Exam 2 PNB
1/207
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
208 Terms
Resting Potential
The state of a resting neuron where the inside is approximately -70 mV relative to the outside, due to unequal ion distribution by the Na+/K+ pump and selective membrane permeability to K+.
Na+/K+ pump
An active transport mechanism that pumps 3 Na+ ions out of the cell for every 2 K+ ions pumped in, creating concentration gradients.
Selective Permeability (at rest)
The membrane's characteristic at rest, where it is much more permeable to K+ than to Na+ due to open K+ 'leak' channels, causing the inside of the cell to become negative.
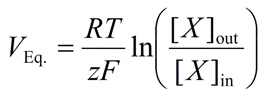
Nernst Potential (Ex)
The equilibrium potential for a single ion; the membrane voltage where the electrical force exactly opposes the chemical concentration gradient, resulting in no net ion flow.
Membrane Potential (Vm)
An electrical voltage difference across a cell's plasma membrane, essentially a tiny battery.
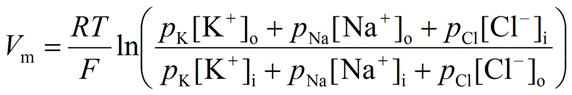
Goldman-Hodgkin-Katz (GHK) Equation
An equation used to calculate the actual membrane potential (Vm) by considering the concentrations and relative permeabilities of multiple relevant ions.
Action Potential (AP)
A rapid, 'all-or-none' electrical signal that travels down an axon without losing strength; the fundamental unit of long-distance communication in the nervous system.
Graded Potential
Most relevant to dendrites, under myelin, and at the neuromuscular junction (NMJ).
Differ from action potentials by being variable in value (amplitude), width (duration), and sign (de- or hyperpolarizing).
Decrease in size with distance traveled (decrementally).
Can summate (spatially or temporally) to affect the membrane threshold
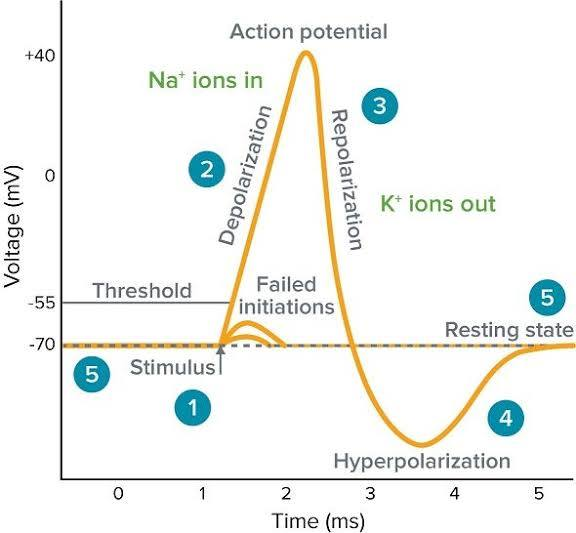
Threshold Voltage
The membrane potential (around -55 mV) that a stimulus (like summed graded potentials) must reach to trigger an action potential.
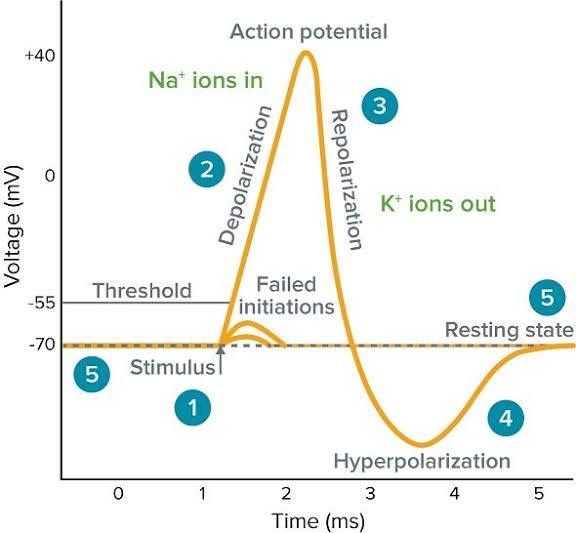
Rising Phase (Depolarization)
The phase of an action potential where voltage-gated Na+ channels open quickly, Na+ rushes into the cell, and the membrane potential rapidly shoots up towards ENa (e.g., +30 mV).
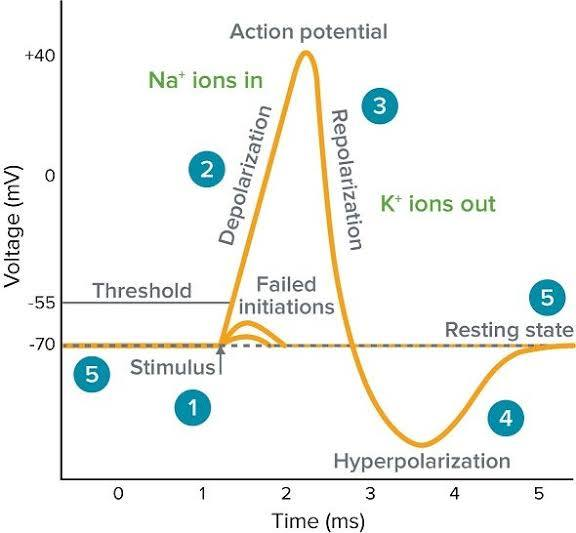
Falling Phase (Repolarization)
The phase of an action potential where Na+ channels' inactivation gates close, and slower voltage-gated K+ channels open, causing K+ to rush out and the membrane potential to become negative again.
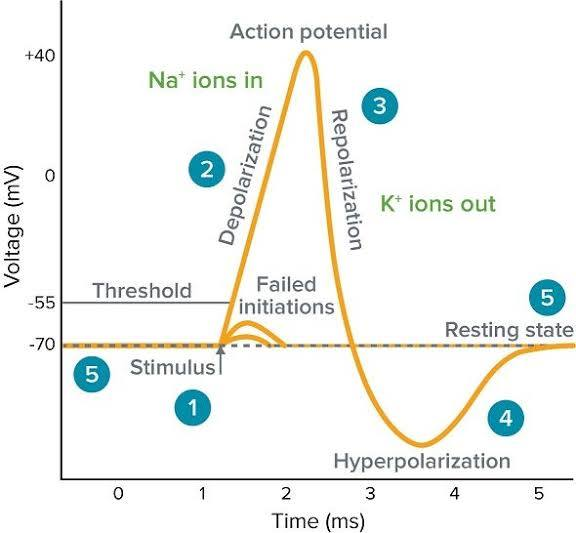
Undershoot (Hyperpolarization)
A brief period after repolarization where K+ channels are slow to close, driving the Vm even closer to EK, causing a temporary dip below the resting potential.
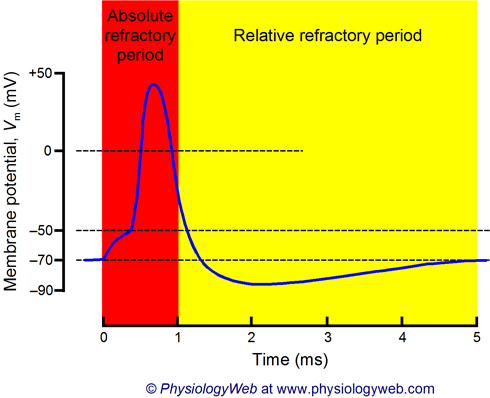
Absolute Refractory Period
A period during the rising and falling phases of an AP when Na+ channels are open or inactivated and cannot be reopened, preventing APs from overlapping or traveling backward.
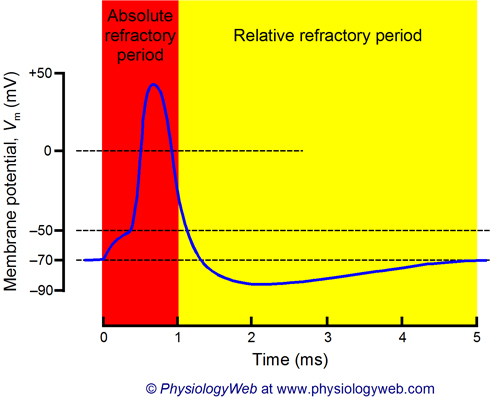
Relative Refractory Period
A period during hyperpolarization when Na+ channels have reset, but the cell is more negative than usual, requiring a stronger-than-normal stimulus to reach threshold.
Propagation (of Action Potential)
The process where the influx of Na+ during an AP creates a local current that depolarizes the adjacent patch of the axon to its threshold, triggering a new AP there, repeating down the axon.
Myelin Sheath
An insulating layer around myelinated axons that concentrates voltage-gated channels at gaps.
Nodes of Ranvier
Gaps in the myelin sheath where voltage-gated channels are concentrated, allowing the AP to 'jump' from node to node.
Saltatory Conduction
The process by which an action potential 'jumps' from node to node in myelinated axons, making propagation much faster than in unmyelinated axons.
Synapse
The junction where a neuron communicates with another cell (neuron, muscle, etc.).
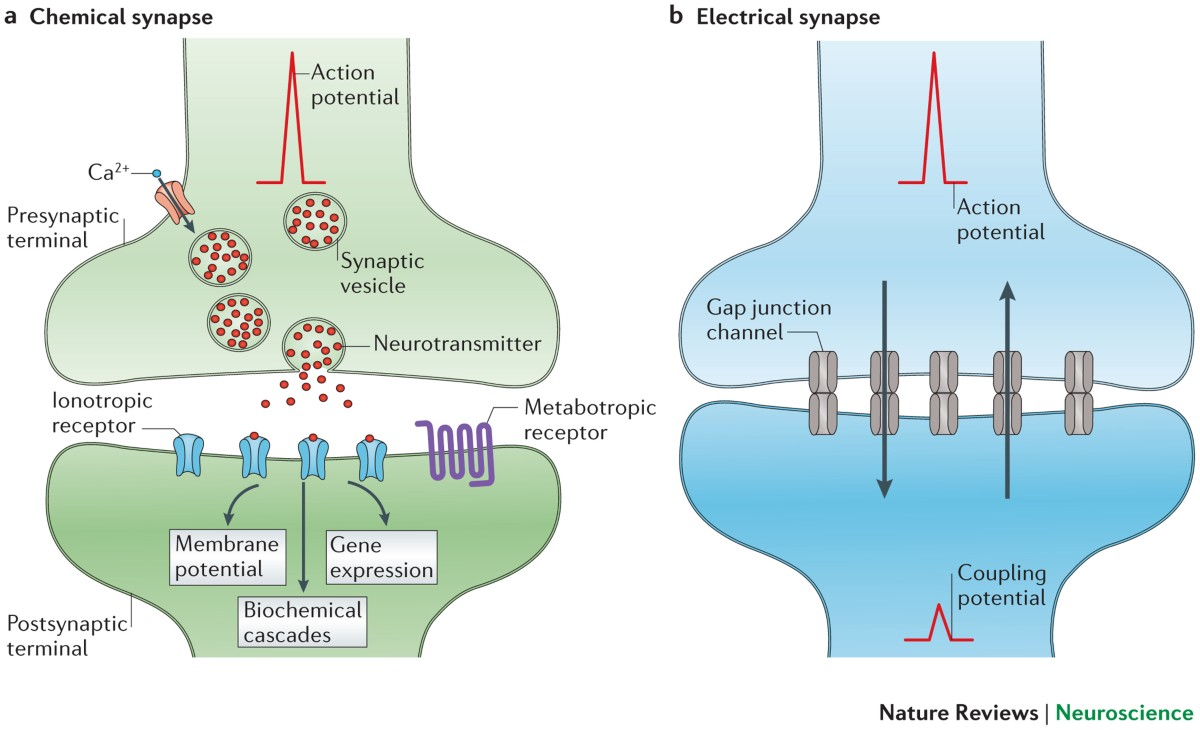
Electrical Synapse
A type of synapse where cells are directly connected by gap junctions, allowing ions to flow freely between them for extremely fast and bidirectional signaling.
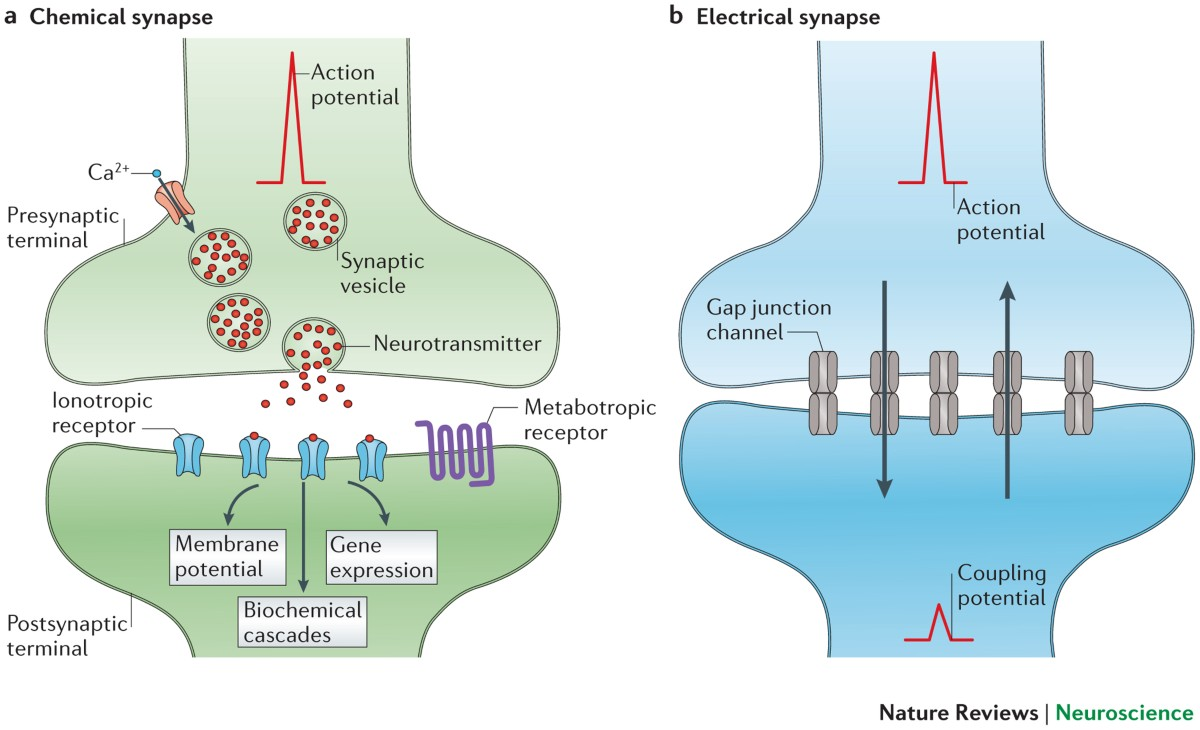
Chemical Synapse
The most common type of synapse where neurons are separated by a synaptic cleft and communicate using chemical messengers called neurotransmitters.
Neurotransmitters
Chemical messengers released from the presynaptic terminal that diffuse across the synaptic cleft to bind to receptors on the postsynaptic membrane.
SNARE proteins
Proteins critical for the fusion of synaptic vesicles (filled with neurotransmitter) with the presynaptic membrane during exocytosis.
Ionotropic Receptors
Responsible for fast synaptic transmission ( < 100 ms).
The receptor and ion channel are part of the same protein complex.
Neurotransmitter binding directly opens the ion channel, allowing ion flow and causing a rapid change in membrane potential.
Exhibit little amplification; one or two neurotransmitters typically open one channel.
Metabotropic Receptors
Responsible for slow synaptic transmission ( > 100 ms).
The receptor and the ion channel (if involved) are separate proteins.
Neurotransmitter binding activates a G protein, which then initiates intracellular second messenger pathways.
These pathways can lead to various actions, including opening or closing ion channels, modifying existing proteins, or regulating protein synthesis.
Exhibit significant amplification; one neurotransmitter may affect many channels and downstream effectors.
Example: Muscarinic Acetylcholine (mACh) receptors at the heart, which indirectly open K+ channels via G protein signaling.
Excitatory Postsynaptic Potential (EPSP)
Occurs when the membrane becomes more positive relative to rest (depolarizes).
This typically involves an increase in Na+ permeability, leading to Na+ influx (e.g., glutamate activating non-selective cation channels).
Inhibitory Postsynaptic Potential (IPSP)
Occurs when the membrane becomes more negative relative to rest (hyperpolarizes).
This typically involves an increase in K+ out or Cl– in (e.g., GABA activating chloride channels).
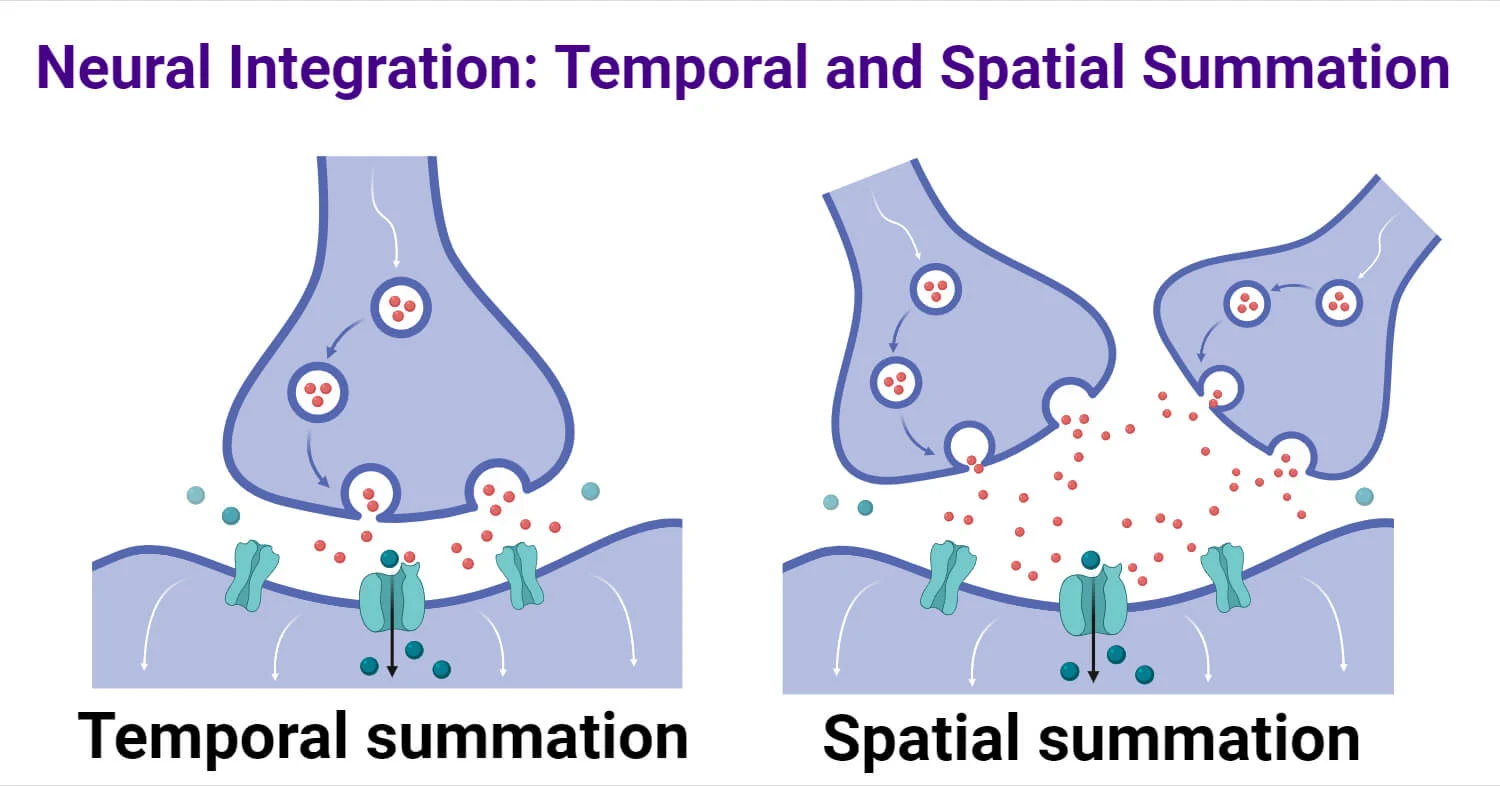
Temporal Summation
The adding up of multiple graded potentials from the same synapse arriving in rapid succession.
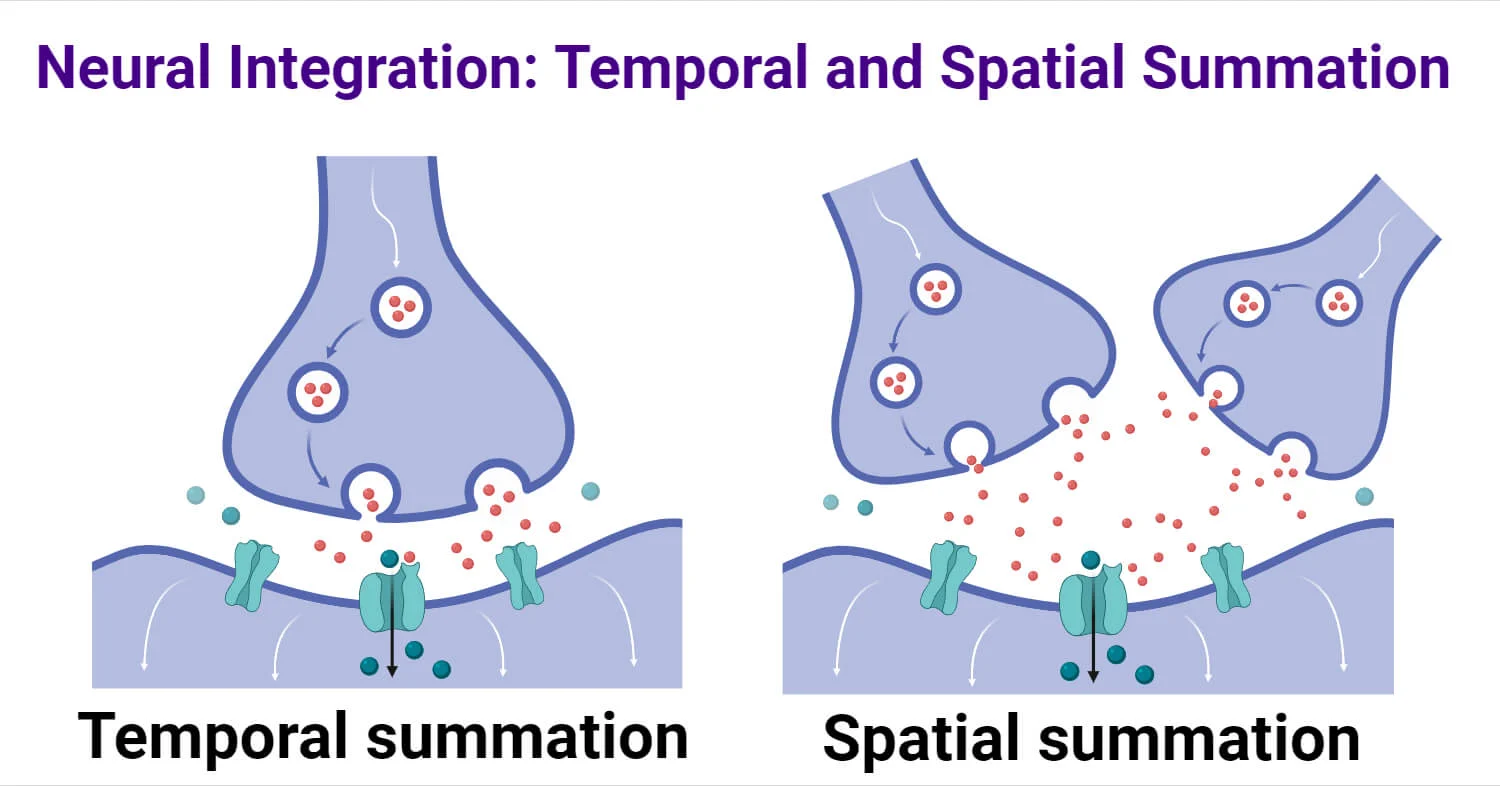
Spatial Summation
The adding up of simultaneous graded potentials from different synapses.
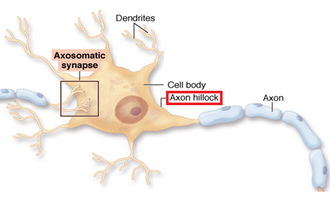
Axon Hillock (integrator)
The region of a neuron that integrates all incoming EPSPs and IPSPs; if the sum depolarizes it to threshold, an action potential is fired.
Enzymatic degradation (NT termination)
A mechanism to terminate neurotransmitter signaling where enzymes break down the neurotransmitter in the synaptic cleft (e.g., Acetylcholinesterase breaking down ACh).
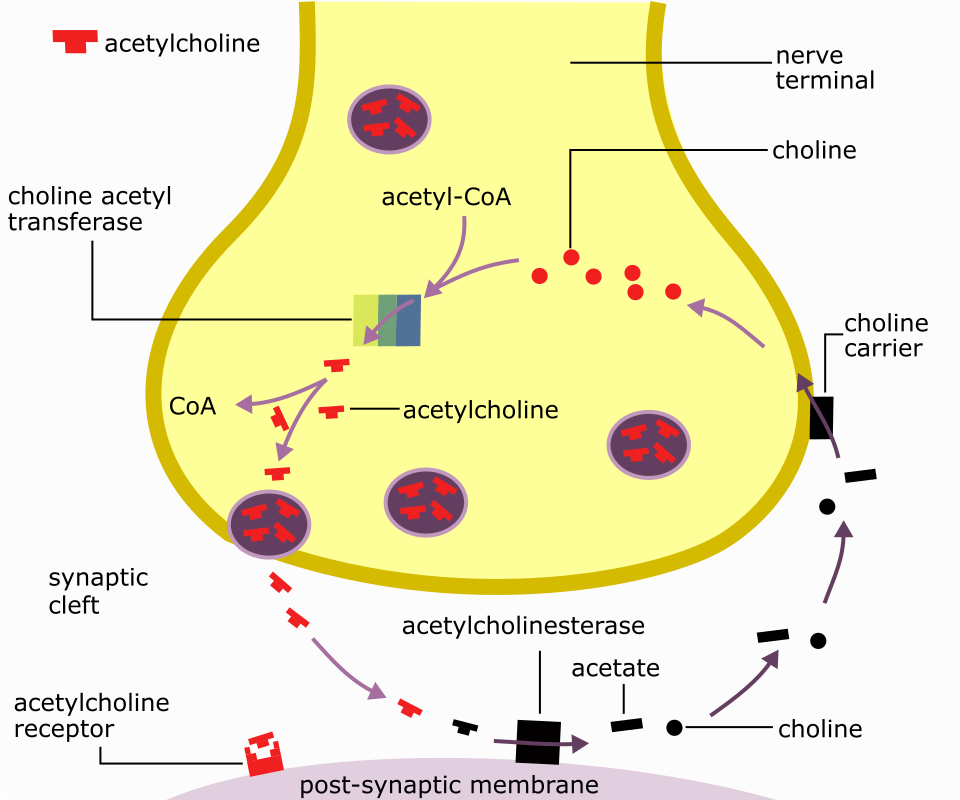
Re-uptake (NT termination)
A mechanism to terminate neurotransmitter signaling where the neurotransmitter is transported back into the presynaptic terminal or glial cells.
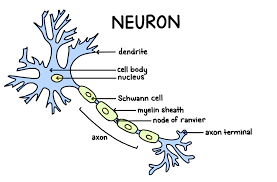
Neuron
A fundamental cell of the nervous system specialized for transmitting electrical and chemical signals.
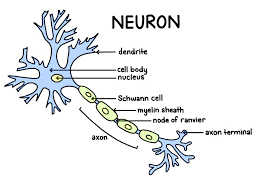
Dendrites
Branching extensions of a neuron that receive signals from other cells and transmit them towards the cell body.
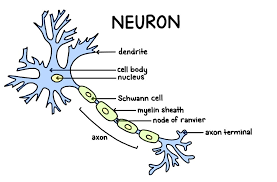
Cell Body (Soma)
The main part of a neuron containing the nucleus; it integrates incoming signals and carries out basic cellular functions.
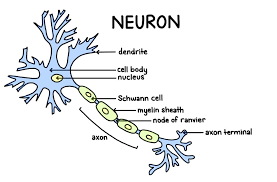
Axon
A long, slender projection that conducts electrical impulses (action potentials) away from the cell body to other neurons, muscles, or glands.
Depolarization
A decrease in the absolute value of a cell's membrane potential (e.g., from -70 mV to -50 mV or +30 mV), making the inside less negative or even positive.
Hyperpolarization
An increase in the absolute value of a cell's membrane potential (e.g., from -70 mV to -90 mV), making the inside more negative.
Presynaptic Terminal
The axon terminal of the neuron sending the signal, where neurotransmitters are released.
Postsynaptic Membrane
The membrane of the neuron or cell receiving the signal, containing receptors for neurotransmitters.
Synaptic Cleft
The narrow extracellular space between the presynaptic terminal and the postsynaptic membrane across which neurotransmitters diffuse.
All-or-none principle
Action potentials are generated when a threshold is reached and propagate without decrement.
They are largely depolarizing events.
End Plate Potentials
Localized changes in membrane potential at the neuromuscular junction, caused by neurotransmitter binding, which can trigger action potentials.
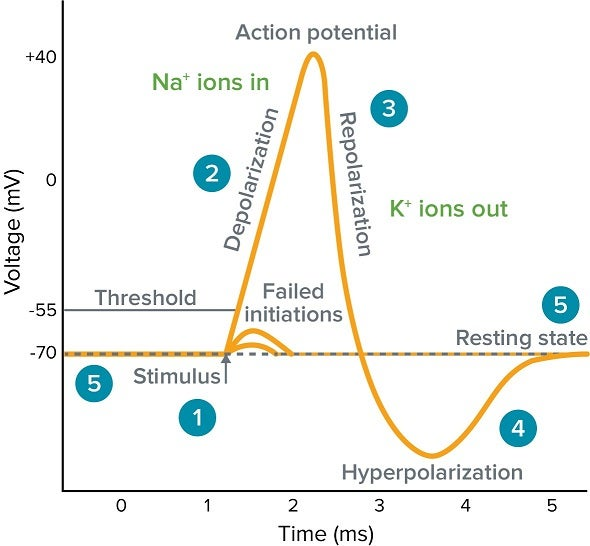
Action potential generation
Resting Membrane Potential
Rising Phase (depolarization)
Falling Phase (repolarization)
Undershoot (hyperpolarization)
VGCCs vs VGNCs
depolarization opens and blocks VGCCs, but the opening and blocking kinetics are slower.
Resting Membrane Potential
Maintained by leak K+ channels (high permeability to K+) and to a lesser extent leak Na+ channels and leak Cl- channels.
Rising Phase (Depolarization)
Voltage-gated Na+ channels (VGNCs) rapidly activate (open) in response to depolarization.
Massive Na+ influx occurs, leading to rapid depolarization and the rising phase of the action potential.
VGNCs activate relatively fast.
Na channels at peak of action potential
are mostly inactivated, stopping Na+ influx. This allows the membrane potential to begin repolarizing.
Falling Phase (Repolarization):
VGNCs inactivate (block) slowly over time in response to sustained depolarization. This stops Na+ influx.
Voltage-gated K+ channels (VGKCs) activate (open) slowly in response to depolarization.
K+ efflux (out of the cell) re-polarizes the membrane.
VGKCS vs VGNCs
VGKCs activate and deactivate relatively slowly compared to VGNCs.
What unblocks VGNCs over time?
repolarization of the membrane potential allows VGNCs to recover from inactivation and become available for reopening.
Undershoot(hyperpolarization)
VGKCs close slowly, leading to a transient period where too much K+ leaves the cell, causing the membrane potential to become more negative than the resting potential
Fast Potentials
( < 100 ) involve quick ion channel responses.
Slow Potentials
( > 100 ) involve second messenger pathways and longer signal outcomes.
Action Potential Coupling
action potential arriving at the presynaptic terminal is coupled to vesicular release via a calcium signal; leads to a rapid Ca2+ influx
Vesicle Cycle (Exocytosis and Endocytosis):
Filling: Neurotransmitters are synthesized and transported into synaptic vesicles.
Trafficking: Vesicles move towards the active zone of the presynaptic terminal.
Docking/Priming: Vesicles become physically attached to the presynaptic membrane and prepared for fusion. this process involves SNARE proteins (e.g., synaptobrevin on the vesicle, syntaxin and SNAP-25 on the plasma membrane) that mediate vesicle fusion.
Fusion (Exocytosis): Upon Ca2+ influx, synaptotagmin (the calcium sensor protein on the vesicle) binds calcium, triggering the fusion of the vesicle membrane with the presynaptic membrane.
Neurotransmitters are released into the synaptic cleft.
Membrane Translocation & Protein Clustering: After fusion, portions of the vesicle membrane are retrieved.
Clathrin Coating: The retrieved membrane is coated by clathrin, forming clathrin-coated pits.
Endocytosis/Pinching Off: The clathrin-coated pit pinches off from the presynaptic membrane, a process facilitated by the protein dynamin, forming a new vesicle.
De-coating & Refilling/Recycling: The newly formed vesicle sheds its clathrin coat and is either refilled with neurotransmitter or sent for degradation and new vesicle synthesis.
NT binding causes
activation of receptor proteins on the postsynaptic membrane, leading to changes in membrane potential.
Steps of Synaptic Transmission
Ion channels in the postsynaptic cell open, increasing ion permeability (e.g., Na+). Resulting in EPSP or IPSP depending on the ions involved.
The altered open state of ion channels can activate second messenger pathways, particularly with metabotropic receptors.
Ion channels can close, decreasing ion flow (e.g., decreasing Na+ in and K+ out).
Changes are made in the postsynaptic cell in response to neurotransmitter action, including immediate electrical responses and biochemical cascades.
Step 5: Long-term modifications and protein synthesis regulation potentially occur, contributing to synaptic plasticity.
Excitatory Neurotransmitters
Chemicals that increase the likelihood of an action potential by depolarizing the postsynaptic membrane.
Glutamate(Glu)
major excitatory neurotransmitter in the CNS (found everywhere).
activates non-selective cation channels at fast chemical synapses, leading to neuronal firing (EPSP).
Acetylcholine (ACh)
excitatory or inhibitory depending on where
At nerve-muscle connections (Neuromuscular Junction), it stimulates muscle contraction by binding to nicotinic ACh receptors (nAChRs), which are ligand-gated cation channels.
At the heart, ACh binds to muscarinic ACh receptors (mAChRs), which are metabotropic and indirectly open K+ channels, causing hyperpolarization and slowing heart rate.
Norepinephrine(NE)
Facilitates “fight or flight” situations, often through metabotropic receptors.
Primarily excitatory
Inhibitory Neurotransmitter
neurotransmitters that reduce neuronal excitability by hyperpolarizing the postsynaptic membrane
GABA
major inhibitory neurotransmitter in the CNS (found everywhere).
it activates chloride channels at fast chemical synapses, leading to inhibition of neuronal firing (IPSP).
Glycine
provides inhibition throughout the central nervous system, also often by activating chloride channels.
How to get rid of neurotransmitters
Diffusion: Neurotransmitters diffuse away from the synaptic cleft.
Reuptake: Neurotransmitters are actively transported back into the presynaptic terminal or glial cells by specific pumps and transporters.
Cleavage by Enzymes: Neurotransmitters are broken down into inactive metabolites by enzymes in the synaptic cleft (e.g., Acetylcholinesterase breaking down ACh).
What 4 key things do muscles have?
Excitability
Contractility
Extensibility
Elasticity
Excitability
Ability of muscle tissue to respond to stimuli (e.g., action potentials).
Contractility
The capability of muscle fibers to shorten when stimulated.
Elasticity
Tendency of a muscle to return to its original shape after contraction.
Extensibility
Ability of muscle tissue to stretch beyond normal length depending on its situation.
Components of a muscle belly
Fascicles
Muscle Cells( Fibers)
Myofibrils
Myofilaments
Fascicles
Bundles of muscle fibers that make up the muscle belly, surrounded by perimysium.
Perimysium
The connective tissue that surrounds fascicles within a muscle, providing support and protection.
Muscle Fibers
Individual long cylindrical cells containing multiple nuclei; are full of myofibrils.
Myofibrils
Cylindrical bundles of filamentous contractile proteins( myofilaments) (e.g., myosin and actin), synonymous with "muscle cell".
Myofilaments
Individual protein bundles within myofibrils (double-helical actin thin filament and myosin thick filament).
Sarcolemma
The cell membrane surrounding a muscle fiber, providing structural support and playing a role in muscle contraction.
T-Tubules(Transverse Tubules)
Extensions of the sarcolemma; rich with leak K+ and voltage-gated N+ & K+ & channels, and DHP receptors (DHPRs); allow for action potentials to propagate throughout the fiber.
Sarcoplasmic Reticulum (SR)
Specialized smooth endoplasmic reticulum that stores calcium ions; features extensive terminal cisternae surrounding T-tubules in a "triad" arrangement.
Sarcoplasm
Muscle fiber cytoplasm rich in organelles (e.g., mitochondria and glycogen granules).
Sarcomere
Defined by the area between two adjacent Z-discs, acts as the functional contractile unit
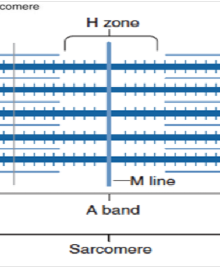
M-line
Center of the A-band, anchoring thick filaments.
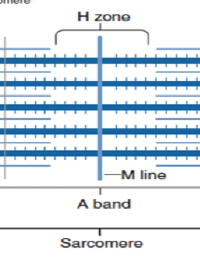
H-zone
Center of the A-band where only thick filaments(myosin) are present.
Thick filaments
Composed of myosin with its own fast endogenous ATPase activity to grant spring potential energy.
thin filaments
Composed of actin (double-helical), troponin C, and tropomyosin.
Accessory Proteins
titin (elastic, anchors myosin to Z-discs), nebulin (aligns the actin double helix thin filaments), and CapZ (holds actin to Z-discs).
titin
A large protein that functions as a molecular spring, providing elasticity and stability to muscle fibers by anchoring myosin filaments to Z-discs.
Nebulin
a giant protein in skeletal muscle, acting as a molecular ruler to set thin filament length and regulate muscle contraction by interacting with actin
CapZ
a capping protein that caps the barbed end of actin filaments in muscle cells.
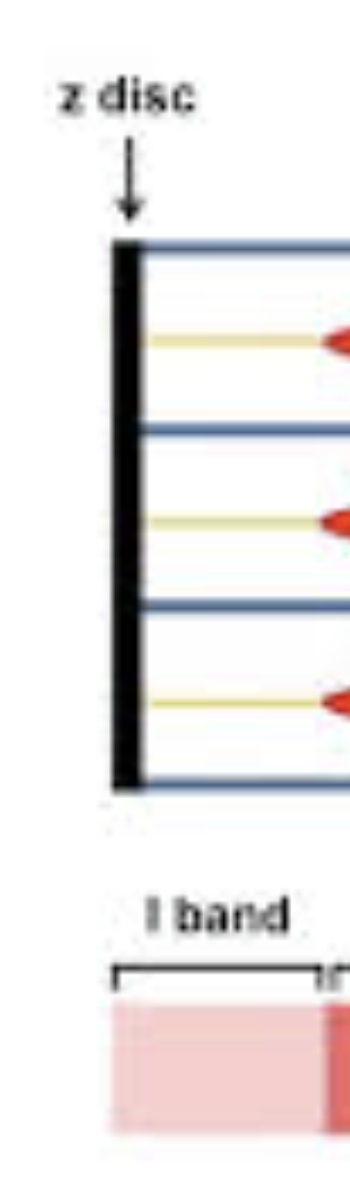
I-band
Region containing only thin filaments(actin), bisected by the Z-disc
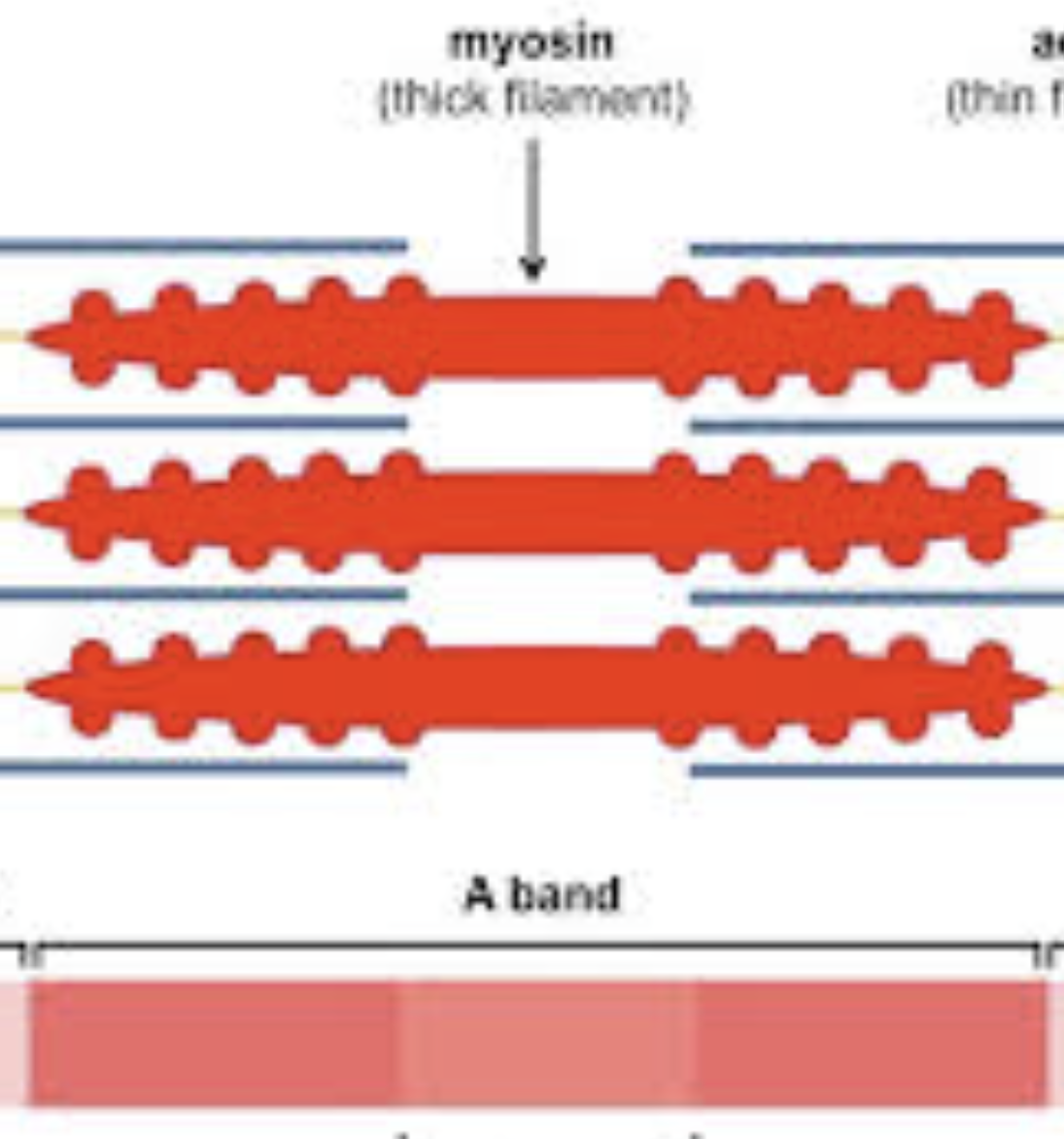
A-band
region corresponding to the length of the thick filaments.

Z-discs
Anchor thin filaments and mark the boundaries of a sarcomere.
Motor unit
single motor neuron and the muscle fibers it controls. usually contains just a few muscle fibers in an entire muscle. a muscle may have many motor units.
Larger muscles have
more motor units than smaller muscles
Muscle Fibers & All or None Principle
Each muscle fiber obeys the all or none principle. Either the whole muscle fiber contracts, or not at all.
Henneman’s Size Principle
As force increases, more and more motor units are recruited to generate larger force
S-Type/ Slow-oxidative fibers (Slow Twitch)
Fatigue resistant, high endurance, easily excitable.
Fast-fatigue resistant fibers (Fast Twitch)
Bigger, Average excitability; resistant to fatigue, helps you maintain an action.
Fast-fatigable fibers (Fast Twitch)
Very big, low excitability. Not recruited unless needed. High power output but fatigue quickly