OPT 112 Neurotransmitters; G Proteins
1/130
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
131 Terms
excitatory neurotransmitters
lower postsynaptic membrane potential to increase firing of EPSPs
Examples of excitatory neurotransmitters
glutamate, aspartate, acetylcholine
inhibitory neurotransmitters
actions will stabilize or raise the postsynaptic potential to decrease firing rate (IPSPs)
Examples of inhibitory neurotransmitters
gamma aminobutyric acid (GABA) and glycine
What neurotransmitters can be both IPSPs and EPSPs
dopamine depending on neuron location
Neuromodulation
release of chemicals from one cell that alter or regulate the reponse of neurons to neurotransmitters
allosteric regulation
The binding of a regulatory molecule to a protein at one site that affects the function of the protein at a different site.
Agonist
modulator mimics action of neurotransmitter by binding to the receptor
Antagonist
modulator blocks action of neurotransmitter by binding to the receptor
facilitation
modulator enhances effect of neurotransmitter by its increased concentration in synaptic cleft, slower degradation, slower reuptake
inhibition
reduces effect of neurotransmitter by decreased concentration in synaptic cleft, faster degradation, faster reuptake
examples of neurotransmitters
nitric oxide, enkephalins, adenosine, endocannabinoids
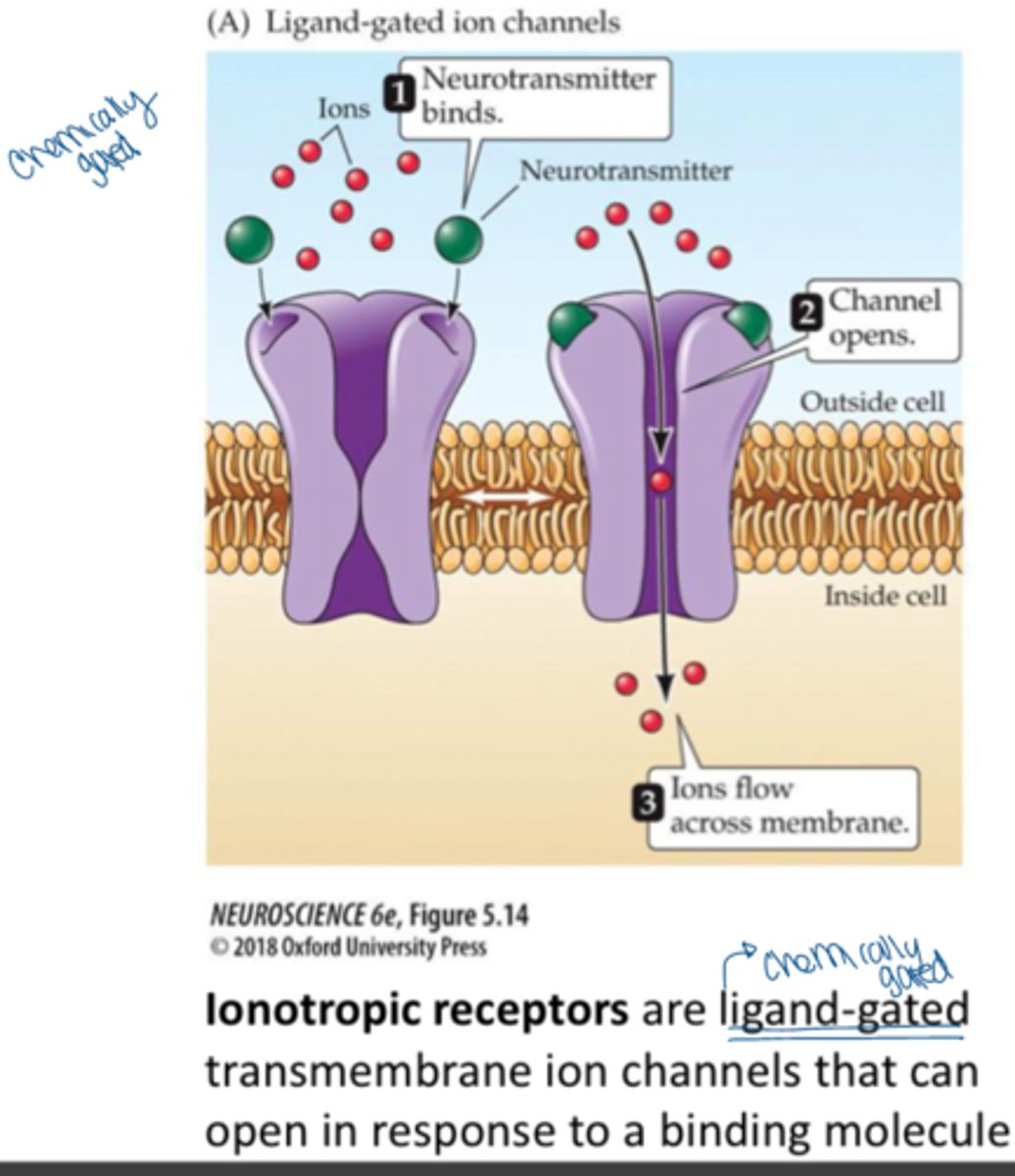
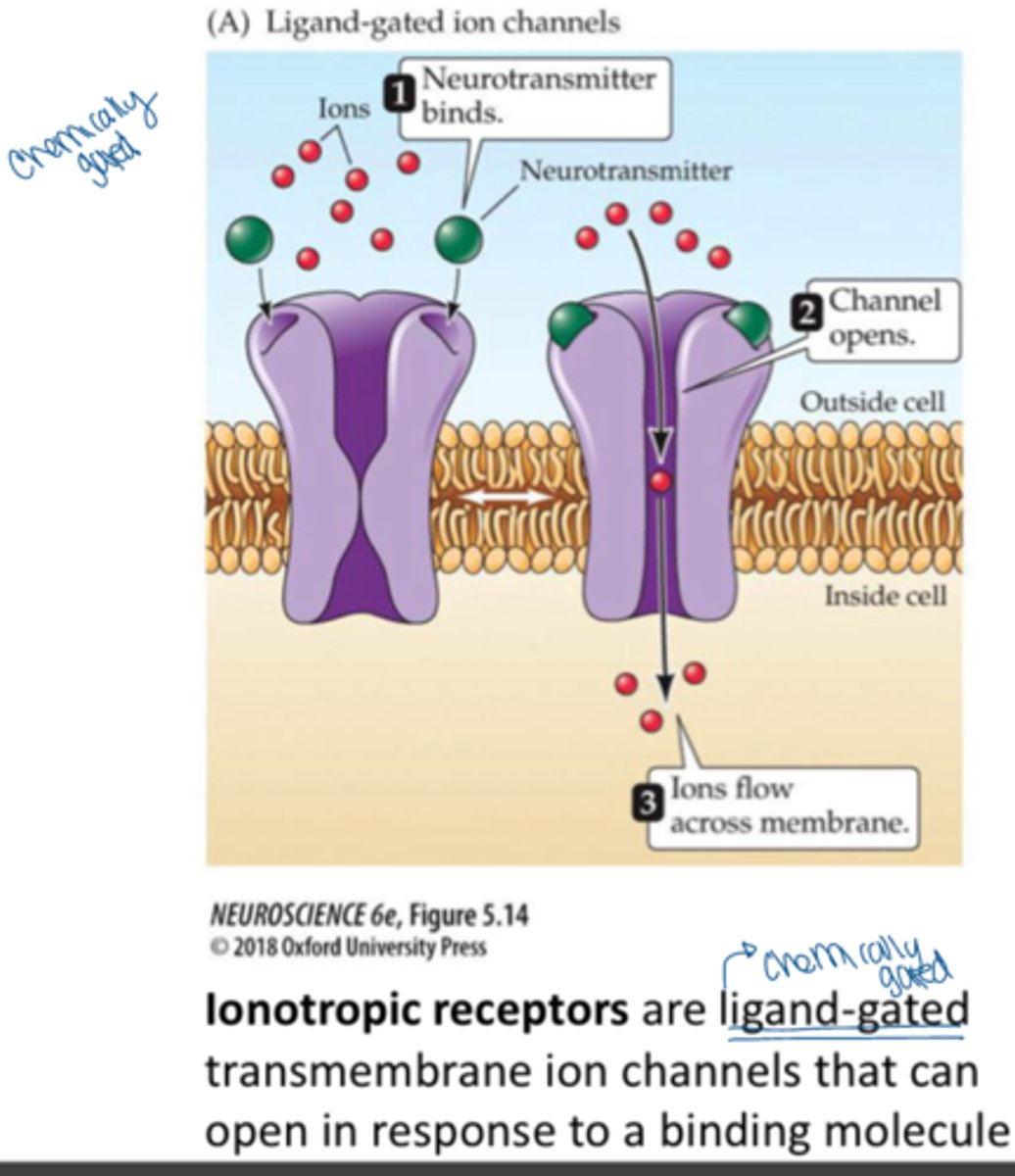
ionotropic receptors
receptors that are coupled to ion channels and affect the neuron by causing those channels to open
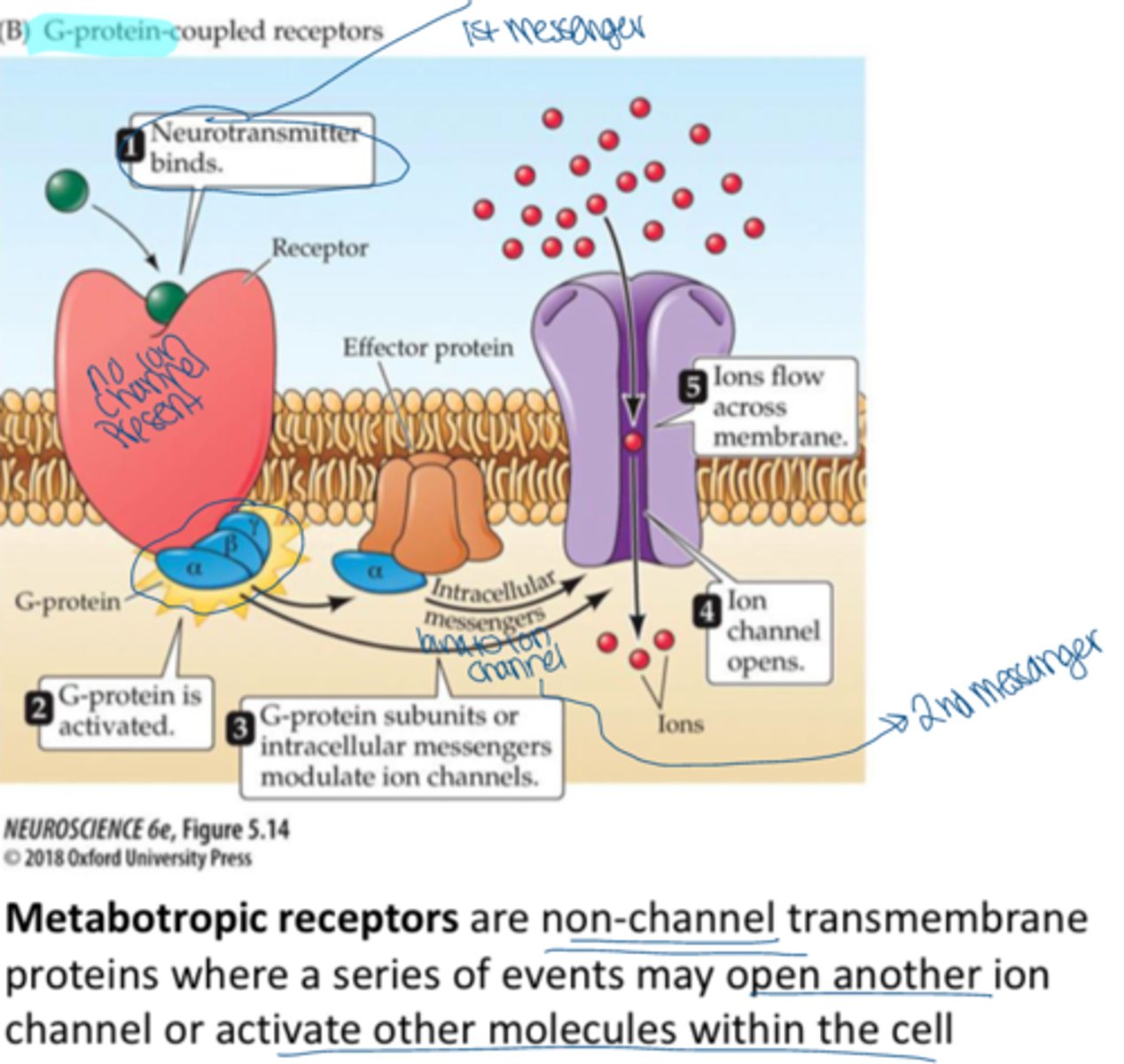
metabotropic receptors
receptors that are associated with signal proteins and G proteins
Acetylcholine (ACh)
A neurotransmitter that enables learning and memory and also triggers muscle contraction
biogenic amines
- modified amino acids
Catecholamines examples
dopamine, norepinephrine, epinephrine, synthesized by tyrosine
Indolamines examples
serotonin and histamine (synthesized by tryptophan and histidine)
amino acids
include glutamate, glycine, aspartate, and GABA
Neuropeptides
2-40 amino acids long, include endorphins as well as others relating to memory regulation and satiety and pain transmission to CNS
Where is acetylcholine found?
PNS and CNS
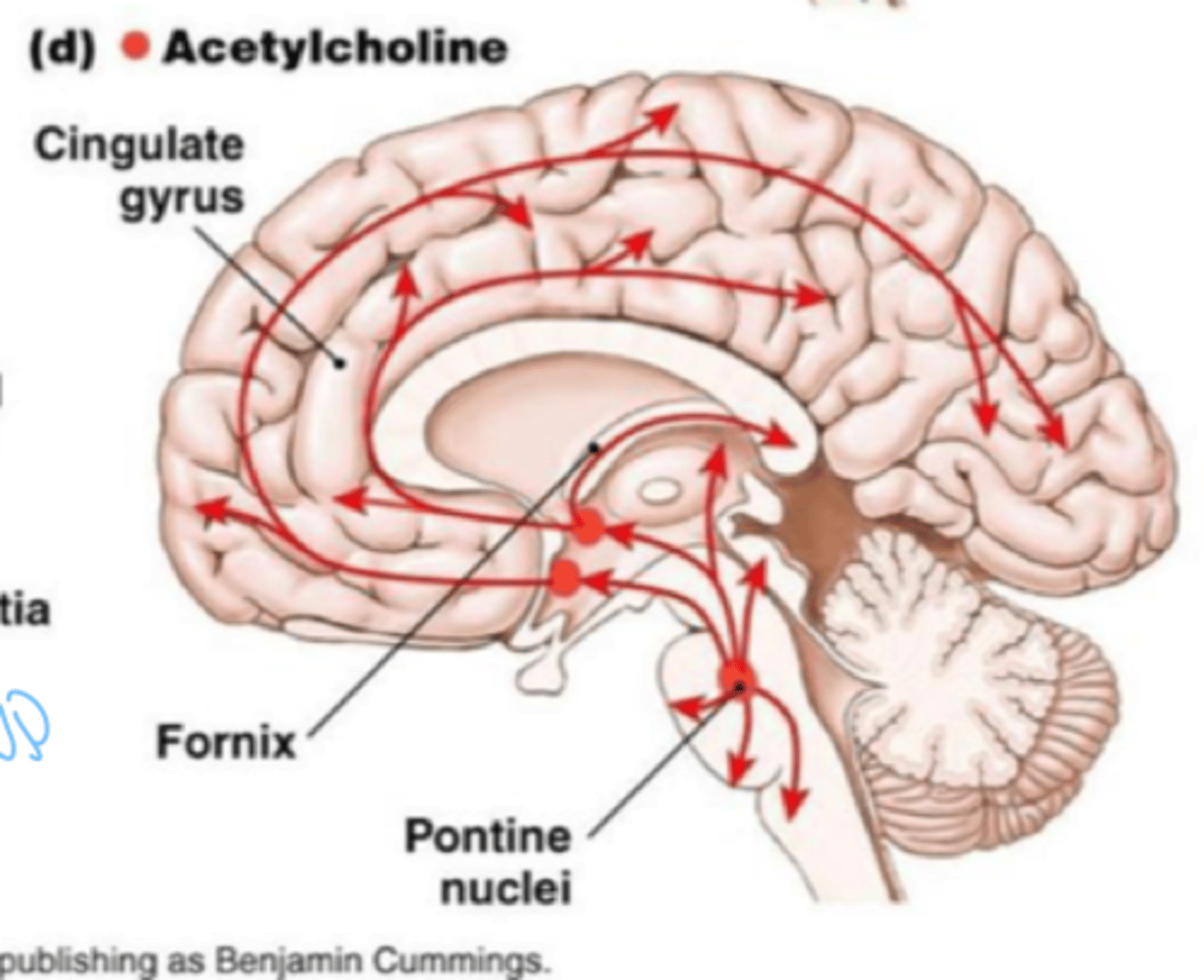
neurons utilizing ACh as primary neurotransmitter are called
cholinergic neurons
how to create ACh
choline acetyl transferase: acetyl coA + choline --> acetylcholine + CoA
What happens when botulism toxin binds to SNARE proteins?
prevents vesicle from releasing excitatory Ach
Where does degradation of Ach occur? what enzyme degrades it?
synaptic cleft, acetylcholinesterase
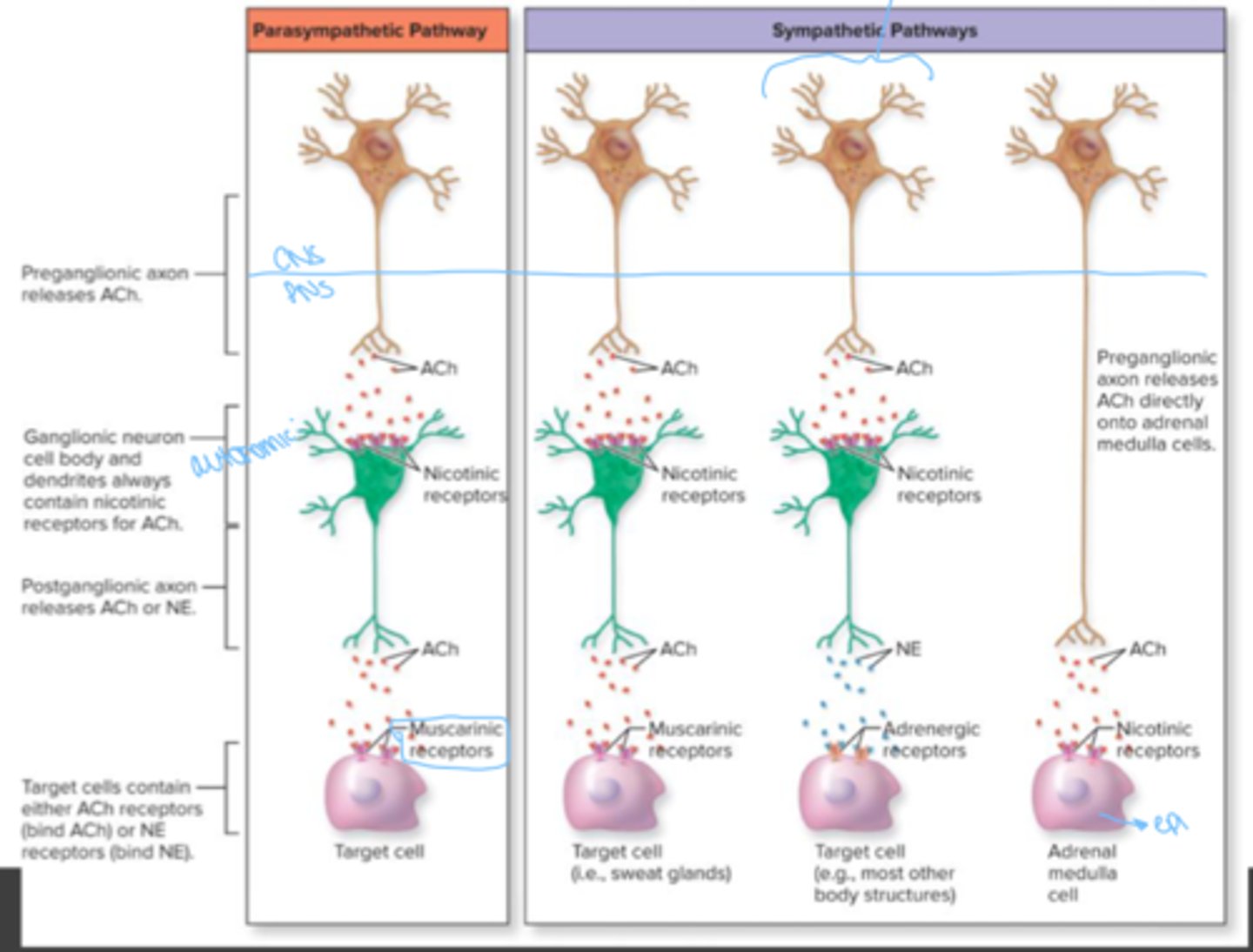
muscarinic receptors
found in CNS, autonomic effector (cardiac and smooth muscle) at its synapse with the postganglionic neuron of ANS
nicotinic receptors
somatic effector (skeletal muscle) at neuromuscular junctions in somatic nervous system. postganglionic neurons at synapse with preganglionic neurons in the autonomic ganglia of the ANS
Other than ACH degradation, what other ways can the neurotransmitter be removed from the synaptic cleft
-reuptake into presynaptic cell
-uptake by glial cells
- diffusion away from synaptic cleft
Neurons associated with ACh system degenerate in people with
alzheimers
Alzheimer's disease
decreased amount of ACh in certain areas of the brain and even the loss of postsynaptic neurons that would have responded to it
examples of catecholamines
dopamine, norepinephrine, epinephrine (all derived from tyrosine)
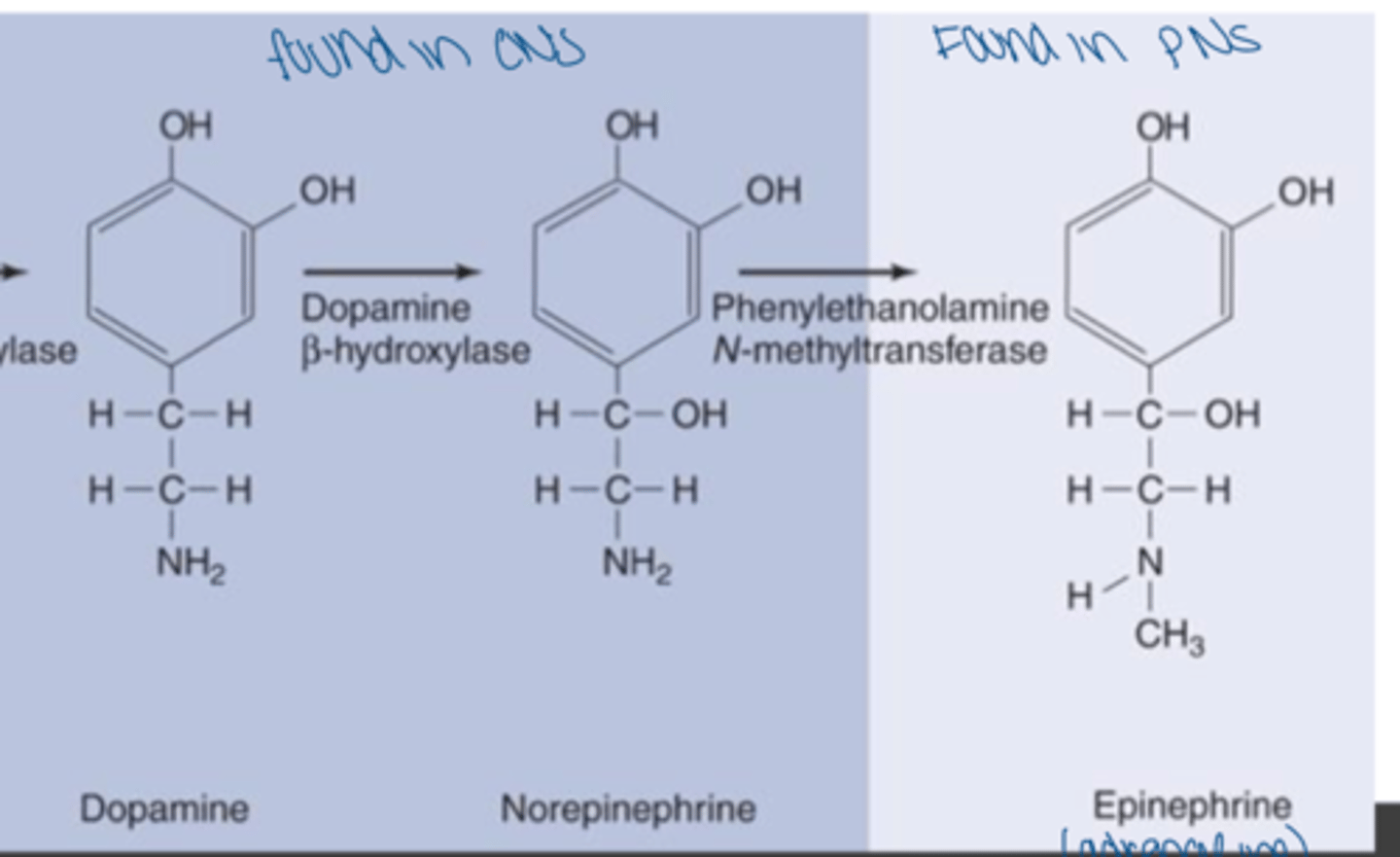
Where are dopamine and norepinephrine commonly found?
neurons of hypothalamus and brainstem of the CNS (norepinephrine can be found in PNS)
Where is epinephrine synthesized?
adrenal medulla (adrenaline)
How are catecholamines degraded?
monoamine oxidase
MAO inhibitors
inhibit the action of an enzyme called MAO, which normally breaks down and deactivates norepinephrine and serotonin.
adrenergic receptors
receptor sites for the sympathetic neurotransmitters norepinephrine (CNS and PNS) and epinephrine (PNS)
G-coupled protein receptors
A type of receptor that can indirectly cause the opening or closing of ion channels; typically do not let ions pass through them
alpha adrenergic receptors
usually excitatory... alpha 1, alpha 2
beta-adrenergic receptors
usually inhibitory... beta 1, beta 2, beta 3
Alpha 1 receptors
smooth muscle contraction
Alpha 2 receptors
smooth muscle contraction and neurotransmitter inhibition
Beta 1 receptors
heart muscle contraction, smooth muscle relaxation, glycogenolysis
Preganglionic fibers of sympathetic division
cholinergic
preganglionic fibers of the parasympathetic division
cholinergic
postganglionic fibers in sympathetic division
mostly adrenergic; a few cholinergic
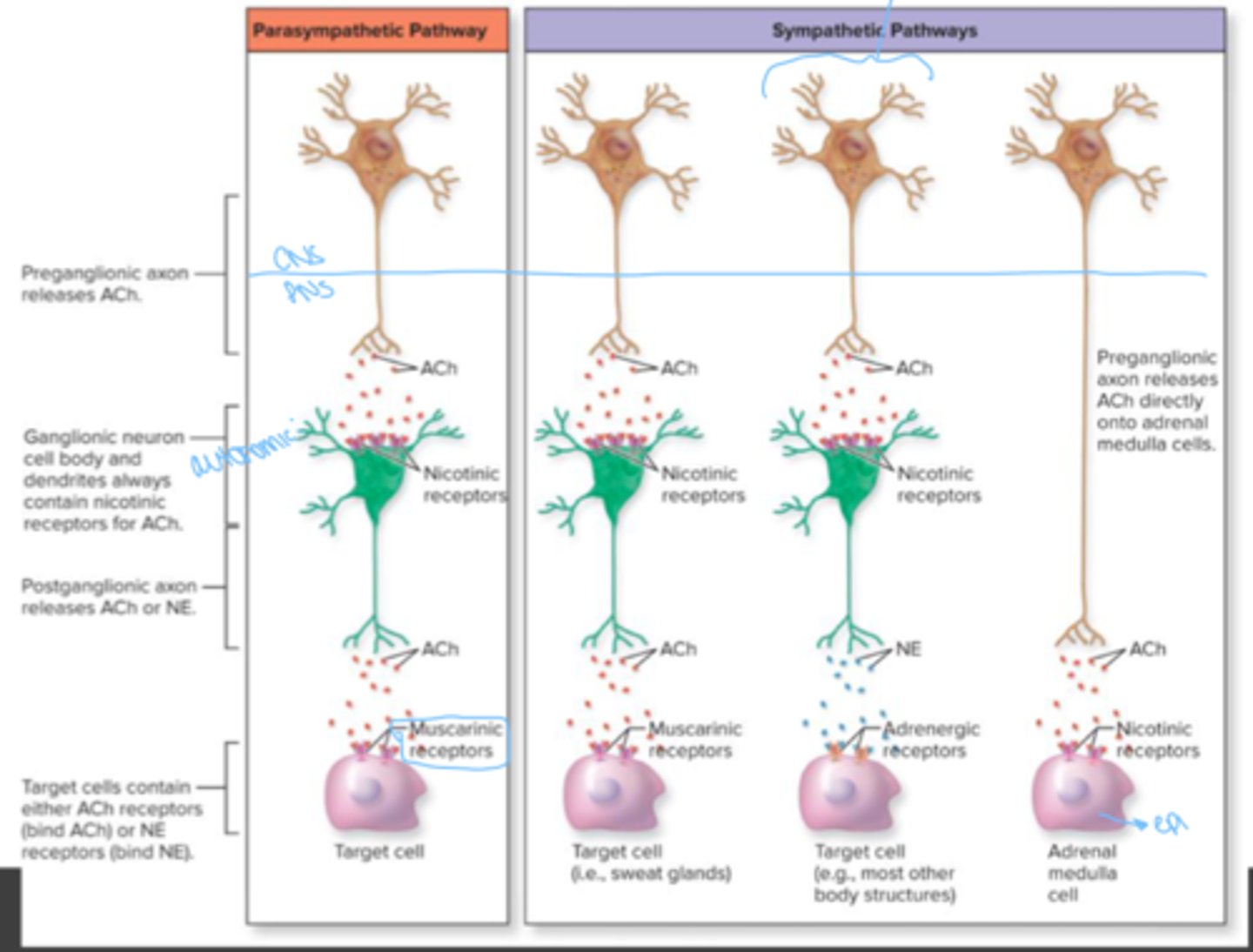
postganglionic fibers in parasympathetic division
cholinergic
Indolamines
serotonin (5-hydroxytryptamine) and histamine
what is serotonin derived from?
Tryptophan
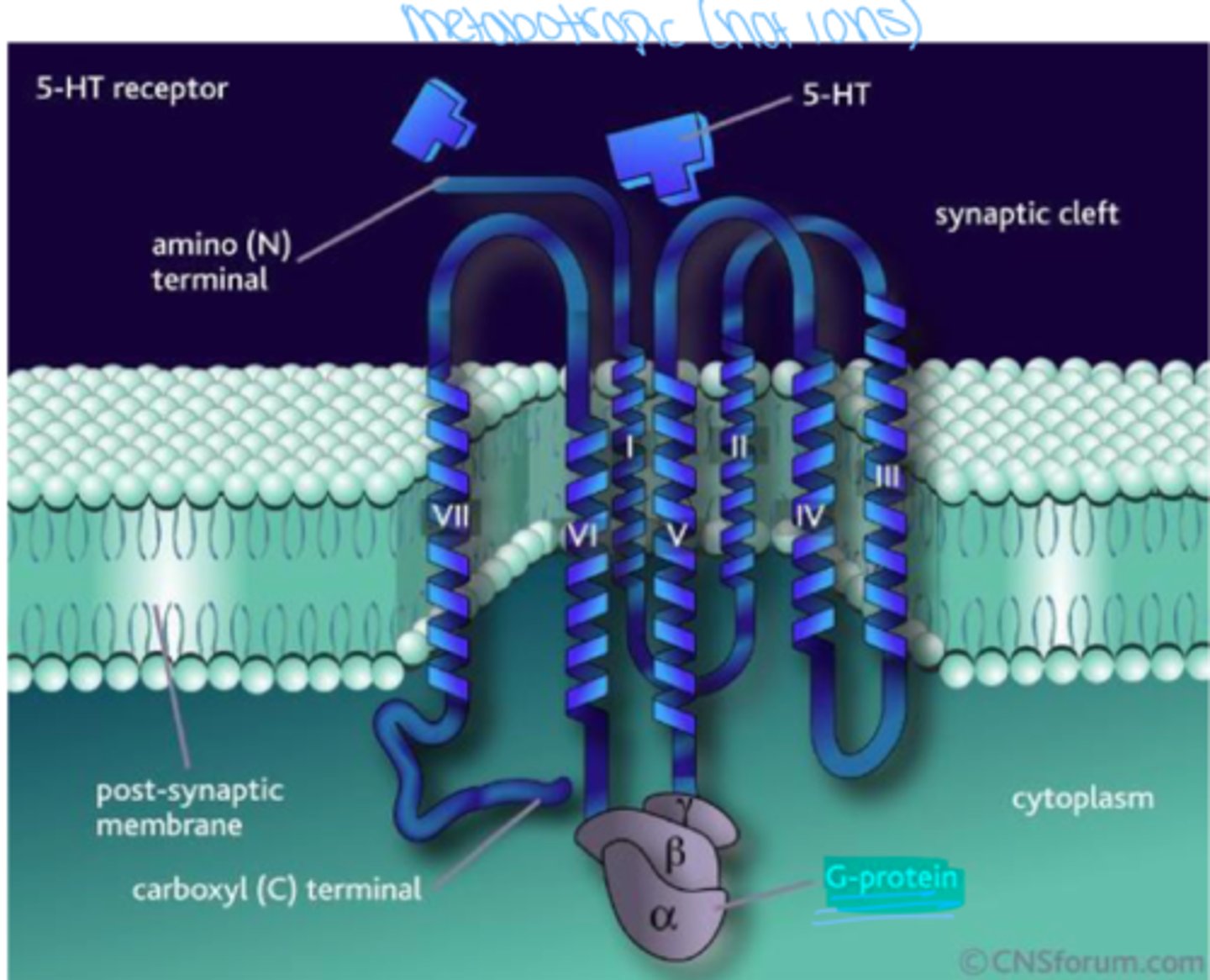
where is serotonin found ?
majority of brain and spinal cord
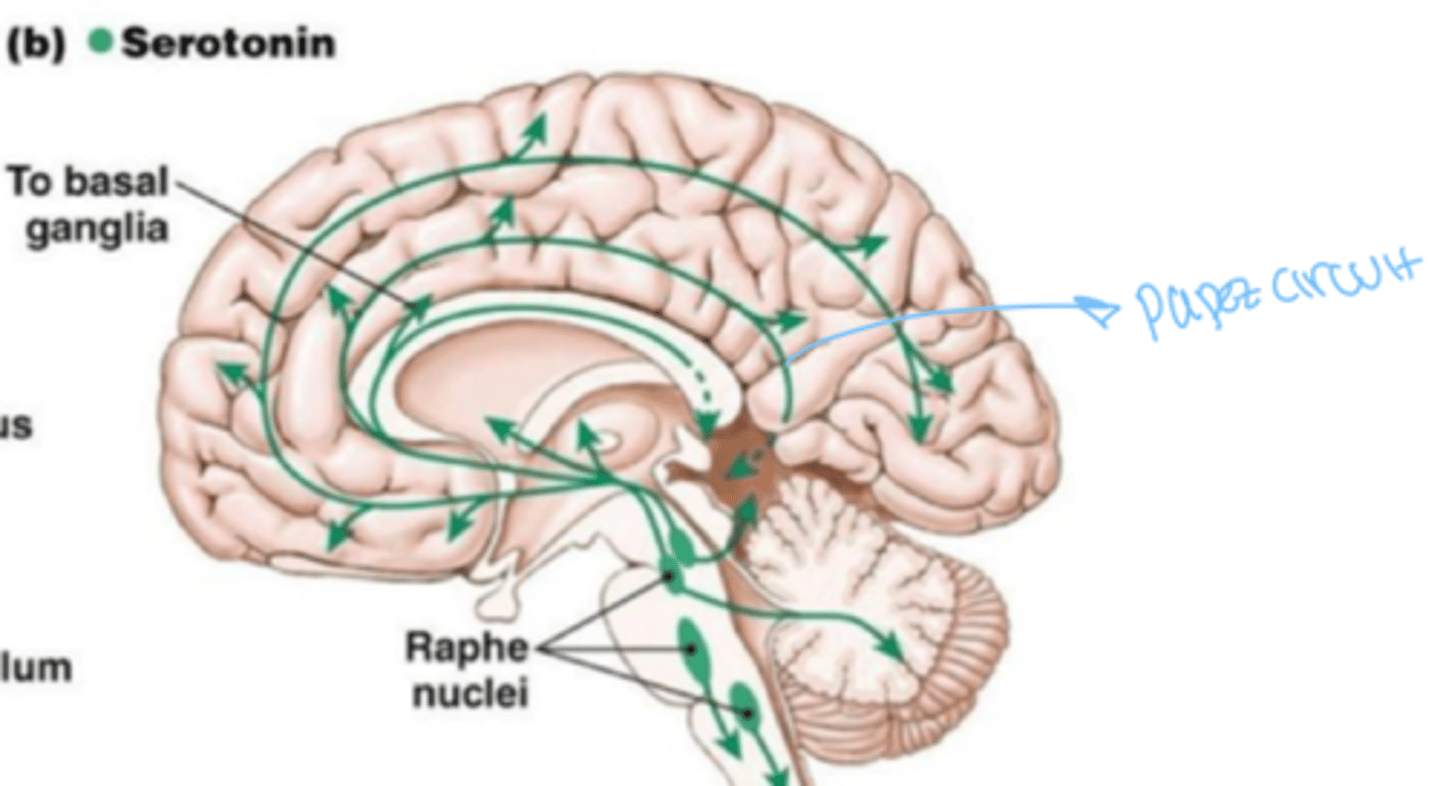
What does serotonin do?
exerts excitatory effect muscle control and inhibitory effect on pathways that mediate sensation
when are serotonin levels lowest? highest?
during sleep, during alertness
SRI (Serotonin Reuptake Inhibitor)
aid in management of depression (paxil)
functions of seratonin
regulating sleep, emotions, regulate cell growth, vascular smooth muscle contraction
What three neurotransmitters serve to regulate cognitive function, mood, and emotion?
serotonin, dopamine, noradrenaline
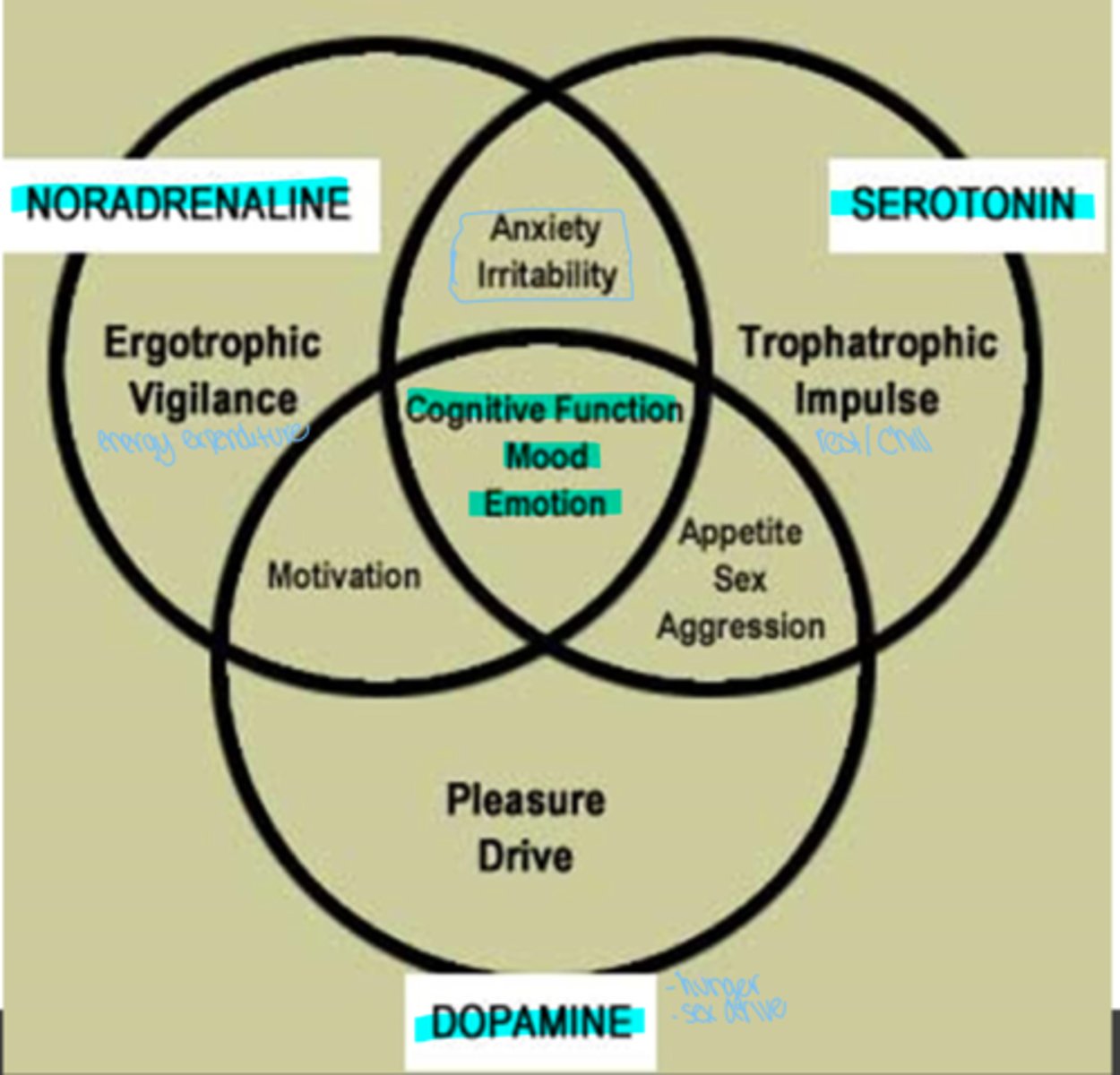
What is noradrenaline responsible for?
ergotrophic vigilance (energy expenditure), motivation, anxiety, irritability
what is dopamine responsible for?
please drive - motivation, appetite, sex, aggression
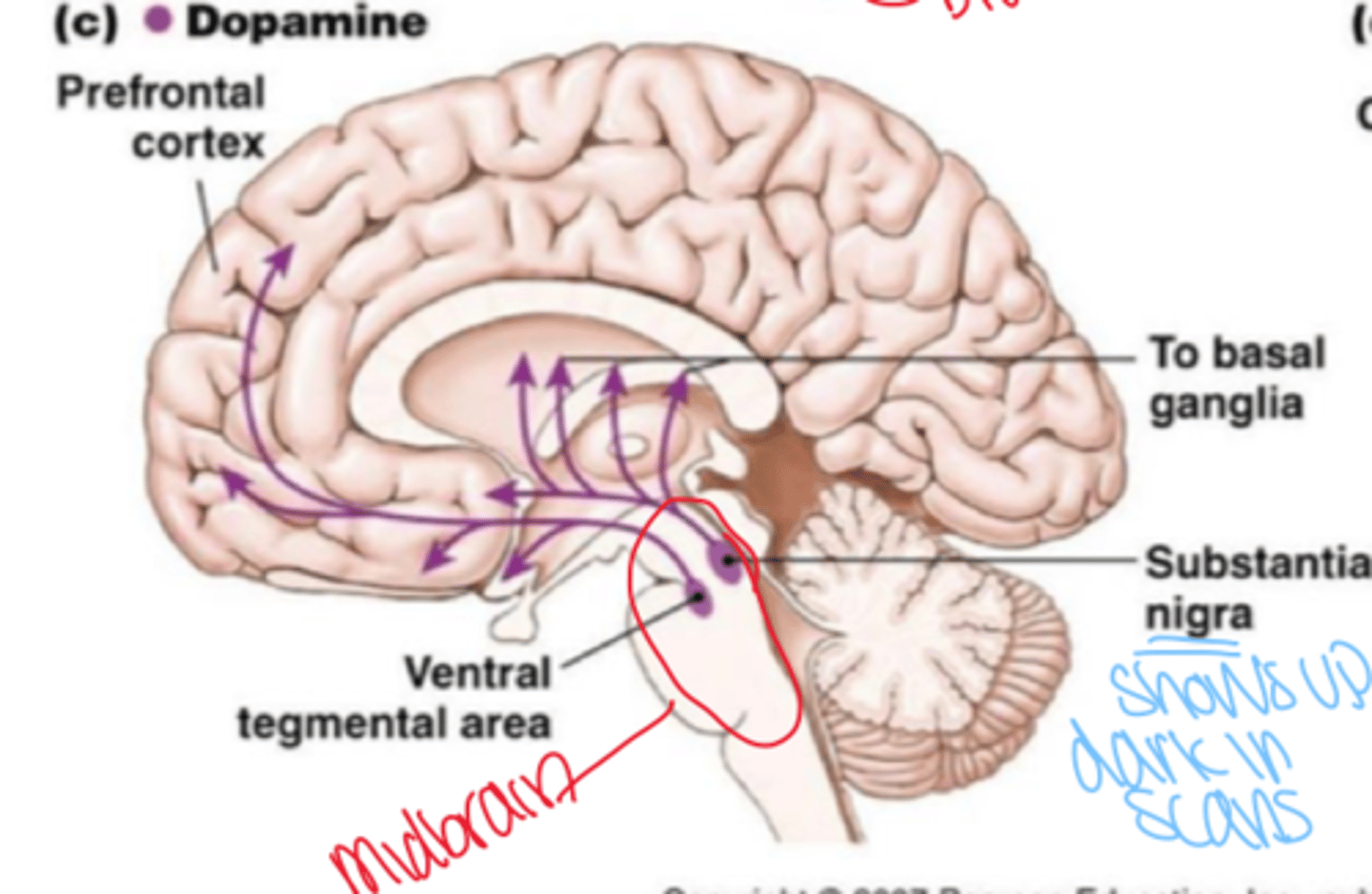
What causes Parkinson's disease?
loss of dopamine releasing neurons in substantia nigra of the midbrain
What drug manages parkinsons?
L-Dopa; alleviates symptoms and often combines with deprenyl which prevents its degradation
amino acid neurotransmitters
glutamate, aspartate, glycine, GABA
Glutamate
A major excitatory neurotransmitter; involved in memory (50% of all ESPS in CNS)
Where is glutamate synthesized?
mitochondria from glucose and glutamine
ionotropic glutamate receptors
learning and memory, AMPA, NMDA
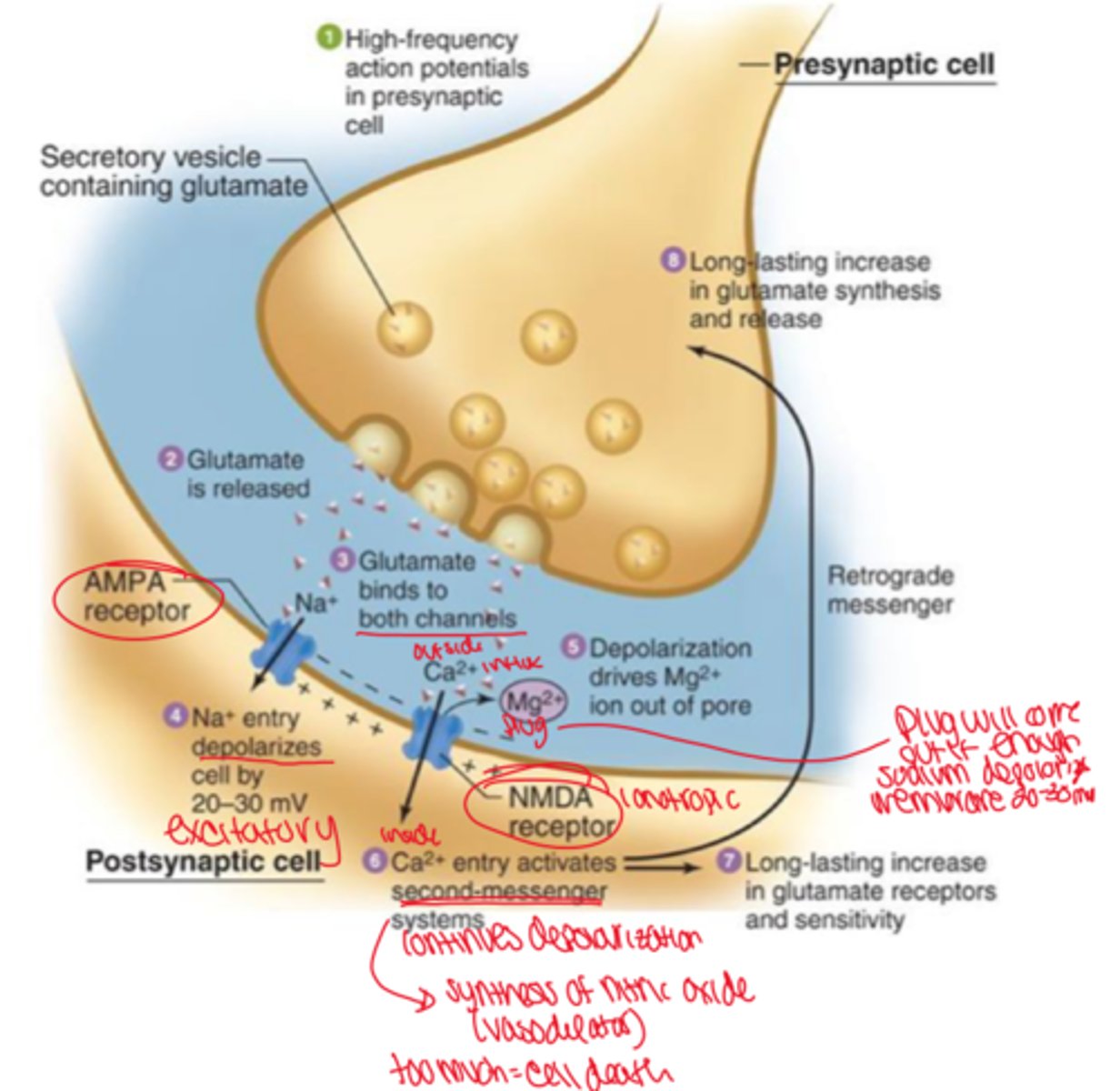
AMPA receptor
fast EPSP, conduction of Na+, depolarizes the membrane
NMDA receptor
bump Mg2+ channel allowing from influx of Ca2+
reuptake
glutamate is recycled by glial cells and converted to glutamine for reuptake by the presynaptic cell
Gamma-aminobutyric acid (GABA) --> GABA a, GABA b
most inhibitory neurotransmitter as it dampens neural activity in the brain
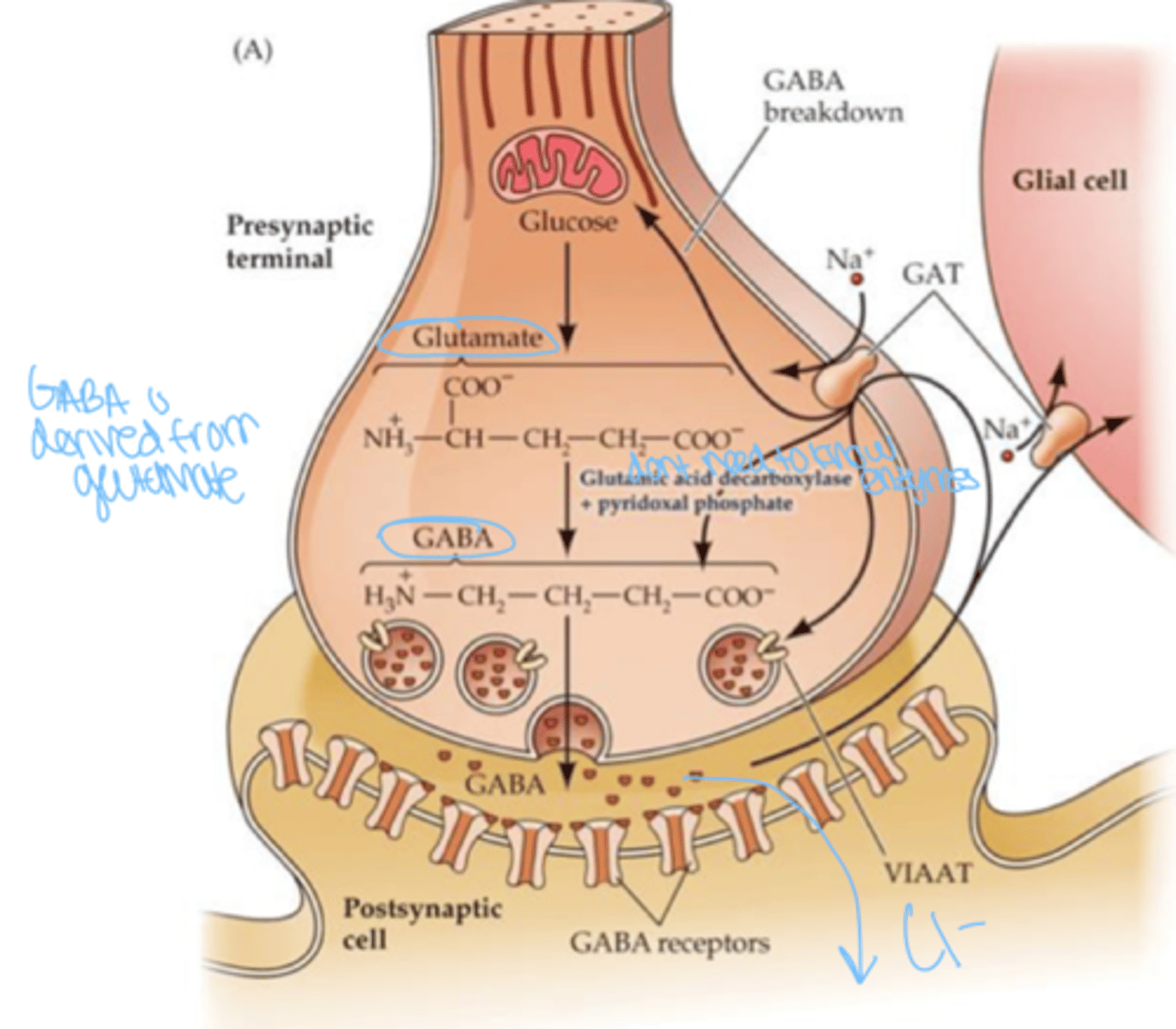
how does GABA alter the membrane potential?
binds to ion channel and shifts to more negative charge as Cl- channels are open ; generates IPSPs @ postsynaptic neuron
binding sites on GABA receptors serve as targets for
steroids, ethanol, and drugs including barbiturates and benzodiazepines
how is glycine formed?
converted from serine by an enzyme
Glycine
inhibitory neurotransmitter of the brainstem and spinal cord
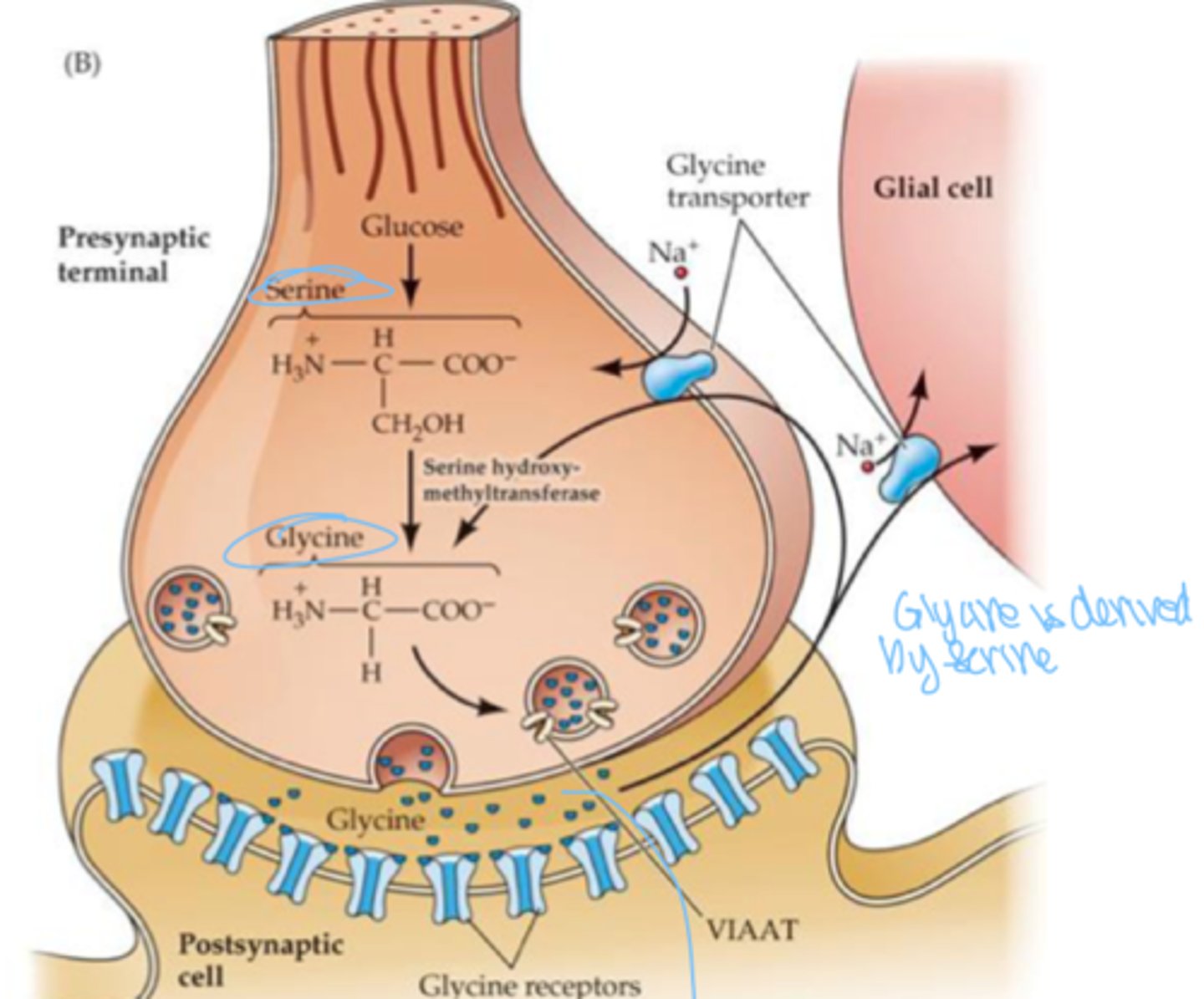
how does glycine work?
cause IPSPs by increasing Cl- influx into postsynaptic cell , regulate skeletal muscle contractions
What is an antagonist for glycine?
neurotoxin strychnine ; causes hyper excitability through nervous system leading to convulsions and spastic contraction of skeletal muscles
examples of neuropeptides
insulin, glucagon, oxytocin, vasopressin
nitric oxide
synthesized in the POSTganglionic neurons and released to cells that can act on PREganglionic (retrograde communication)
What is nitric oxide synthesized from?
Arginine
functions of nitric oxide
vasodilation, long term memory
What happens if too much nitric oxide is synthesized?
proinflammatory, cytotoxic
Neuronal NOS
CNS, skeletal muscle, cell communication
inducible NOS
immune system, cardiovascular system , immunological defense against pathogens (not calcium dependent)
entothelial NOS
endothelium, vasodilation, calcium dependent
Examples of metabolically active molecules
carbon monoxide, hydrogen sulfide, ATP and adenosine
Endocannabinoids
decrease neurotransmitter release from presynaptic neurons altering memory and cognition as well as increase appetite
where are Endocannabinoids synthesized?
in post-synaptic terminals in response to Ca++ influx
Endocannabinoids examples
anandamide (AEA) and 2-arachidonyl-glycerol (2-AG) bind to CB1 and CB2
Ways in which a drug may effect neurotransmitters
see image
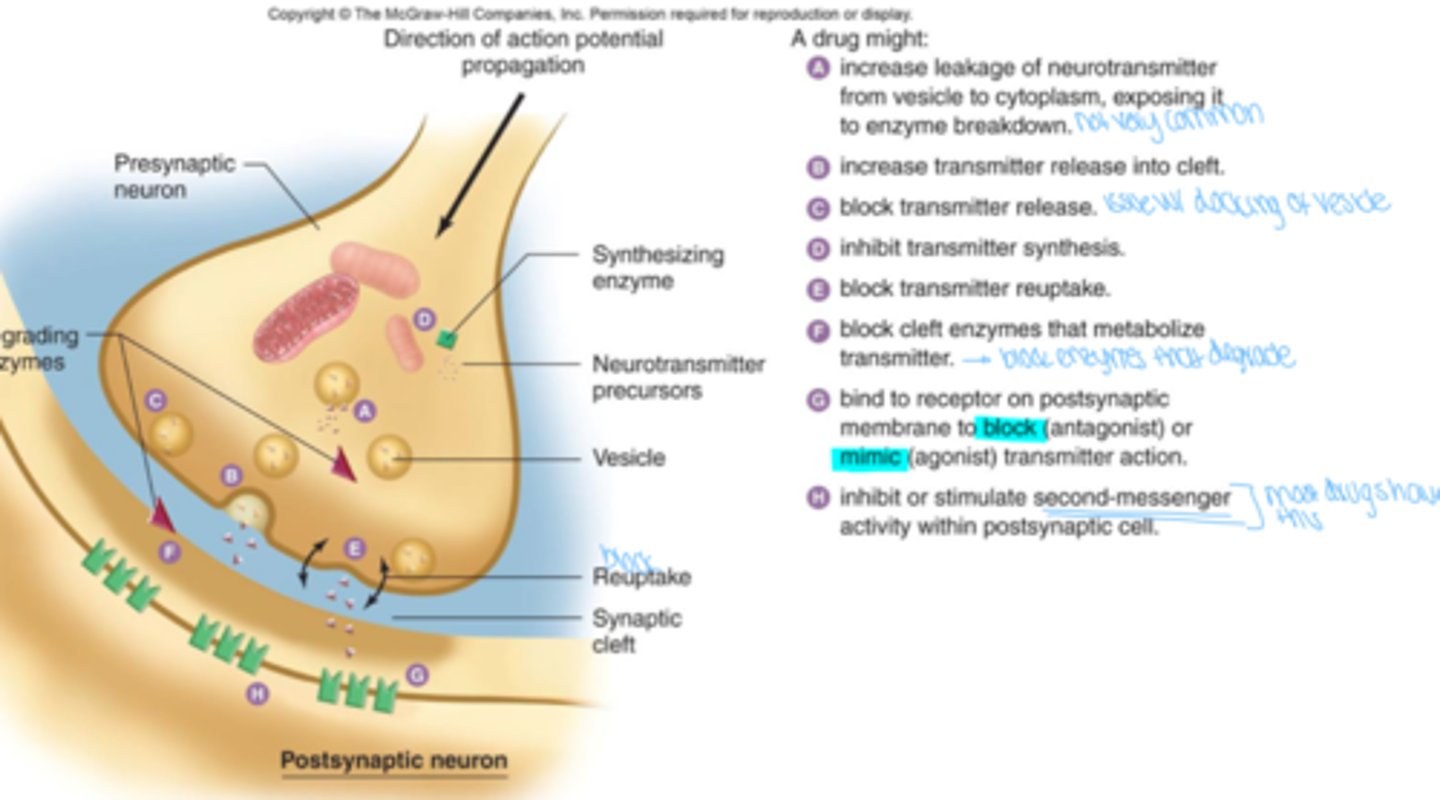
What is the most common effect drugs will have on neurotransmitters
inhibit or stimulate second messenger activity within postsynaptic cell
ACh
excitatory
precursor: choline +acetyl CoA
type of vesicle: small, clear
glutamate
excitatory
precursor: glutamine
type of vesicle: small, clear
GABA
inhibitory
precursor: glutamate
type of vesicle : small, clear
glycine
inhibitory
precursor: serine
type of vesicle: small, clear
epinephrine, norepinephrine, dopamine
excitatory
precursor: tyrosine
type of vesicle: small, dense core or large, and irregular
serotonin (5-HT)
mostly inhibitory
precursor: tryptophan
type of vesicle: large, dense core
histamine
excitatory
precursor: histidine
type of vesicle: large, dense core
ATP
excitatory
precursor: ADP
type of vesicle: small, clear
neuropeptides
excitatory and inhibitory
precursor: amino acids/protein synthesis
type of vesicle: large, dense core
Endocannabinoids
inhibits inhibition
precursor: membrane lipids
vesicle type: none
nitric oxide
excitatory and inhibitory
precursor: arginine
vesicle type: none
Why are G proteins so important?
important for vision, tear formation, aqueous humor formation, responsible for many biochemical pathways
many medications (60%) and various systemic diseases affect them
7 TM (horseman)
Class A-F; play important role in cell physiology and biochemistry, play an important role in medicine
What are 7 TM activated by
light, olfactory stimulants, peptides, hormones and neurotransmitters