bonding 2 and non covalent bonds
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
According to Molecular Orbital (MO) theory, covalent bonds result when
§atomic orbitals combine to form molecular orbitals
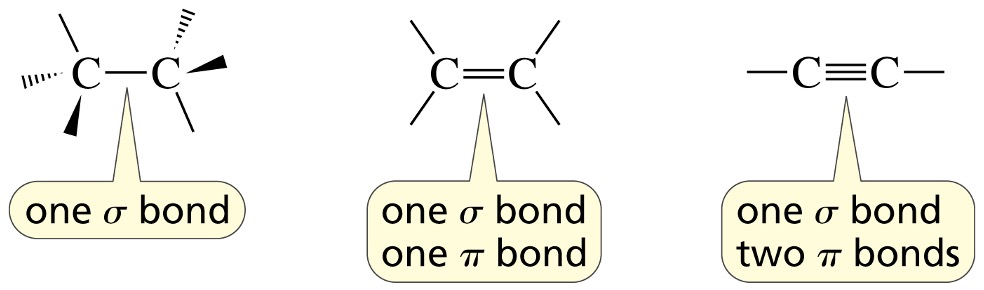
Forming a sigma (σ) bond – “head-on” overlap of atomic orbitals

A molecular orbital (MO) describes
the volume of space around a molecule where an electron is likely to be found
Orbitals are conserved:
§# of Molecular Orbitals = # of Atomic Orbitals Combined
rbitals of the same phase (colour) can overlap constructively (σ bonding MO) or
of different phase (colour) overlap destructively (σ* antibonding MO)
A sigma (σ) covalent bond is formed by the
head on overlap of two atomic orbitals.
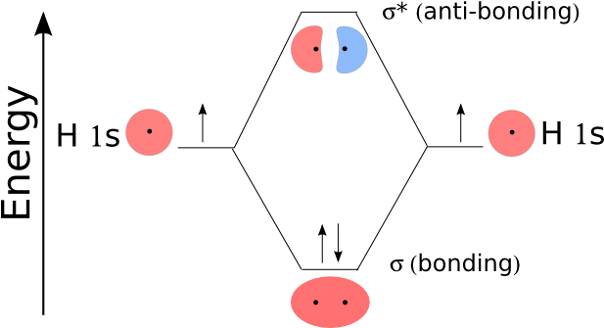
§When 2 AOs combine, 2 MOs are formed, one lower and one higher in E than the original AOs
§Both electrons are in the σ bonding MO. The σ* antibonding MO is empty. - easier to lose energy than gain
Electrons are assigned to MOs using the same rules as for AOs
§Electrons always occupy available orbitals with lowest E (Aufbau principle)
§No more than two electrons can occupy a MO (Pauli exclusion principle)
Side-to-side overlap of p orbitals forms a
π covalent bond
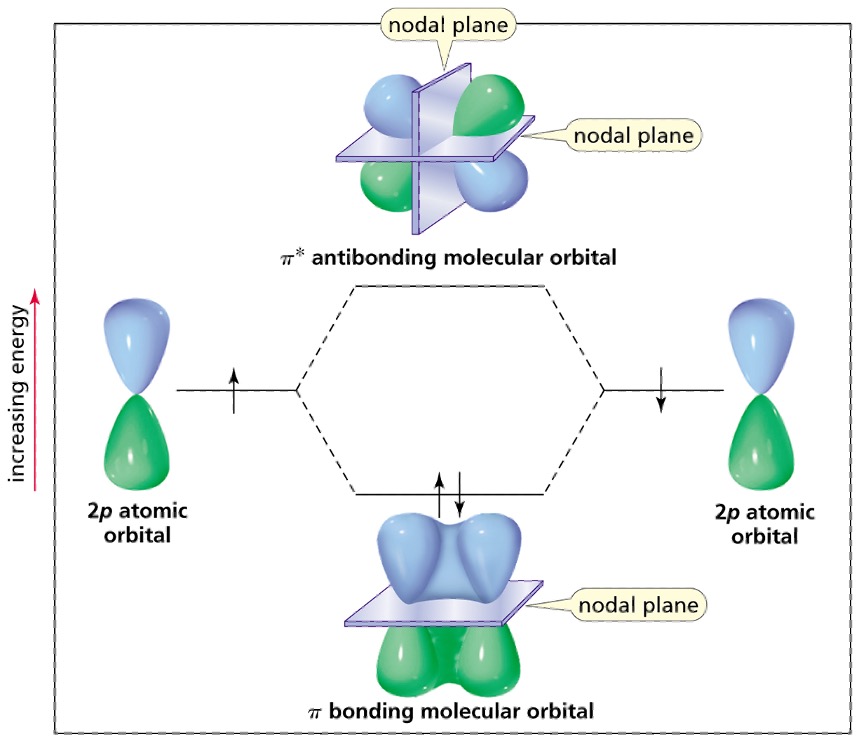
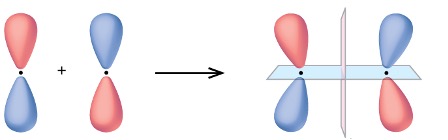
Out-of-phase (different colour)overlap forms a
π* antibonding MO
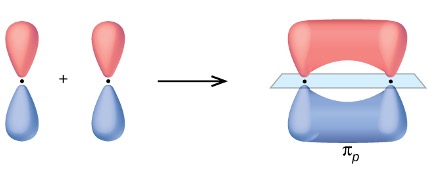
In-phase (same colour) overlap π bonding MO.
hydrid orbitals –
give side-to-side overlap and π covalent bonds
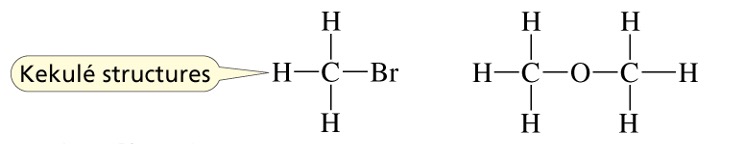
Kekulé structures are like Lewis structures except that lone pairs are normally omitted
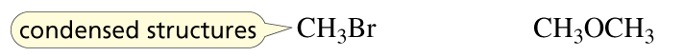
Condensed structures
omit the covalent bonds and the lone pairs
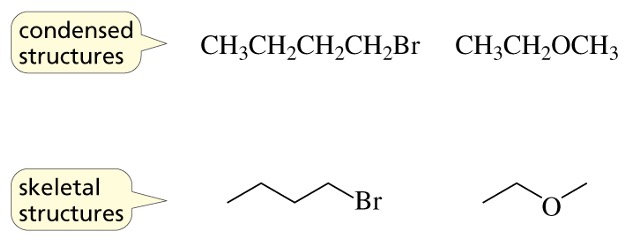
Skeletal structures
show the carbon-carbon bonds as lines, and do not show the hydrogens that are bonded to the carbons
The main macromolecular targets for drugs are
proteins (mainly enzymes, receptors & transport proteins) and nucleic acids (DNA & RNA)
The interaction of a drug with a molecular target involves a process known as
binding
There is usually a specific area of the macromolecule where this takes places – this is known as the
binding site
qMost drugs bind to their targets by forming a network of intermolecular interactions (non-covalent bonds)
Binding typically involve intermolecular interactions between drug and target
Regions within the target binding site involved in binding interactions are called
binding regions
Functional groups on the drug involved in binding interactions are called
binding groups
Binding sites are sometimes a hydrophobic hollow/cleft on the macromolecular surface
Drugs are generally much smaller than their targets
•Most drugs are in equilibrium between being bound and unbound to their target
•Binding interactions usually result in an induced fit where the binding site changes shape to accommodate the drug
The study of how drugs interact with their targets through binding interactions is known as
pharmacodynamics

qIonic bonds are the most important initial interactions as a drug enters the binding site
qTakes place between groups of opposite charge
qIonic electrostatic interactions are generally the strongest of the intermolecular bonds (20-40 kJ mol-1)
The strength of interaction drops off much less rapidly with distance compared to van der Waals interactions – electrostatic interactions are considered long-range interactions
A hydrogen bond takes place between an
electron deficient hydrogen and an electron rich heteroatom (electronegative N or O)
qThe electron deficient hydrogen is usually attached to a heteroatom (O or N)

the electron deficient hydrogen is called a
qhydrogen bond donor (HBD)
The electron rich heteroatom has to have a lone pair of electrons and is called a
qhydrogen bond acceptor (HBA)
Hydrogen bond distances are typically
1.5 – 2.2 Å
a heteroatom is
any atom that is not carbon or hydrogen
Some functional groups can act both as hydrogen bond donors and hydrogen bond acceptors EG
–OH and –NH2
A good hydrogen bond acceptor has to be
electronegative and have a lone pair of electrons.
Nitrogen has one lone pair of electrons and can act as an acceptor for one hydrogen bond; oxygen has two lone pairs and can accept two hydrogen bonds
Any feature that affects the electron density of the HBA is likely to affect its ability to act asaHBA:
the greater the electron density on the heteroatom, the greater its strength as a HBA
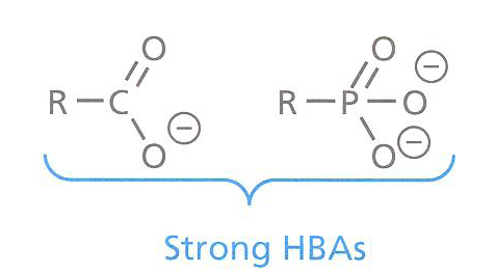
qExamples of strong hydrogen bond acceptors
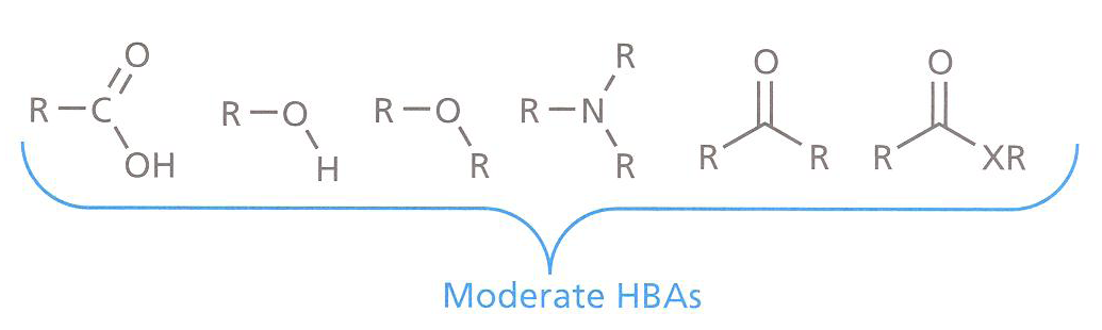
Most HBAs present in drugs and binding sites are neutral
qThese groups will form moderately strong hydrogen bonds

qS also forms weak H-bonds. Its lone pairs are in the 3rd shell, orbitals are more diffuse hence interact less efficiently with the HBD
qF is more electronegative than N or S, it has three lone pairs of electrons, yet it is a weak HBA
qThe pi (π) system in alkynes and aromatic rings are electron rich and hence can act as “hydrogen bond acceptors” – electron density is diffuse so interactions weak
van der Waals interactions
qWeak but very important interactions (2-4 kJ mol-1)
qThe overall contribution of van der Waals interactions to binding can be crucial
qOccur between hydrophobic regions of the drug and the target
Transient (temporary) areas of high and low electron densities cause
temporary dipoles
qInteractions drop off rapidly with distance (short-range interactions)
qDrug must be close to the binding region for interactions to occur
The instantaneous dipole on the drug will induce an instantaneous dipole on the target, favorable for interaction
Dipole-dipole interactions can occur if
the drug and the binding site have dipole moments
dipole-dipole interactions involve “localised” dipole moments in contrast to the “transient” (instantaneous) dipole moments we saw for van der Waals
Dipole moment ( μ ) is the measure of net molecular polarity, which is the magnitude of the charge Q at either end of the molecular dipole times the distance r between the charges. Dipole moments tell us about the charge separation in a molecule.
Ion-dipole interactions occurs when
the charge on one molecule interacts with the dipole moment of another
qStronger than a dipole-dipole interaction