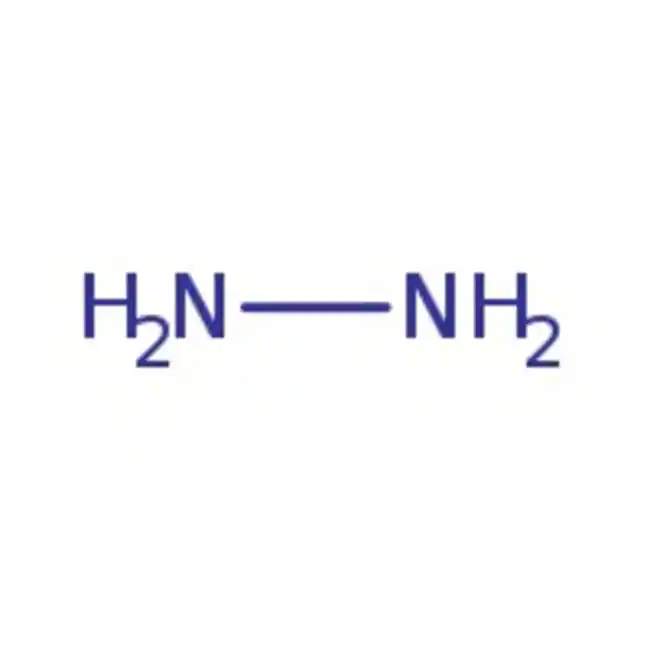
what is hydrazine?
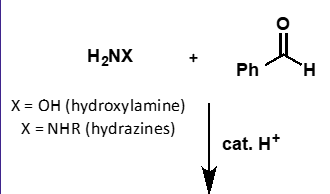
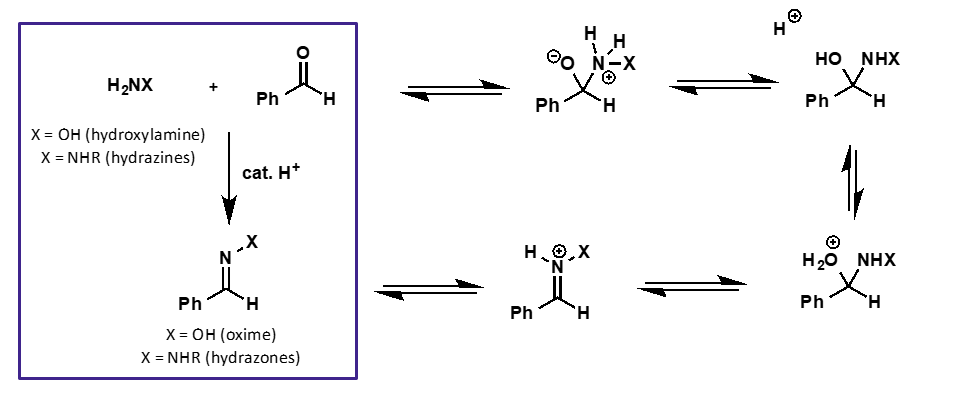
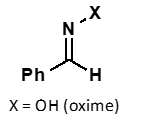
what does hydroxylamine + carbonyl form?
oxime
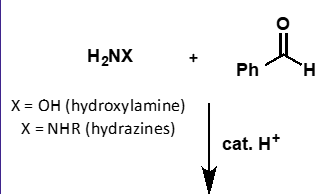
what does hydrazine + carbonyl form?
hydrazone
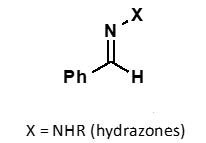
why are hydroxylamine and hydrazines adducts more stable than carbonyls?
products are more stable due to conjugation of N or O lone pair with C=N bond
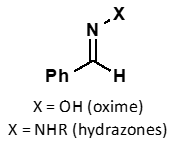
show resonance structures of both compounds

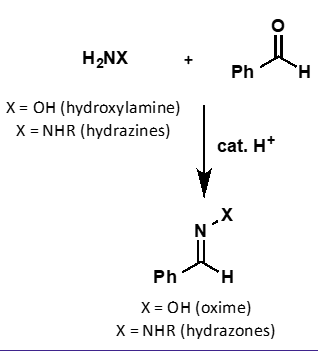
what is the mechanism?`