Muscle III: Fatigue, Metabolism, and Adaptation
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
CSA, Muscle Mass, Fiber area (can be applied to a single fiber or a whole muscle), fiber diameter
things that affect force production per unit of contracting muscle tissue
possible factors in changes in specific force
central activation, NM transmission, sarcolemma excitability, E-C coupling (Ca release and sensitivity), crossbridge formation, force transmission, fiber type changes, fatigue, changes in connective tissue
fatigue
transient decrease in muscle performance, associated with activity as there is a decrease in max force generating capacity, inability to maintain submax force, or inability to continue an activity, and is task specific
muscle fatigue definition 1
starting with maximum effort of an isolated muscle or muscle group that is subject to a fixed time of exercise, fatigue sets in after a relative decrease in percentage of force production, this amount can vary based on what is specified by the study
muscle fatigue definition 2
muscle starts at submax, output is kept fixed, time to task failure is principle failure, and fatigue is binary, example: holding 50% of max effort is held, when max effort can no longer reach that 50% threshold fatigue has been reached
things that relate to fatigue
supply and demand
supply
substrate utilization to regenerate ATP, substrate availability, enzyme abundance/activity, perfusion/vascularity (delivers oxygen)
demand
myosin heavy chain/crossbridges, sarcoplasmic reticulum re-uptake via SERCA, Na+/K+ ATPase
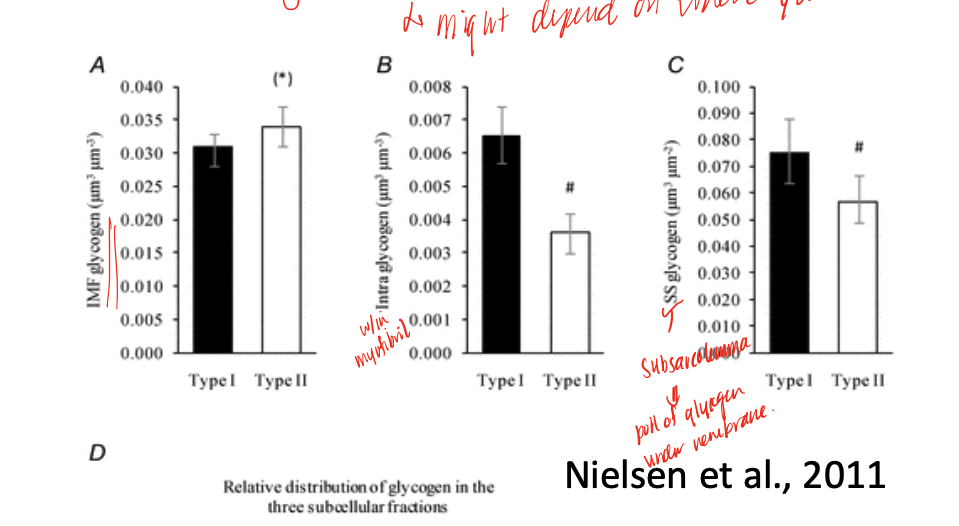
different enzymatic and biochemical profiles
feature of fibers that allows for optimization of resting metabolism vs peak metabolism, related to capillary and myoglobin content (red/dark vs white/light)
characteristics of Type I/IIa fibers
more mitochondria, more capillaries, more oxidative enzymes, more lipid droplets, variable glycogen
variable- may find different levels just depending on where you look
level of glycogen in different fiber types
metabolically expensive and space constraints
reason why muscle does not have the capacity to support maximal demand at all times
resting metabolism features
primarily is driven by oxidative phosphorylation, and is post-prandial (meaning responds to what we eat/our diet)
PCr (phosphocreatine) breakdown through Creatine Kinase
mechanism that is used to replenish ATP during initial transition from rest to activity before oxidative phosphorylation and glycolysis is really able to kick in or catch up
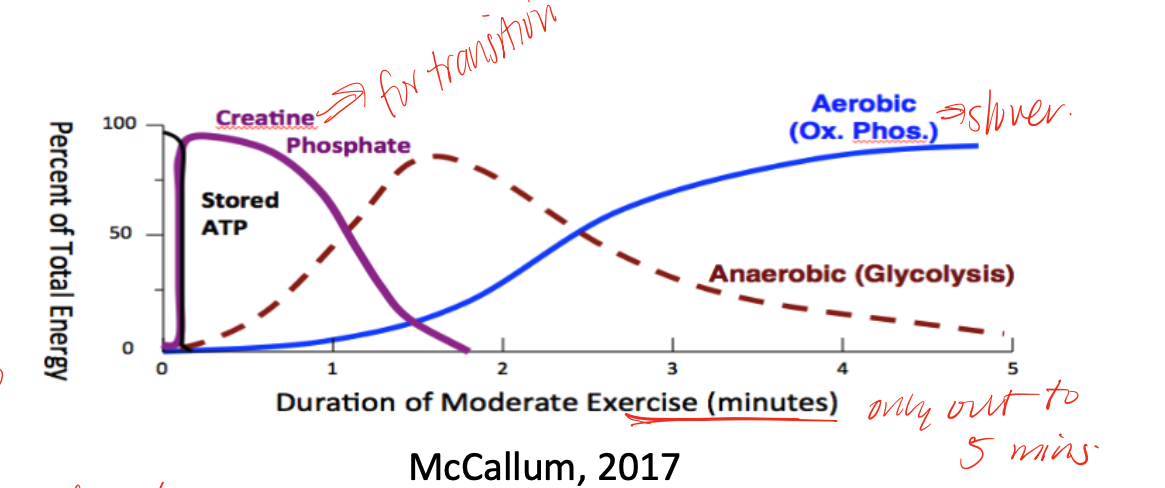
PCr
an energy “capacitor” or buffer, very high in skeletal muscle and some in heart and brain, but low in liver, kidney, testes
FFA (through Oxphos)
during long duration endurance exercises, low intensity exercises body typically uses this supply in higher demands
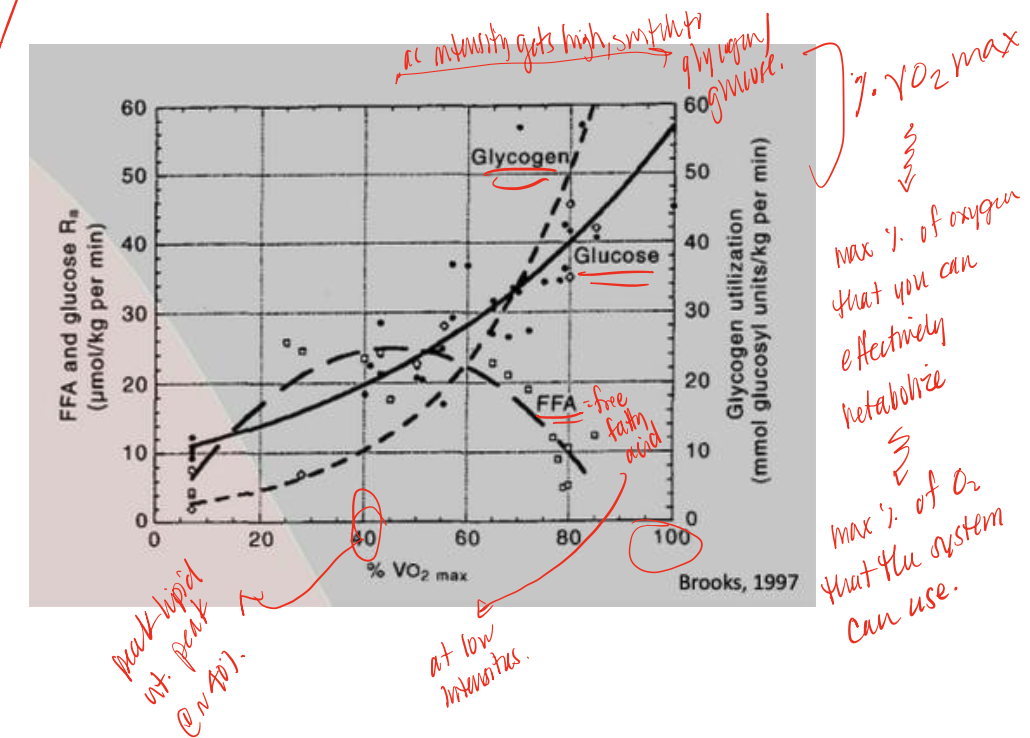
glucose/glycogen
during high intensity exercise, body switches from FFA use to this supply, utilizing glycolysis
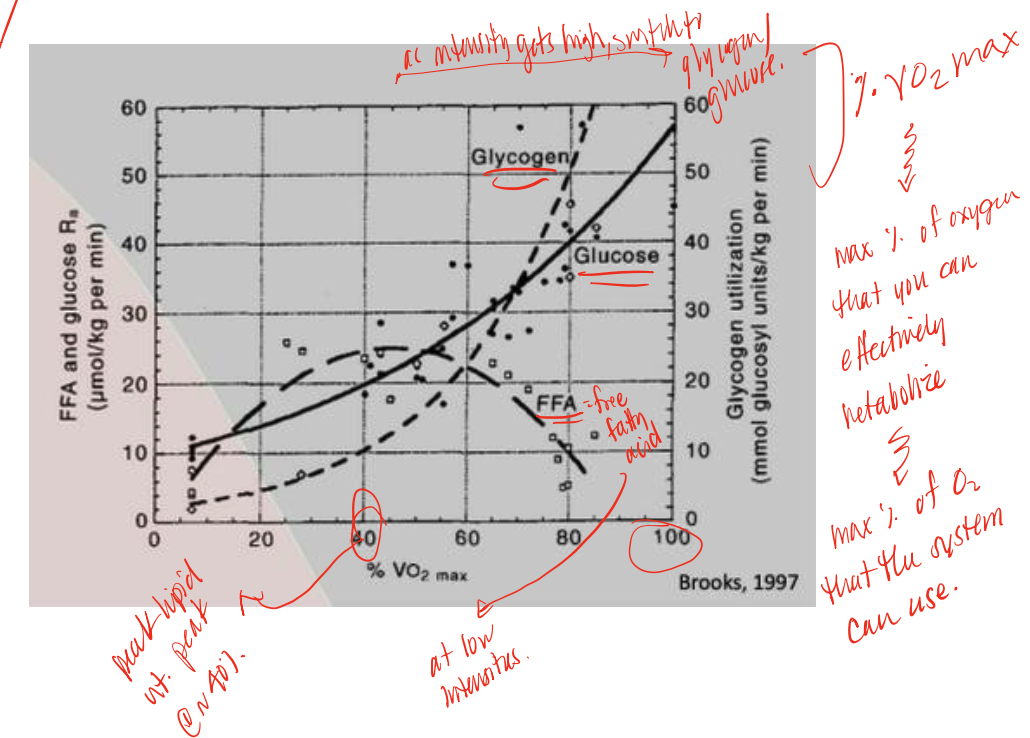
VO2 max
amount of oxygen that your body can effectively metabolize/use
sarcoplasmic reticulum
feature more extensive in fast fibers IIx/b (more so than IIa, though IIa has significant, and more so than I)
mysoin ATPase rate of ATP consumption
differences in this rate is what distinguishes different fiber types, example: ATP consumption is IIx> IIa > I
glycogen depletion
mechanism of fatigue, seems to be a limiting factor for endurance activity, “the bonk”
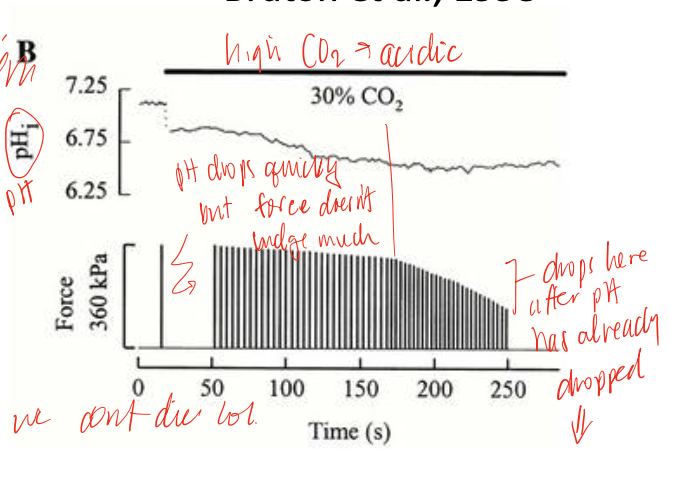
lactic acid
a more correlational that causative factor of fatigue, actually has a number of benefits, acidosis can lower force, but does so minimally as physiological temperatures, but there may be neural involvement in response that leads to central inhibition
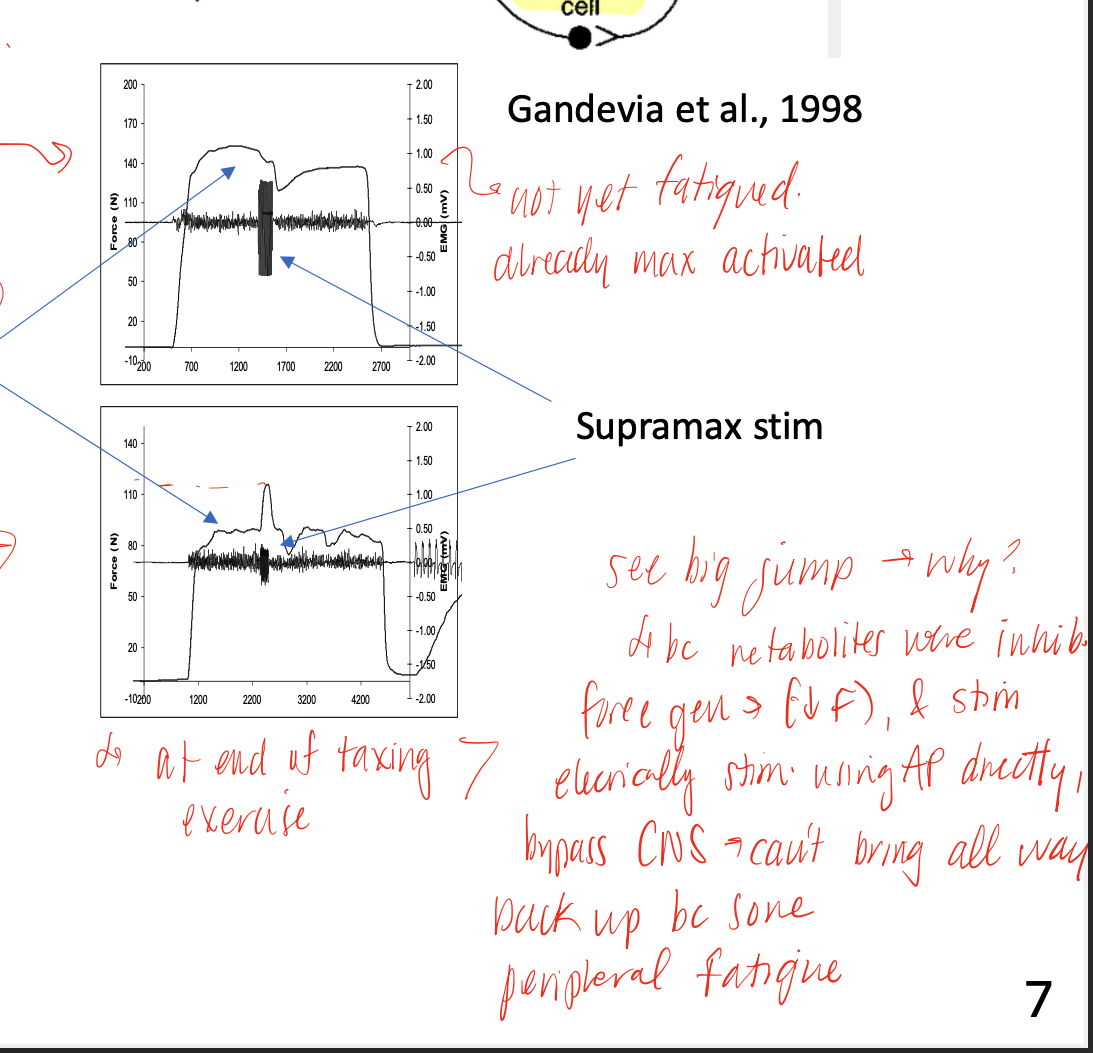
peripheral fatigue
fatigue that happens at the periphery, due to NMJ transmission, electrical fatigue, ADP/Pi buildup that inhibits cross bridge formation, ECC impairments, and environmental effects on proteins (heat up too much will denature them)
central fatigue
fatigue that occurs at or above the alpha MN (cortical, subcortical, spinal)
electrical fatigue
depolarization of the cells becomes diminshes as increasing number of APs sent down the t-tubules, which have only a small concentration of extracellular fluid, depletes its concentrations, “messing” up the concentration gradients and causing this
caffeine
after continuous stimulation, the fibers begin to lose the ability to release calcium due to electrical fatigue, but adding a TON of this directly is able to stimulate the RyR receptors and increase the release again (also shows us that we haven’t run out of calcium stores in the SR)
central fatigue mechanism
feedback from metabolites (H+, lactate, etc) activates GrpIII/IV sensory neurons, which activate inhibitory inter-neurons, reducing MN firing and firing rates, force production, may signal pain/nociocieption/muscle damage
NMES (neuromuscular electrical stimulation)
mechanism that allow us to bypass central fatigue mechanisms and generate more force (not back to baseline max force tho) despite fatigue already setting in
basic adaptations to increase use
hypertrophy, increase oxidative capacity, increased capillarization, transition from fast to slow fibers
SAID (specific adaptation to imposed demand)
gains tend to be greatest for performance variables most similar to those related to the training, ex: if you train at one joint angle you will get strong at that angle and a little bit around it, but not so much at others, only what you activate will adapt!
time course of adaptation
accumulation of protein changes, largely a function of transcriptional activation, protein half life and exercise frequency
basic adaptations to decreased use
reduced size (atrophy), reduced oxidative capacity through decrease mitochondrial volume/number and decrease oxidative enzyme activity, slow to fast fiber transition (jury still out on capillarization)
basic adaptations to aging
reduced size, equivocal change on oxidative capacity, fast to slow fiber transition (jury still out on capillarization)
clinical goals of increasing muscular activity
gain/regain functional independence, improve health and fitness, increase muscular strength/endurance, maximize athletic performance, performance changes that are task specific, increase strength and power (through CSA and/or neural drive), increase tolerance, increase muscle CSA
changes in performance after training low rep (3-5 RM) training
see highest increase in 1 RM strength, but lowest repetitions of 60% RM
changes in performance after high rep (20-28 RM) training
see smallest increase in 1 RM, but highest repetitions of 60% RM
fiber type CSA increases across all fiber types
interestingly, in high rep exercise, see this trend across all fiber types after training
smallest change in CSA of fiber types
in low rep exercise, see this trend across all fiber types after training
most type I, medium IIa, low IIx
fiber type composition of distance runners, this is demonstrated by higher capillary length density, mitochondrial density, concentration of lipids within muscle, and sacroplasmic volume density
high type IIx, medium IIa, low I
fiber type composition in weight lifters, this is demonstrated by highest myofibrilar volume density (contractile fibers), fiber mean cross sectional area, decrease fat content within muscle
hypertrophy
increase in size of existing muscle cells, most data suggest this as the mechanism at work for increasing muscle mass
hyperplasia
increased number of new muscle cells, proposed mechanism is through “splitting” of muscle fibers
De Lorme (1940s) Human Exercise study
study on rehan of soldiers with knee injuries- proposed a strength-endurance continuum, a few reps against a heavy load increases strength/power, many reps against a light load increases muscular endurance
fast to slower myosin isoforms
conversion of MHC with exercise follow this trend, generally, which takes longer and requires more intense training that changes in metabolic enzymes
Possible mechanims for change to slow to fast with training
study of scandinavian sprinters- training is characterized bu heavy resistance and intervals, tapering period when stop training before event, see an “overshoot” of fast fibers (small sample, results haven’t been replicated)
manifestations of decreased use
reduced capacity for work, decreased force, power, endurance, increased fatigue, decrease in functional outcomes and QOL, altered task performance
whole muscle changes with reduced muscle use
decrease muscle mass/CSA, especially with anti-gravity muscles (muscles with higher number of slow MHC tend to show greater atrophy than those with more fast MHC), decreased force, fiber type changes- contractile properties, alterations of muscle architecture (length, pennation, tendon/CT changes), metabolic alterations
slow soleas vs fast gastrocnemius
soleus shows faster atrophy with disuse than does gastroc
loss of mass/volume
muscle atrophy is typically a feature of this, may or may not be also relate to CSA
fiber atrophy
specifically describes in terms of CSA, similar to whole muscle, this occurs in areas of greatest fiber populations (MUs) that are most often used
summation of force
as disuse occurs and muscles transition from slow to fast, this causes problems with this since the more rapid a fiber is, the fast it falls from its peak, which will requires higher intensities in order to fuse tetanus
increased glycolytic enzyme activity
disuse causes this shift in enzyme activity, which is consistent with fiber type changes
oxidative enzyme activity with disuse
mechanism is less clear, declines in both phasic and anti-gravity muscle have been observed (succinate dyhyrdogenase- SDH), in models with denervation component, no loss of SDH observed, even when MHC converts
proteolysis
what atrophy is first driven by (during the first 5 days)
protein synthesis
what atrophy is mainly driven by after 2 weeks of disuse
downregulated translation
mechanism that is proposed to be the cause of reduction in synthesis of protein
3 main proteolytic pathways in muscle breakdown
cathespins (lysosomal)
calpains (calcium-dependent)
ubiquitin-proteasome (ATP-dependent)
ubiquitin-proteasome pathway
where bulk of proteolysis occurs, ubuquitin binds to proteins to mark it for breakdown, then proteasome breaks up the protein into peptide fragments
calpain systems
proteasome can’t degrade intact myofibrils, but rather only soluble actin and myosin, and since this system targets myofibrillar proteins, which will disrupt myofibrils, it may be needed for ubiquitin-proteosome action
loss of strength > loss of mass
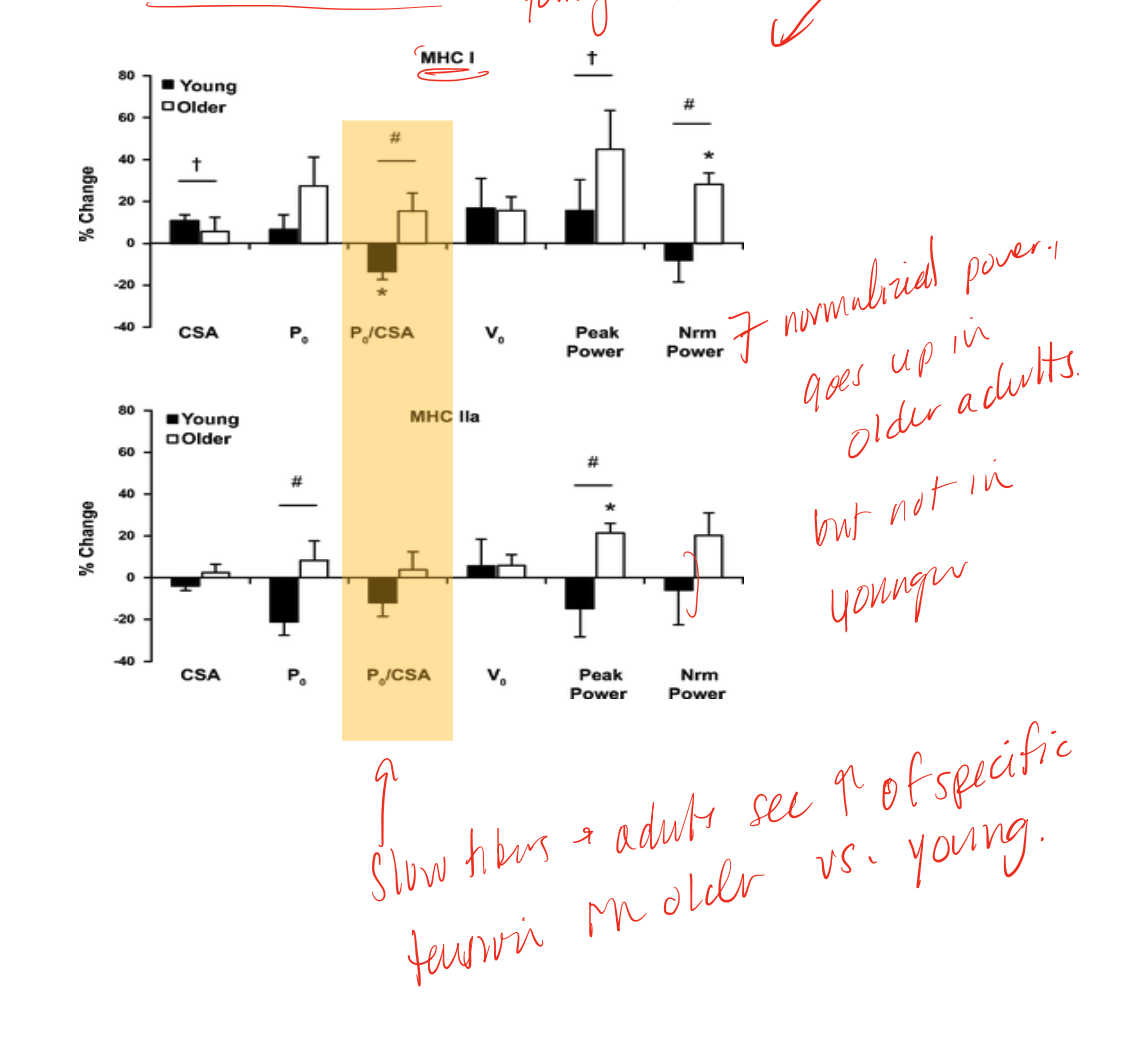
as we age, we see this pattern regarding strength and loss of mass, may be due to denervation/re-innervation as we lose some alpha MN and see compensatory larger MUs as a response, which decreases specific force
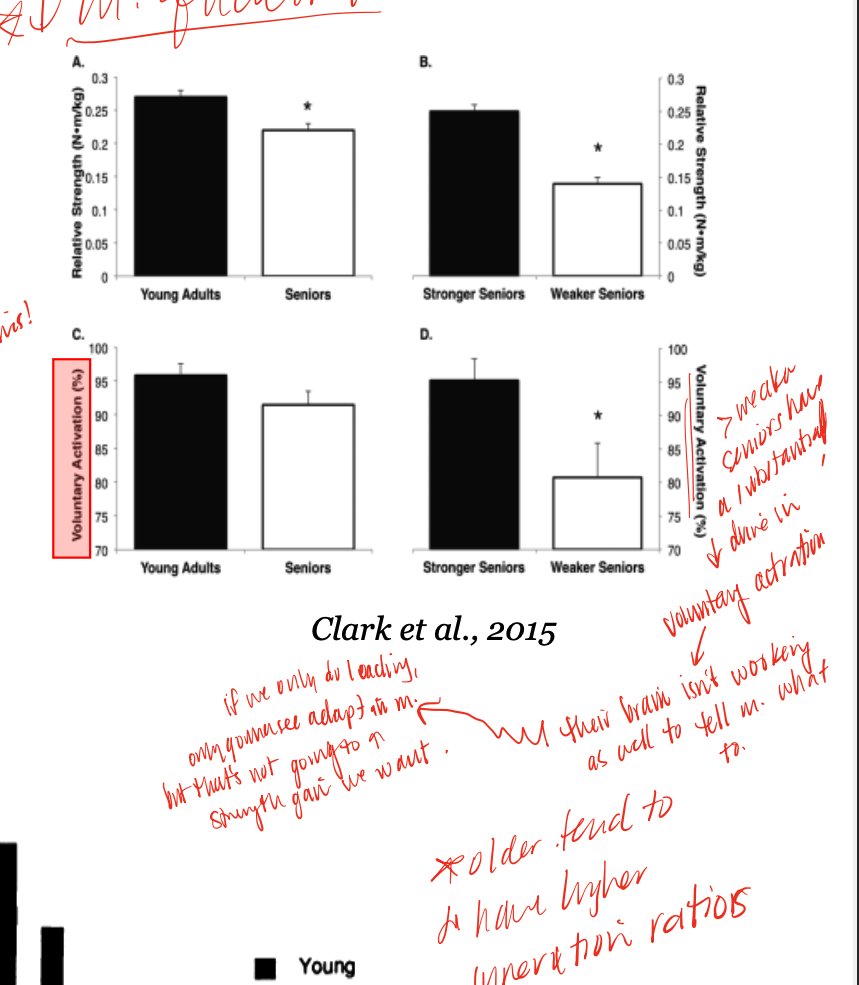
endurance training
exercise that older muscle responds better to (increased power and CSA) while young muscle responds the opposite way