Parasitology INM module
1/111
Earn XP
Description and Tags
1. Malaria 2. Leishmaniasis 3. Toxoplasmosis 4. Filariasis
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
112 Terms
Define high receptive areas of Malaria
Areas with high densities of the major vector Anopheles culicifacies
What are the 2 hosts necessary for the Plasmodium sp. to complete their life cycle?
Definitive host
Intermediate host
What is the definitve host of the Plasmodium sp.?
The female Anopheles mosquito where the sexual phase of the parasite’s life cycle takes place.
What is the intermediate host of the Plasmodium sp.?
Man, where the asexual phase of the Malaria parasite’s life cycle takes place.
What the advantages of serology in diagnosis of malaria?
A tool to screen blood donors
Screening blood donors involved in cases of transfusion induced malaria when donor’s parasitemia may be below detectable level of blood film
What are the disadvantages of serology in diagnosis of malaria?
Doesn’t detect current infection (only measures past exposure)
Not practical for routine diagnosis of acute malaria (time required for antibody development and persistence of antibodies
Cross reactions between Plasmodium sp. and Babesia sp.
Why is serology not suitable for diagnosis of acute infections of malaria?
Detectable levels of anti-malaria antibodies do not appear until after the infection and persist long after parasitemia has resolved
What are the central features of Pathogenesis of P.falciparum malaria?
Cytoadherence
Rosetting
Agglutination
What are the complications of P.falciparum malaria?
Jaundice
Cerebral malaria
Generalized convulsions
Severe normocyctic anaemia
High fever (39-40oC)
Hypoglycemia
Acute renal failure
Hyperparasitemia
Malaria hemoglobinuria
Cicruclatory collapse, shock, septicemia
Acute pulmonary edema and ARDS
Abdominal bleeding
Metabolic acidosis with respiratory distress
Fluid electrolyte imbalance
What are the drugs used to treat acute malaria in Sri Lanka?
Chloroquine
Quinine
Artemisinin derivatives
Lumefantrine
What are the drugs available to treat acute malaria besides those used in SL?
Halofantrine
Proguanil
Pyrimethamine
Sulphones and sulphate
Mefloquine
Name the drugs used in chemoprophylaxis of malaria in SL
Mefloquine
What are the adverse effects in choloroquine?
NVAD
Corneal deposits (dose and time related and usually occurs when cumulative dose >100g )
Retinopathy/maculopathy
Qt prolongation
Headaches
Dizziness
Convulsions
Hyperpigmentation (skin,nails)
Hair loss and depigmentation
Pruritus , skin reactions
Hemolysis in G6PD deficiency
What are the cautions to be considered when administering chloroquine?
Diabetes
Psoriasis
Myasthenia graves
Long term therapy
Neurological disorders- especially epilepsy hx.
Severe GI disorders
Acute porphyrias
G6PD deficiency
What is chloroquine effective against?
Blood schizonts of
a. P. vivax
b. P. Ovale
c. P.malarie
Gametocytes of
a. P.vivax.
What is chloroquine NOT effective against?
Blood schizonts of P.falciparum (due to drug resistance)
Hypnozoites of P.vivax and P.ovale
Gametocytes of P.falciparum
What are the clinical uses of chloroquine?
Treatment of
P vivax
P Ovale
P malariae
What is the dosage of chloroquine administered?
Chloroquine base at a dosage of 25 mg/kg over three days
Day 1 and 2(10mg/kg)
600mg CQ sulphate (4tabs) - single dose each day
Day 3 (5mg/kg)
300mg CQ sulphate (2tabs) - single dose
What does relapse mean in the context of malaria?
Infection without an infective bite/Reactivation of malarial infection via hypnozoites
What is recrudescence in malariae?
The situation in which parasitemia falls below detectable levels and then later increases to a patent parasitemia
What is the average incubation period of malaria?
7-30 days
How many merozoites are released in the rupture of a Plasmodium falciparum erythrocytic schizont?
24-32 merozoites
How many merozoites are released in the rupture of a Plasmodium vivax erythrocytic schizont?
12-16
State the virulence factors of P.falciparum that result in sever malaria.
Hn
How long can the hypnozoite lay dormant in P.vivax?
Up to 5 years
What is the distribution of toxoplasmosis?
Worldwide
State the definitive host of toxoplasmosis
Cat
State the intermediate host of toxoplasmosis
Warm blooded animals including humans, birds and rodents
What is the causative agent of toxoplasmosis?
Obligate intracellular parasite Toxoplasma gondii
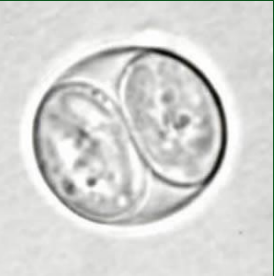
What is depicted in the image?
Toxoplasma gondii sporulated oocyst in an unstained wet mount

What is depicted in the image?
Toxoplasma gondii tachzyoites stained with Giemsa
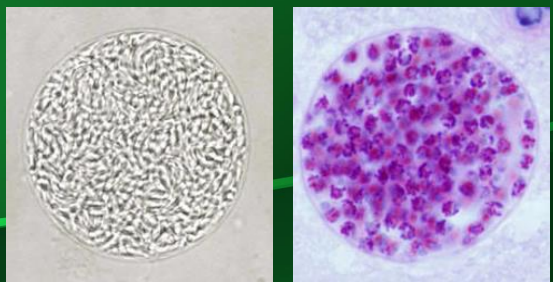
What is depicted in the image?
Toxoplasma gondii tissue cyst with bradyzoites seen within the cyst
How does the definitive host get infected with toxoplasmosis?
By ingestion of tissue cysts or sporulated oocysts
How long do Toxoplasmosis oocysts take to become infective?
Once shed in cat feces, they take 1-5 days to sporulate in the environment and become infective
How do intermediate hosts become infected with toxoplasmosis?
After ingestion of plant matter, soil or water contaminated with oocysts
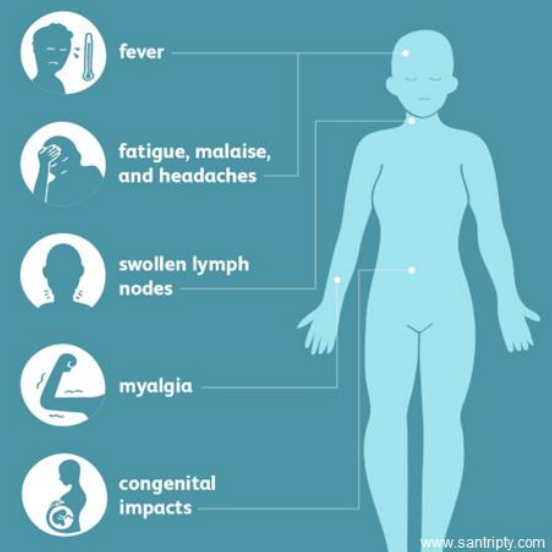
What is the clinical manifestation of toxoplasmosis in otherwise healthy individuals?
Majority of patients (85-90%) are asymptomatic
Febrile illness with malaise
Fatigue
Headache
Muscle pain
Cervical and axillary lymphadenopathy
What is the clinical manifestation of toxoplasmosis in immunosuppressed individuals?
Severe infection with high fever and skin rash
Meningo-encephalitis
Pneumonitis
Myocarditis
Hepatitis
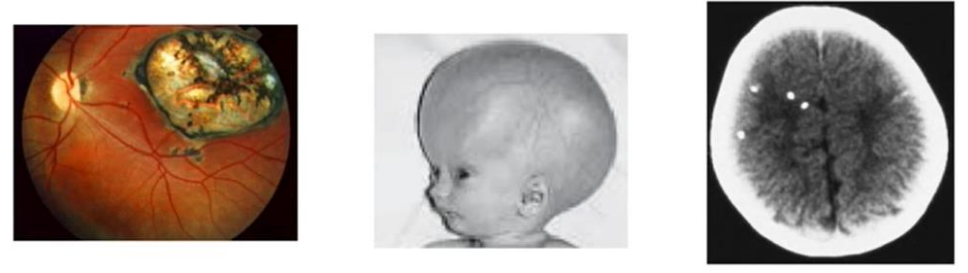
What is the classical triad for congenital infections of toxoplasmosis?
Chorioretinitis
Hydrocephalus
Intracranial calcifications
What is the clinical manifestation in relapse of toxoplasmosis in immunosuppressed individuals?
Headache
Confusion
Nausea/vomitting
Fever
Poor cordination
When can ocular toxoplasmosis come about?
Reactivation of congenital acquired toxoplasmosis infections
Reactivated retinal cysts cause tissue damage and inflammation
Commonly seen in teenagers and young adults
Describe the immune response in toxoplasmosis.
Stimulates both cellular and humoral immune response
Cell mediated immune response is important in controlling the infection
IgM and IgA appear early in the infection
IgG appear 2-3 weeks after and peak at 6-8 weeks
List the diagnostic methods of toxoplasmosis.
Serologic tests
Molecular techniques
Direct visualization of the parasite and/or its antigens'
By isolation of the parasite
What are the serological tests that can be used for the diagnosis of toxoplasmosis?
Enzyme linked immunosorbent assay (ELISA)
TORCH screen
Lateral flow chromatographic assay
Indirect fluorescent test (IFT)
Agglutination tests
Complement fixation tests (CFT)
State methods of direct demonstration of the toxoplasmosis parasite.
Mouse inoculation
Cell cultures
Autopsy material - Brain, fetal tissue
Biopsy material - Bone marrow, lymph nodes
What is the treatment for toxoplasmosis in immunocompetent symptomatic patients?
Sulfadiazine+pyrimethamine+Folinic acid OR
Trimethoprim sulfamethoxazole
How is ocular disease of toxoplasmosis in adults treated?
Sulfadiazine+pyrimethamine+folinic acid OR
Intravitreal clindamycin + dexamethasone
Why and how should folinic acid (leucovorin) be given in the treatment of toxoplasmosis?
For prevention of hematological toxicity
Should be given during and for one week after pyrimethamine therapy
How are immunocompromised patients with toxoplasmosis treated?
Sulfadiazine + pyrimethamine + folinic acid OR
Pyrimethamine + folinic acid + clindamycin OR
Trimethoprim sulfamethoxazole
How is a congenital infection of toxoplasmosis treated?
Sulfadiazine + pyrimethamine + folinic acid
Started ASAP after birth and continued for at least one year
How is toxoplasmosis treated in pregnancy if POA < 14 weeks with no fetal infection?
Spiramycin
Can pyrimethamine be given for treatment of toxoplasmosis in first trimester?
No, pyrimethamine is teratogenic in the first trimester
List the primary prevention methods of toxoplasmosis.
Dispose of cat feces daily
Eating well cooked meat
Wash hands thorougly with soap and water after handling raw meat or gardening
Washing vegetables and fruits before consumption
How would you prevent transmission of toxoplasmosis through blood transfusions and organ transplants?
Screening potential organ donors
Transfusing antibody negative blood to high risk patients
Transfusing leucocyte depleted blood components
State the causative agent for visceral leishmaniasis.
Leishmania donovani complex
State the causative agent for muco-cutaneous leishmaniasis.
Leishmania braziliensis
State the causative agent for cutaneous leishmaniasis
Leishmania major
Leishmania tropia
Leishmania aethiopica
State the morphological forms of the causative agent of leishmaniasis.
Amastigote
Promastigote
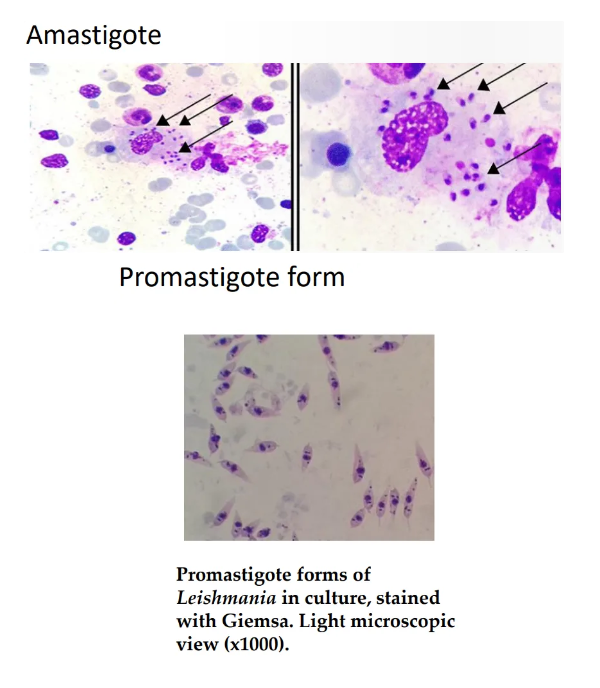
State the morphological features of amastigote.
Rounded shape
Non flagellated
Non motile
Found in host cells
Enters sandfly vector during a blood meal
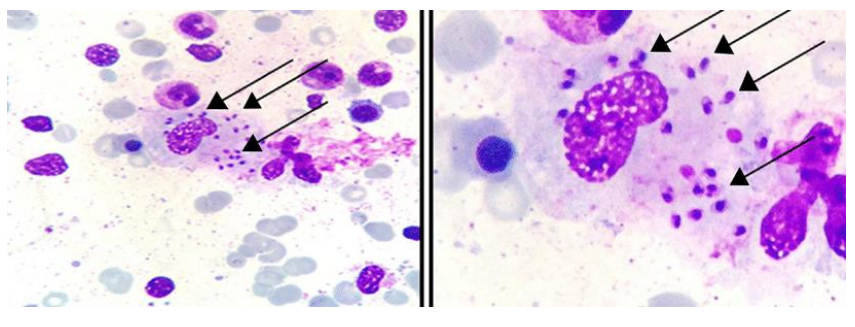
State the morphological features of promastigote.
Thin and elongated in shape
Motile
Flagellated
Found inside sandfly or in culture
Enters host when infected sandfly bites the host
State the vector of leishmaniasis.
Infected female sand fly
Smaller than mosquitoes, light brown in color

State the incriminated vector species of leishmaniasis in humans.
Phlebotomus in the Old World
Lutzomyia in the New World
State the probable vector of Leishmaniasis in Sri Lanka.
Phleobotomus argentipes
State the hosts of Leishmania parasites.
Mammals:
Humans
Rodents
Cattle
Dogs
State the methods of leishmania transmission
Zoonotic
Anthropronotic
What are the clinical types of cutaneous leishmaniasis?
Papules
Nodules
Plaques
Ulcers
What are papules in the context of cutaneous leishmaniasis?
Very small palpable lesions raised above the skin
Longest diameter is less than 1 cm
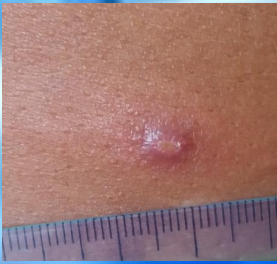
What are nodules in the context of cutaneous leishmaniasis?
palpable lesions raised above the skin
Longest diameter is more than 1 cm
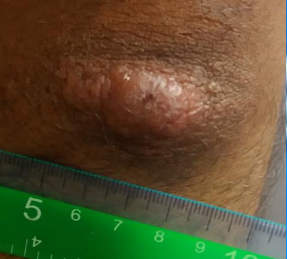
What are plaques in the context of cutaneous leishmaniasis?
A palpable flat lesion more than 1 cm in diameter
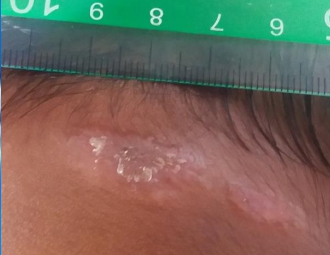
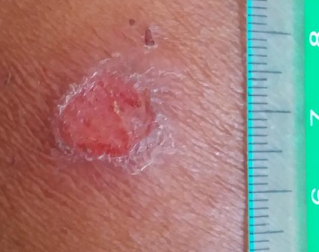
What are ulcers in the context of cutaneous leishmaniasis?
‘Volcanic’ in appearance with a raised border and a central crater
What are the main organs affected by visceral leishmaniasis?
Liver
Spleen
Bone marrow
State common clinical signs and symptoms of visceral leishmaniasis.
Rigors and chills
Lymphadenopathy
Pancytopenia
Non tender splenomegaly with or without hepatomegaly
Weight loss
How is mucocutaneous leishmaniasis caused?
Cutaneous lesions extending directly to adjacent mucuos membranes
Metastasis of cutaneous lesions via lymphatic or haematagonous spread to mucosal layer of mouth and upper respiratory tract
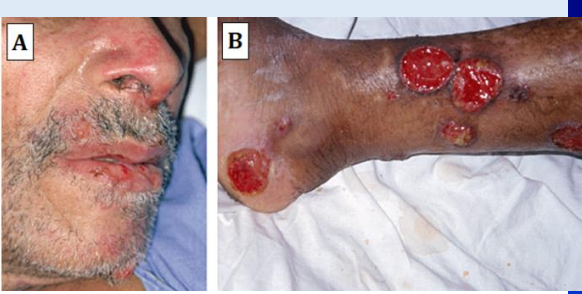
What are the signs of nasal involvement in mucocutaneous leishmaniasis?
Stuffed nose
Nasal bleeding
Where are the lesions of mucocutaneous leishmaniasis commonly seen?
Mouth
Nose
Throat
What is post kalar-azar dermal leishmaniasis (PKDL)?
It is a cutaneous sequela of visceral leishmaniasis, common with VL caused by L.donovani
What is the appearance of Post kalar-azar dermal leishmaniasis?
Hypopigmented or erythematous macules papules or nodules appearing on exposed parts of body such as face, arms and upper part of body
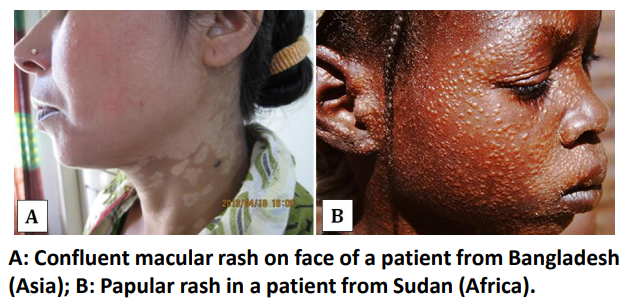
Wha is the most common clinical form of leishmaniasis seen in leishmaniasis HIV co -infection?
Visceral leishmaniasis
What is disseminated cutaneous leishmaniasis?
Co existence of different types of lesions such as papules, nodules and ulcers
What is diffuse cutaneous leishmaniasis?
Chronic
Non ulcerating
Non necrotising
Multiple skin lesions widespread over the body except on
Scalp
Axillae
Inguinal folds
Palms
Soles
Where are samples obtained for testing in CL, MCL and VL?
CL and MCL - samples are obtained from active edge of lesion
VL
Bone marrow aspirate
Liver aspirate
Lymph node aspirate
Spleen aspirate
Buffy coat of whole blood
List methods of sampling used in testing for leishmaniasis.
Tissue scraping/ split skin smear
Biopsy of lesion
Blood for serology
Tissue-impression smear
Bone marrow aspirate
Fine needle aspiration
State laboratory methods used to confirm the diagnosis of leishmaniasis.
Parasitological
Light microscopy
Culture
Molecular methods
PCR
Serological methods
Dipstick assays
ELISA
What is the most commonly used method in parasitological diagnosis of Leishmaniasis currently?
Demonstration of amastigotes by light microscopic examination of Giemsa stained lesion material

What are smears prepared from for light microscopy?
Slit skin scrapings
Lesion aspirates
Bone marrow aspirates
Impression smears from biopsies

In which type of leishmaniasis are serological methods used?
Visceral leishmaniasis since most patients don’t develop a significant antibody response in CL.
What is the specific antigen used in serological methods to detect the specific antibody against the L.donovani complex?
rK39 antigen in ELISA and ICT
What is the currently used main molecular diagnostic method of leishmaniasis?
Detection of parasitic DNA using PCR-based assays
List the challenges faced in the diagnosis of leishmaniasis.
Lack of pathognomic clinical features
Wide spectrum of clinical features
Existing as co-infections with other diseases
Overlapping with clinical features of other common diseases in the region such as malaria, leprosy and tuberculosis
What are tissue impression smears?
They are ‘imprints’ of the biopsy
Describe how a tissue impression smear is prepared and used in leishmaniasis diagnosis.
It is prepared by rolling the freshly cut surface of the biopsy on a clean glass slide
The glass slide is stained with Giemsa to observe under microscope
If there is a lot of blood, it must be blotted prior to making the smear.
What are the measures taken for control and prevention of leishmaniasis?
Personal protective measures to reduce contact with sandflies
Early diagnosis and effective treatment
Vector control measures targeting adult sandflies
Health education
Notification and active surveillance
What is the treatment for intestinal amoebiasis?
Metronidazole for 5 days
Diloxanide furoate (500 mg tds - 10 days)
What is the treatment for extra intestinal amoebiasis?
Metronidazole for 5-10 days
Diloxanide furoate (500 mg tds - 10 days)
List the available laboratory tests for amoebic diseases
Concentration technique
Floatation technique
Direct smears
Saline
Iodine
Staining - help differentiating amoebae
Iron-hematoxylin
Trichome
Imaging
Serology - useful in invasive forms - esp extra intestinal amoebiasis
Blood - leucocytosis
Stool culture - not routinely used
Colonoscopy - may reveal colonic ulcers and can take biopsies from lesion for microscopy/histopathology