Radioactivity
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Some isotopes are unstable because of their _____
large size or because they have too many or too few neutrons
Unstable nuclei can emit ____ to become more stable + in what form?
radiation
Radiation can be in the form of a high energy particle or wave

How does this make it more stable?
As the radiation moves away from the nucleus, it takes some energy with it
This reduces the overall energy of the nucleus
This makes the nucleus more stable
What is this called?
radioactive decay
What does random decay mean?
This means it is not possible to know exactly when a particular nucleus will decay
Radioactive decay is defined as:
The spontaneous disintegration of a nucleus to form a more stable nucleus, resulting in the emission of an alpha, beta or gamma particle
Radioactive decay is a random process, which means that:
There is an equal probability of any nucleus decaying
It cannot be known which particular nucleus will decay next
It cannot be known at what time a particular nucleus will decay
The rate of decay is unaffected by the surrounding conditions
It is only possible to estimate the proportion of nuclei decaying in a given time period
Radioactive decay is a spontaneous process, which means that:
The decay of nuclei is not affected by the presence of other nuclei in the sample
External factors such as pressure do not have an effect on the decay

What are alpha particles made up of?
Alpha (α) particles are high energy particles made up of 2 protons and 2 neutrons (the same as a helium nucleus)
alpha particles are usually emitted when __
nuclei that are too large

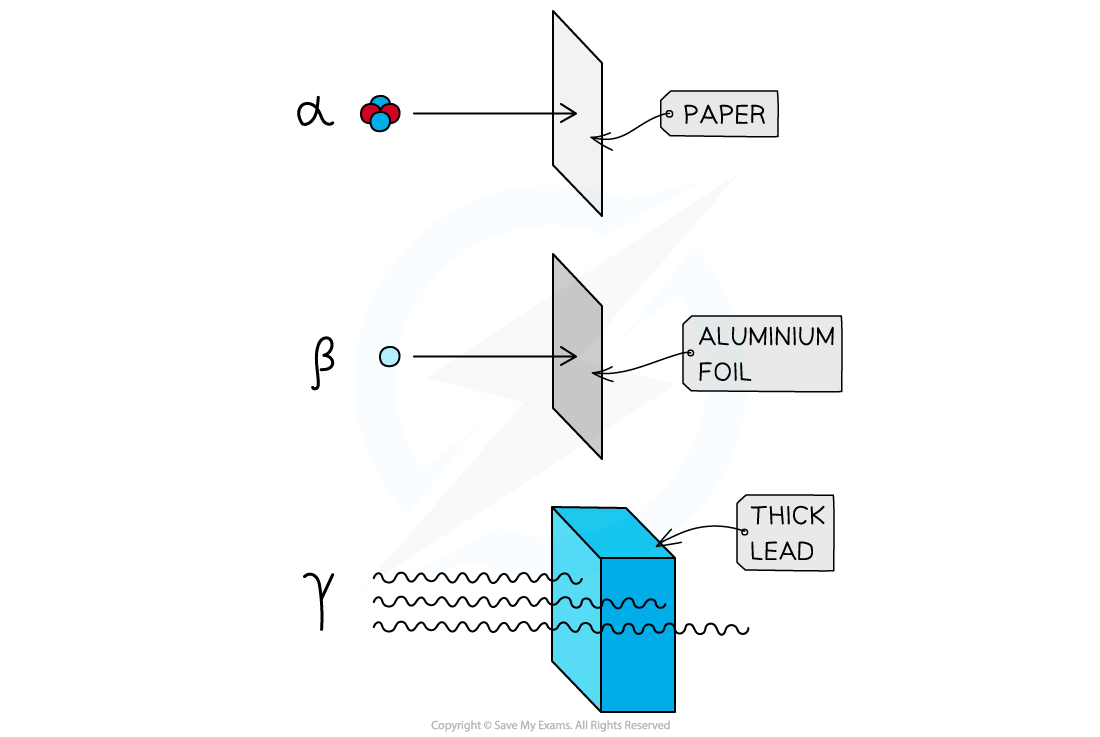
Alpha is a ___ penetrating type of radiation
Alpha particles have a range of ____
low
a few cm in air
What are beta particles?
Beta (β−) particles are high energy electrons emitted from the nucleus
When are beta particles emitted?
β− particles are emitted by nuclei that have too many neutrons
Beta is a _______ ionising type of radiation
moderately
This is due to it having a charge of +1e
This means it is able to do some slight damage to cells (less than alpha but more than gamma)
Beta is a _______ penetrating type of radiation
Beta particles have a range of around __ cm - _ m in air, depending on their energy
moderaletly
20 -3
Beta can be stopped by a ________
few millimetres of aluminium foil
Gamma (γ) rays are ______
high energy electromagnetic waves
gamma rays are emitted by ____ that need to lose some energy
nuclei

Gamma is a ____ penetrating type of radiation
Gamma particles have a range of around _ - __cm in lead or several metres in concrete
highly
1-10
If these particles hit other atoms, they can knock out electrons, ionising the atom
This can cause chemical changes in materials and can damage or kill living cells
aim of this experiment is to investigate the penetration powers of different types of radiation using either radioactive sources or simulations
Independent variable = Absorber material
Dependent variable = Count rate
Control variables:
Radioactive source
Distance of GM tube to source
Location / background radiation
Connect the Geiger-Müller tube to the counter and, without any sources present, measure background radiation over a one minute period
Repeat this three times, and take an average
Now place a radioactive source a fixed distance of 3 cm away from the tube and take another reading over a one minute interval
Now take a set of absorbers: some paper, several different thicknesses of aluminium (increasing in 0.5mm intervals) and different thickness of lead
One at a time, place these absorbers between the source and the tube and take another reading over a one minute interval
Repeat the above experiment for other radioactive sources
Results
Alpha radiation will be absorbed by the paper
Beta radiation will be absorbed by the aluminium foil
Some gamma radiation will be absorbed by the thick lead
Safety Considerations
When not using a source, keep it in a lead lined container
When in use, try and keep a good distance (a metre or so) between yourself and the source
When handling the source, do so using tweezers (or tongs) and point the source away from you
Alpha Decay
During alpha decay an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process

Beta Decay
During beta decay, a neutron changes into a proton and an electron
The electron is emitted and the proton remains in the nuclei
A completely new element is formed because the atomic number changes


The decay constant λ is defined as:
The probability, per second, that a given nucleus will decay
When a sample is highly radioactive, this means the number of decays per unit time is very high
This suggests it has a high level of activity
Activity, or the number of decays per unit time can be calculated using:
A = activity of the sample (Bq)
ΔN = number of decayed nuclei
Δt = time interval (s)
λ = decay constant (s-1)
N = number of nuclei remaining in a sample
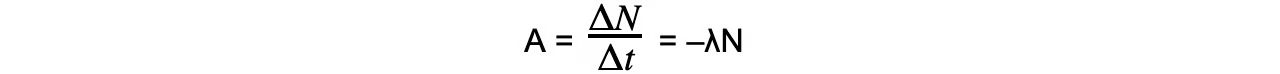
The activity of a sample is measured in ________
Becquerels (Bq)
An activity of 1 Bq is equal to one decay per second, or 1 s-1
This equation shows:
The greater the decay constant, the greater the activity of the sample
The activity depends on the number of undecayed nuclei remaining in the sample
The minus sign indicates that the number of nuclei remaining decreases with time - however, for calculations it can be omitted
Half life is defined as:
The time taken for the initial number of nuclei to reduce by half
this means when a time equal to the half-life has passed, the activity of the sample will ___ + why?
half
This is because activity is proportional to the number of undecayed nuclei, A ∝ N
To find an expression for half-life, start with the equation for exponential decay:
N = N0e–λt
N = number of nuclei remaining in a sample
N0 = the initial number of undecayed nuclei (when t = 0)
λ = decay constant (s-1)
t = time interval (s)
When time t is equal to the half-life t½, the activity N of the sample will be half of its original value, so N = ½ N0

what does this show:
This equation shows that half-life t½ and the radioactive decay rate constant λ are inversely proportional
Therefore, the shorter the half-life, the larger the decay constant and the faster the decay
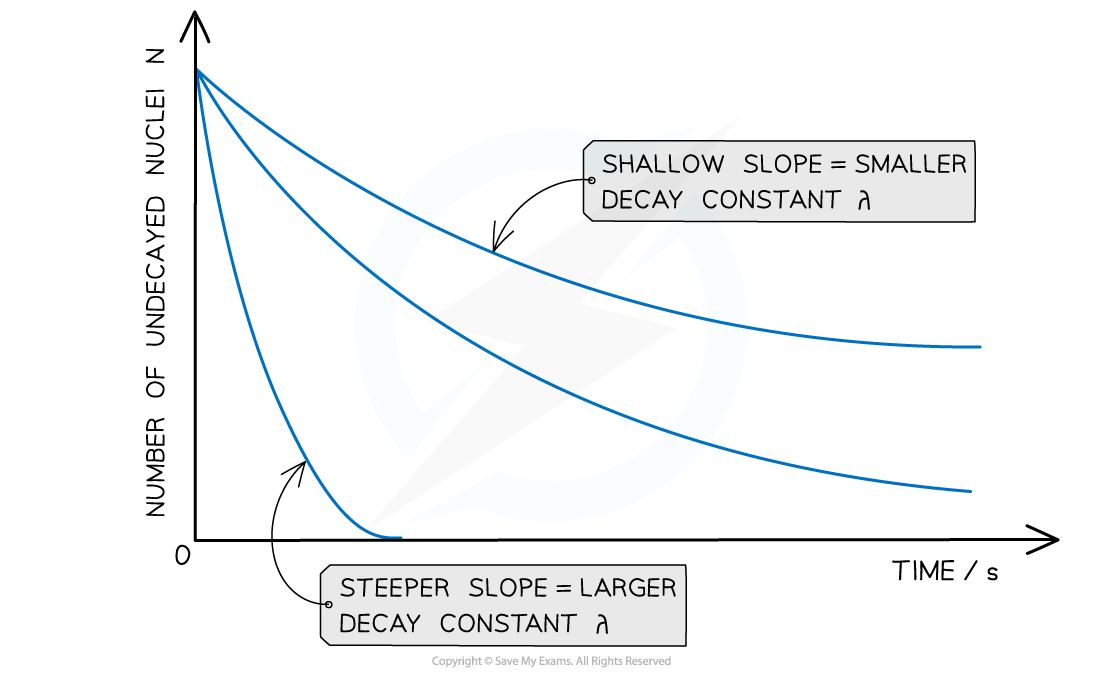
graph of number of undecayed nuclei against time
The steeper the slope, the larger the decay constant λ (and vice versa)
The decay curves always start on the y-axis at the initial number of undecayed nuclei (N0)
The received count rate C is related to the activity of the sample, hence it can also be represented in exponential form by the equation:
C = C0 e–λt
C = count rate at a certain time t (counts per minute or cpm)
C0 = initial count rate (counts per minute or cpm)
spreadsheet to model the exponential decay of nuclei:
Start with a given number of undecayed nuclei, N0 in the sample
N0 = 1000 is a logical number to start with
2. Choose a very small interval of time, Δt
This should be significantly shorter than the half-life of the isotope chosen
3. Calculate the number of nuclei decaying, ΔN during the time period
∆N∆t = -λN
So, ΔN = (λΔt) x N
4. Calculate the number of undecayed nuclei, N now left at the end of the time period, Δt
N0 - ΔN = N
5. Repeat this process by iterating your value for N as your new N0 for many values of Δt
The isotope ______ is commonly used in radioactive dating
carbon-14
how is it formed:
It forms as a result of cosmic rays knocking out neutrons from nuclei, which then collide with nitrogen nuclei in the air:
1n + 14N → 14C + 1p
How does carbon-14 get it stuff?
Plants take in carbon dioxide from the atmosphere for photosynthesis, including the radioactive isotope carbon-14
Animals and humans take in carbon-14 by eating the plants
Therefore, all living organisms absorb carbon-14, but after they die they do not absorb any more
is amount constant?
The proportion of carbon-14 is constant in living organisms as carbon is constantly being replaced during the period they are alive
When they die, the activity of carbon-14 in the organic matter starts to fall, with a half-life of around __ years
5730
how does it work ?
Samples of living material can be tested by comparing the current amount of carbon-14 in them and compared to the initial amount (which is based on the current ratio of carbon-14 to carbon-12), and hence they can be dated
problems:
Carbon dating is a highly reliable ageing method for samples ranging from around 1000 years old up to a limit of around 40 000 years old
Therefore, for very young, or very old samples, carbon dating is not the most reliable method to use
This can be explained by looking at the decay curve of carbon-14:
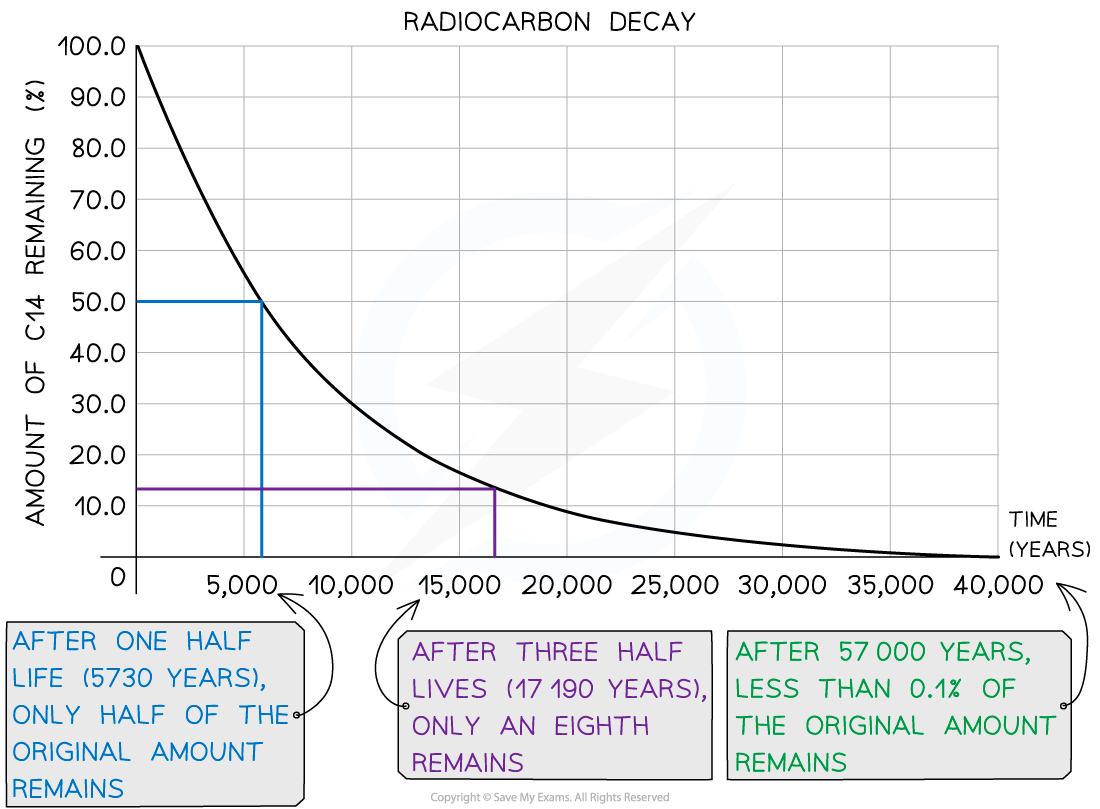
If the sample is less than 1000 years old:
The activity of the sample will be too high
So, it is difficult to accurately measure the small change in activity
Therefore, the ratio of carbon-14 to carbon-12 will be too high to determine an accurate age
If the sample is more than 40 000 years old:
The activity will be too small and have a count rate similar to that of background radiation
So, there will be very few carbon-14 atoms remaining, hence very few decays will occur
Therefore, the ratio of carbon-14 to carbon-12 will be too small to determine an accurate age
other prob:
Carbon dating uses the currently known ratio of carbon-14 to carbon-12, however, scientists cannot know the level of carbon-14 in the biosphere thousands of years ago
Therefore, this makes it difficult to age samples which are very old