Chemistry: Unit 11 Review
1/19
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
pOH
measure of hydroxide ion (OH-) concentration
pH
measure of hydrogen ion (H+) concentration
pOH and pH are similar in the sense that they both…
are measured from 0-14
have an inverse relationship (pOH=12, then pH=2)
Arrhenius acids and bases
compounds that form (H+) and (OH-) when dissolved in water
*Acids release hydrogen ions when dissolved in water
Bronsted-Lowry acids and bases
acids are substances that can donate protons (H⁺ ions) to another substance, known as a base. In this model, an acid is considered a proton donor
*not just for aqueous solutions
Bronsted-Lowry base pairs with…
Conjugate acid
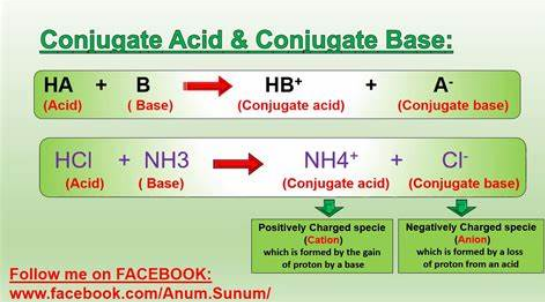
Bronsted-Lowry acid pairs with…
Conjugate base
pH of 0-6
Acid
pH of 8-14
Base
pH=7
neutral
pH 0-3
concentrated acid
The lower the pH…
concentrated acid
dilute base
The higher the pH…
dilute acid
concentrated base
strong means…
100% ionized in aqueous solutions
weak means…
ANYTHING less than 100% ionization.
this incudes 99%
Amphoteric
a substance that can act as either an acid or a base
acid/base indicators
litmus paper, cabbage test, phenolphthalein,
neutralization
results in an acid and a base
KOH
Arrhenius base
How to find pH from an equation
