Asthma - mechanisms of hyperactivity
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Describe the incidence of asthma
- 1:12 adults have asthma and 1:11 children have asthma
- 4 people die daily from asthma, 2 of which are preventable (could be due to lack of diagnosis? Medication may not be taken effectively?)
- Large portion of NHS budget
- 750,00 hospital admissions annually
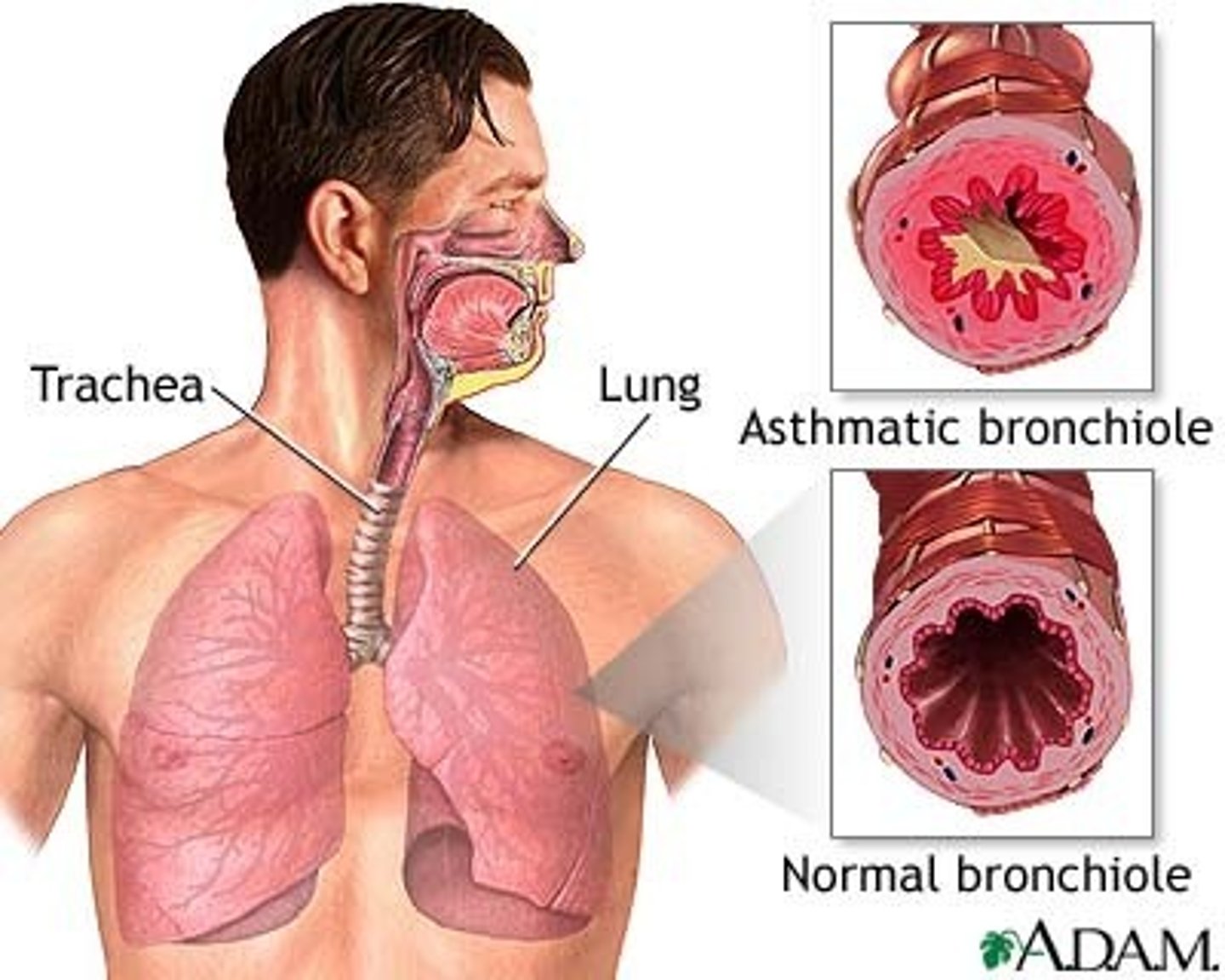
What is asthma?
A chronic inflammatory disorder of the airways
What is the main difference between asthma and COPD?
The main difference between asthma and COPD is that asthma has variable air flow obstruction.
Describe the main pathological features of asthma
- it has variable airflow obstruction; the obstruction is often reversible spontaneously or with treatment.
- Airways have increased response to several stimuli (hyperreactivity/ hyperresponsiveness)
How can we test lung function?
1. PEFR - peak flow meter (measures peak expiratory flow rate). Blow hard and fast to measure.
2. FEV1 - max volume of air to blow out in 1 sec (forced expiratory volume in 1 second).
If either measurement is reduced, it indicates poor lung function
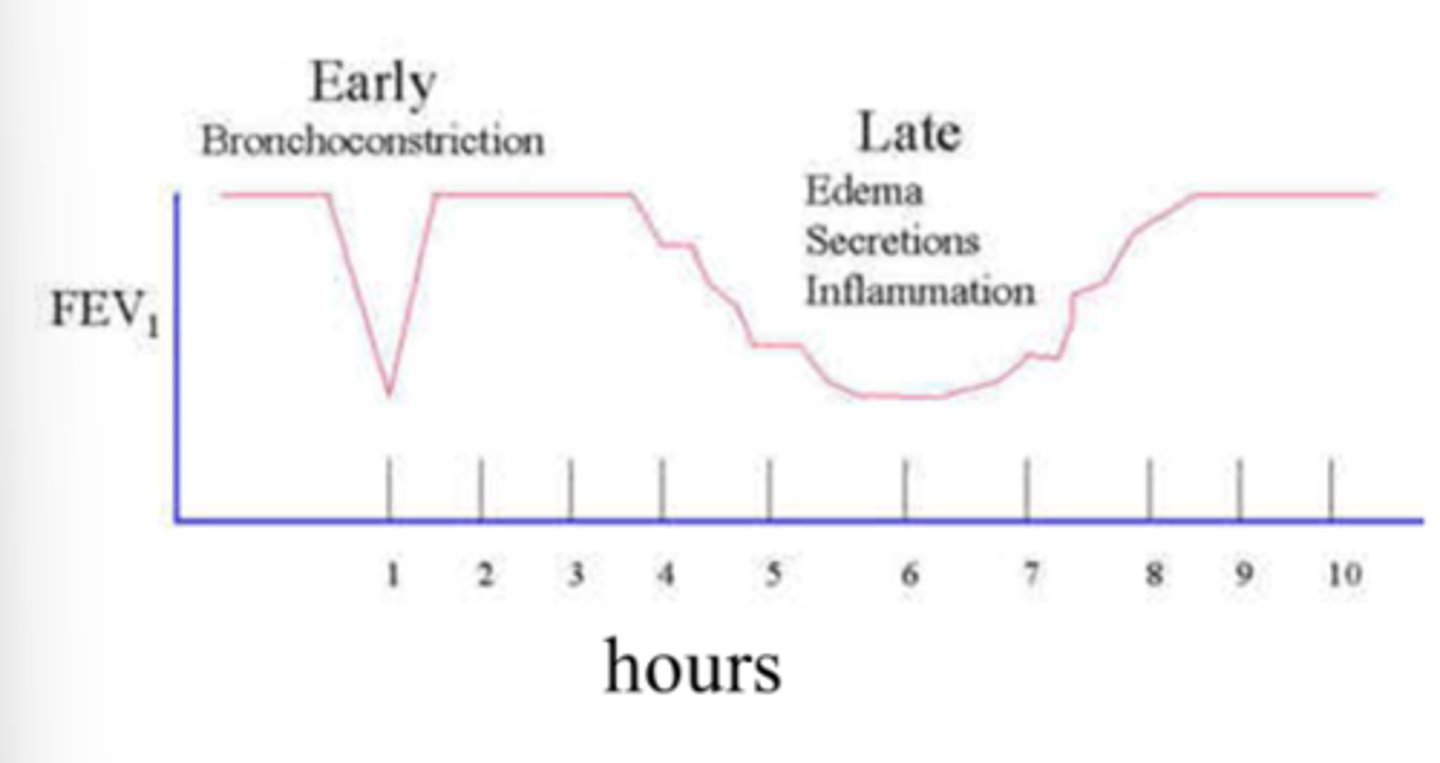
Describe variable airflow obstruction in asthma
In asthmatic patients there are two phases of airflow reduction:
1. Early reduction (asthma attack) - likely due to bronchoconstriction. This is a dramatic decrease in airflow- noticeable to individual. This is variable so it can return to normal airflow.
2. Late reduction - individuals may not recognise the change in lung capacity (less noticeable). This tends to occur over a longer period of time. This tends to be because of inflammation.
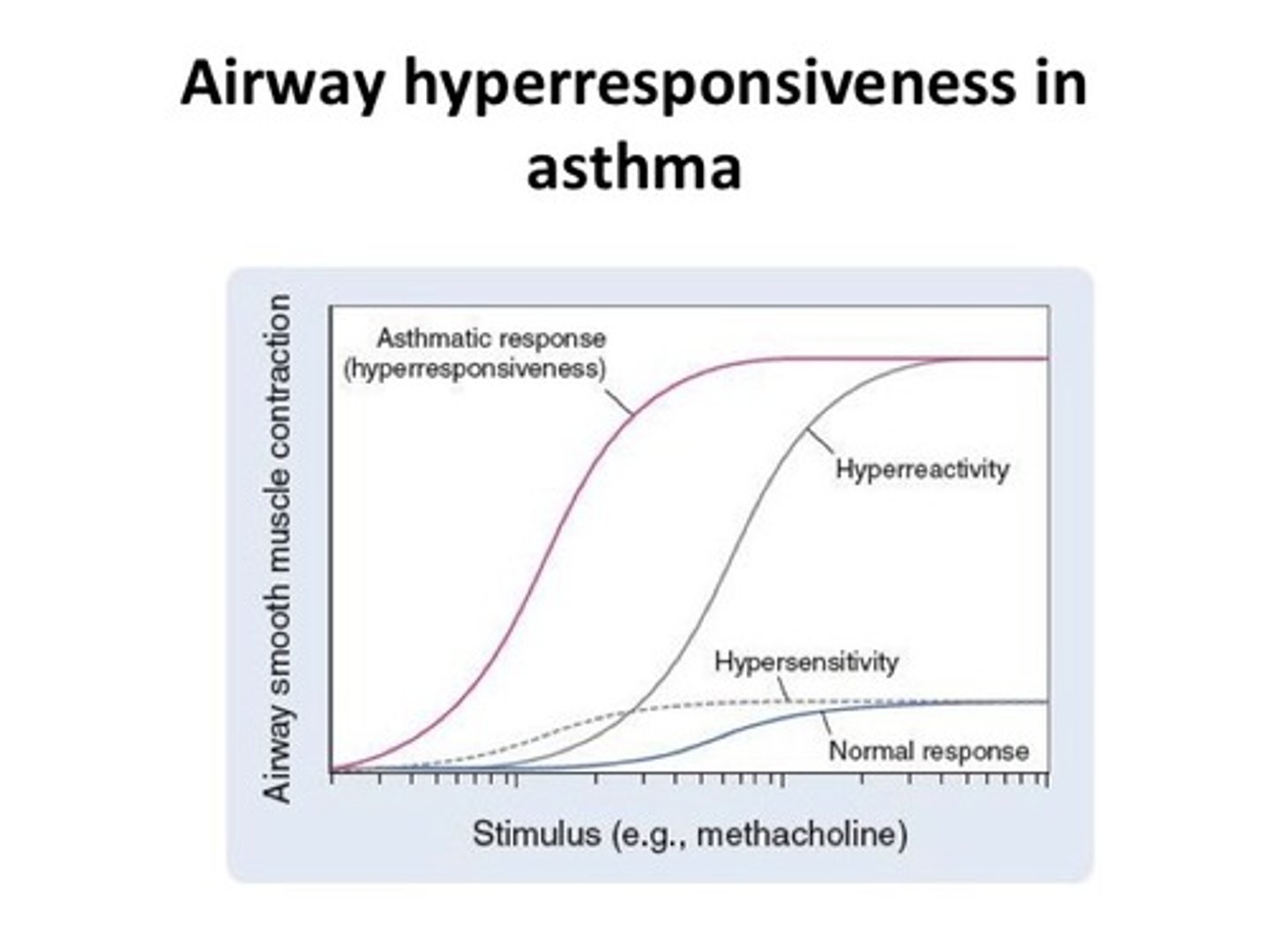
Describe airway hyperresponsiveness in asthmatic patients
In someone who has asthma, if we give a bronchoconstrictor (e.g. histamine) then they will have more bronchoconstriction compared to a healthy individual.
Less of the agent (bronchoconstrictor) is required for bronchoconstriction to be caused in asthmatic patients.
What are the possinle mechanisms of hyperresponsiveness?
1. Increased contractility of smooth muscles
2. Increased excitatory nerve activity
3. Decreased bronchodilator activity
4. Inflammation
Describe how "increased contractility of smooth muscles" is a potential mechanism of hyperreactivity
In asthmatic patients, the smooth muscle layer in the bronchioles (and other airways) is much larger than in patients without asthma, so the lumen (where the air flows) is smaller causing airflow obstruction.
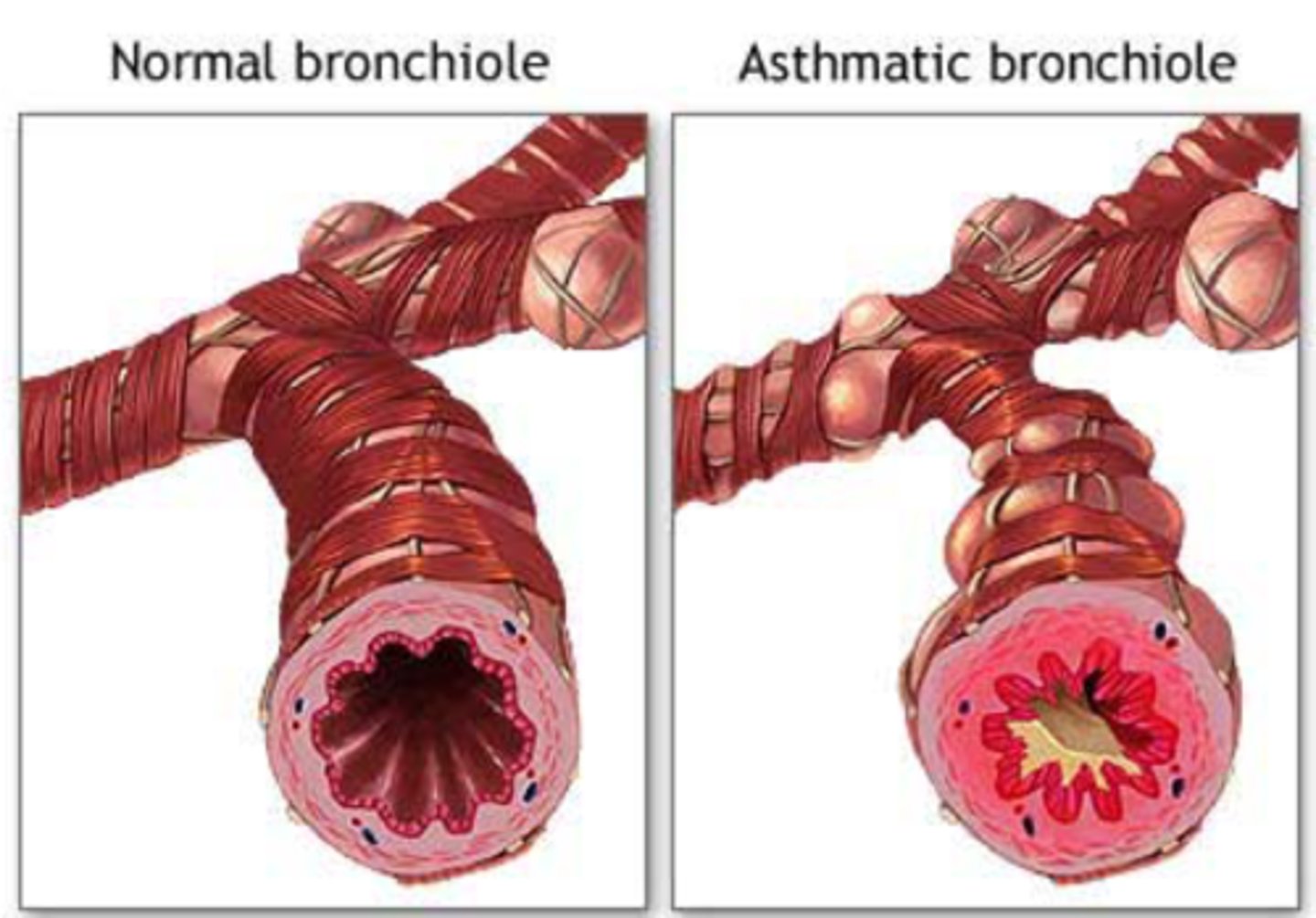
Why is the smooth muscle layer in airways larger in asthmatic individuals?
The smooth muscle layer is larger because:
- More muscle cells dividing - hyperplasia
- Cells undergo hypertrophy. Each cell increases in size.
The muscle layer gets larger through both mechanisms due to inflammation (release of cytokines).
Describe how "increased excitatory nerve activity" is a potential mechanisms of hyperreactivity
The airways are innervated by the Vagus which contains:
1. Cholinergic nerves: acetylcholine (Ach) acts on M3 muscarinic receptors which causes bronchoconstriction
2. Excitatory non-adrenergic non-cholinergic transmitters (eNANC): these act on their own neurokinin receptors and also cause bronchoconstriction.
Give examples of excitatory non-adrenergic non-cholinergic transmitters (eNANC)
Neurokinin A, neurokinin B, substance P
Describe the mechanisms of smooth muscle contraction in the airway via activation of M3 receptors
Parasympathetic nerves release acetylcholine to smooth muscles.
Ach binds to M3 receptors on airway smooth muscle.
This activates the Gq coupled receptor which activates phospholipase C (PLC).
PLC cleaves PIP2 to IP3 and DAG.
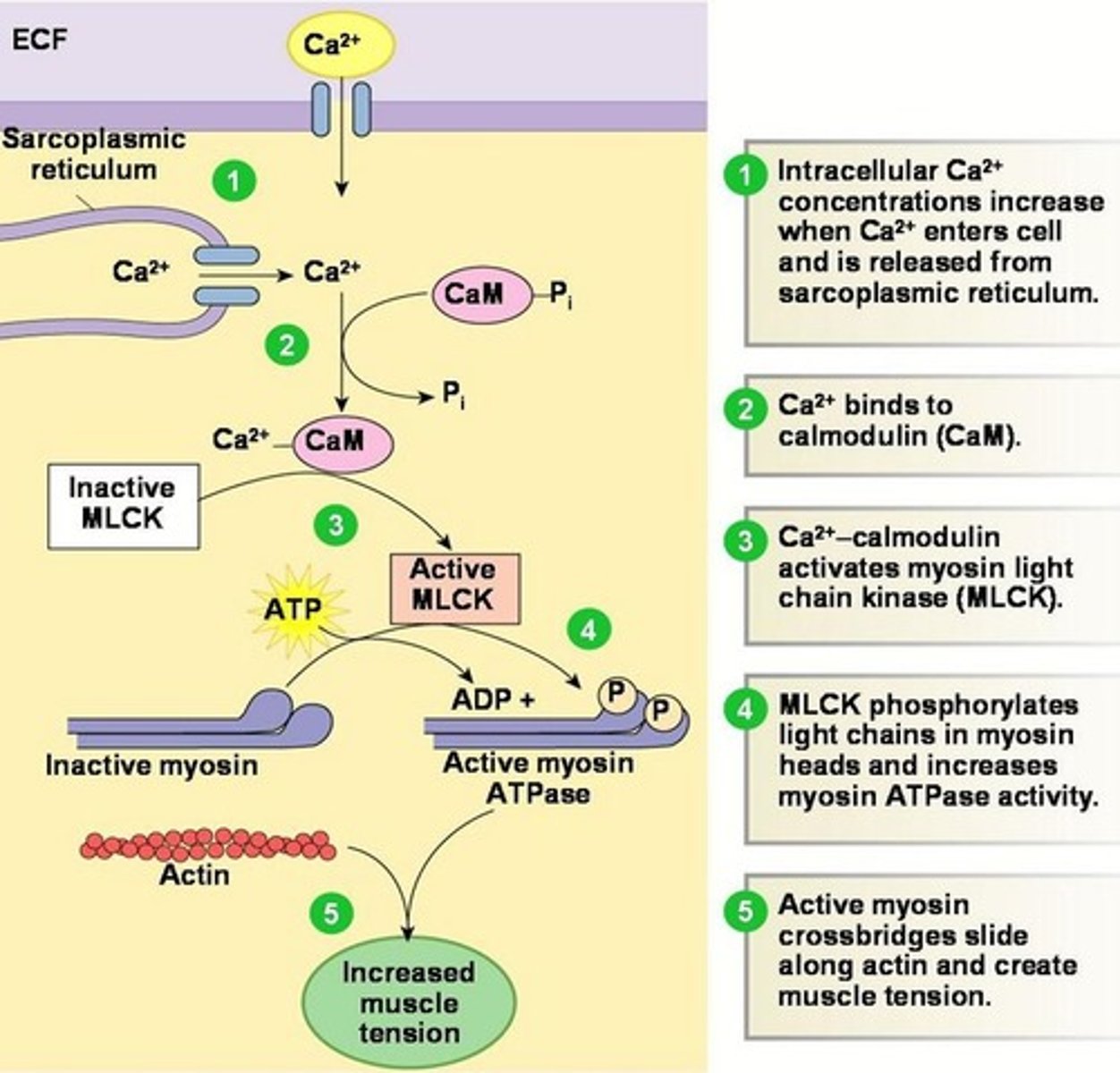
IP3 binds to IP3 receptors on the SR which releases sequestered Ca2+.
CaM binds to Ca2+ which activated Ca2+-calmodulin dependent kinases such as MLCK.
This leads to airway smooth muscle contraction.
DAG also has action in constriction of airway smooth muscle by activating protein kinase C.
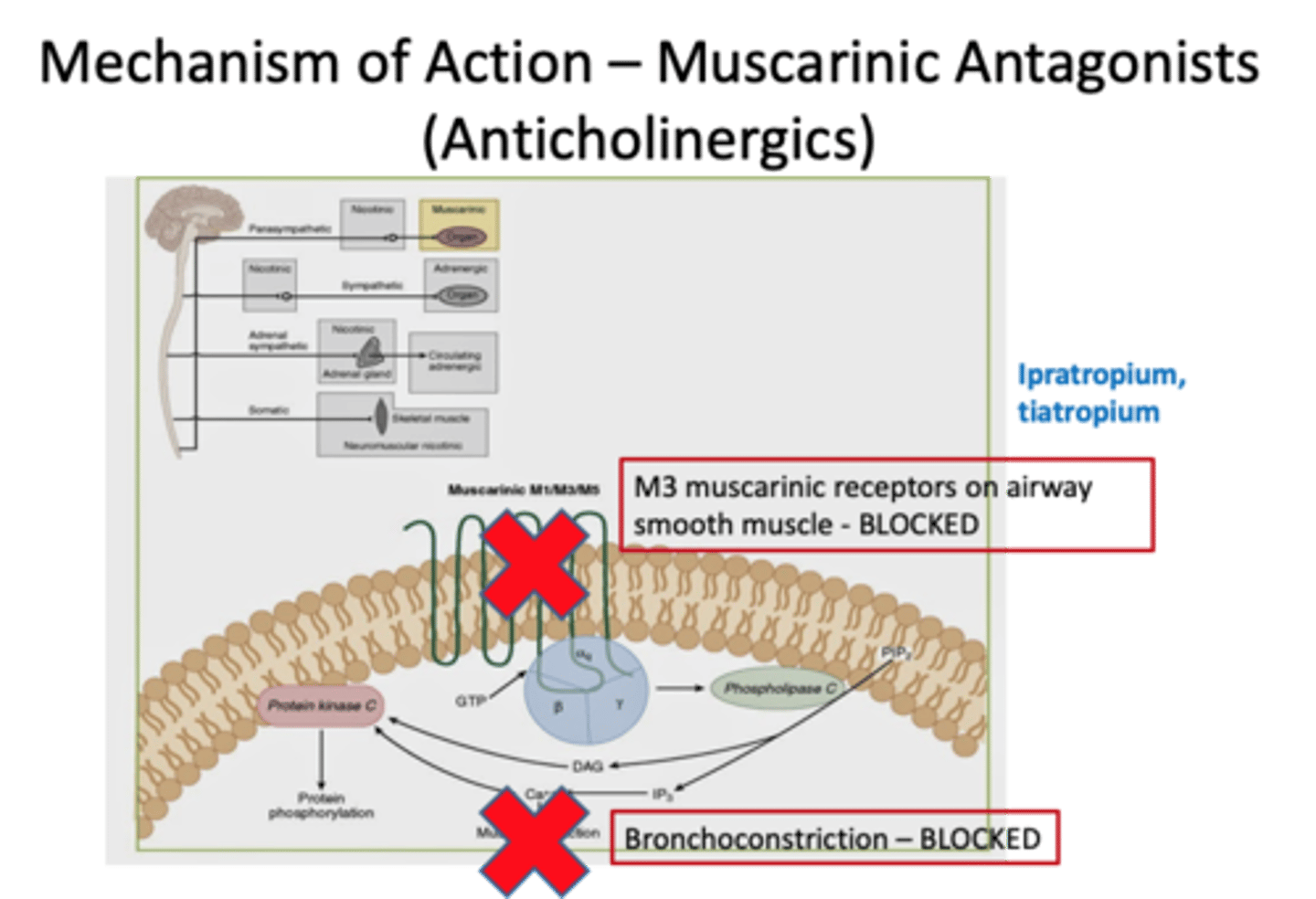
Describe the mechanism of action of muscarinic antagonists
They block M3 receptors to prevent bronchoconstriction.
Give examples of muscarinic antagonists
Aclidinium
Ipratropium
Tiotropium
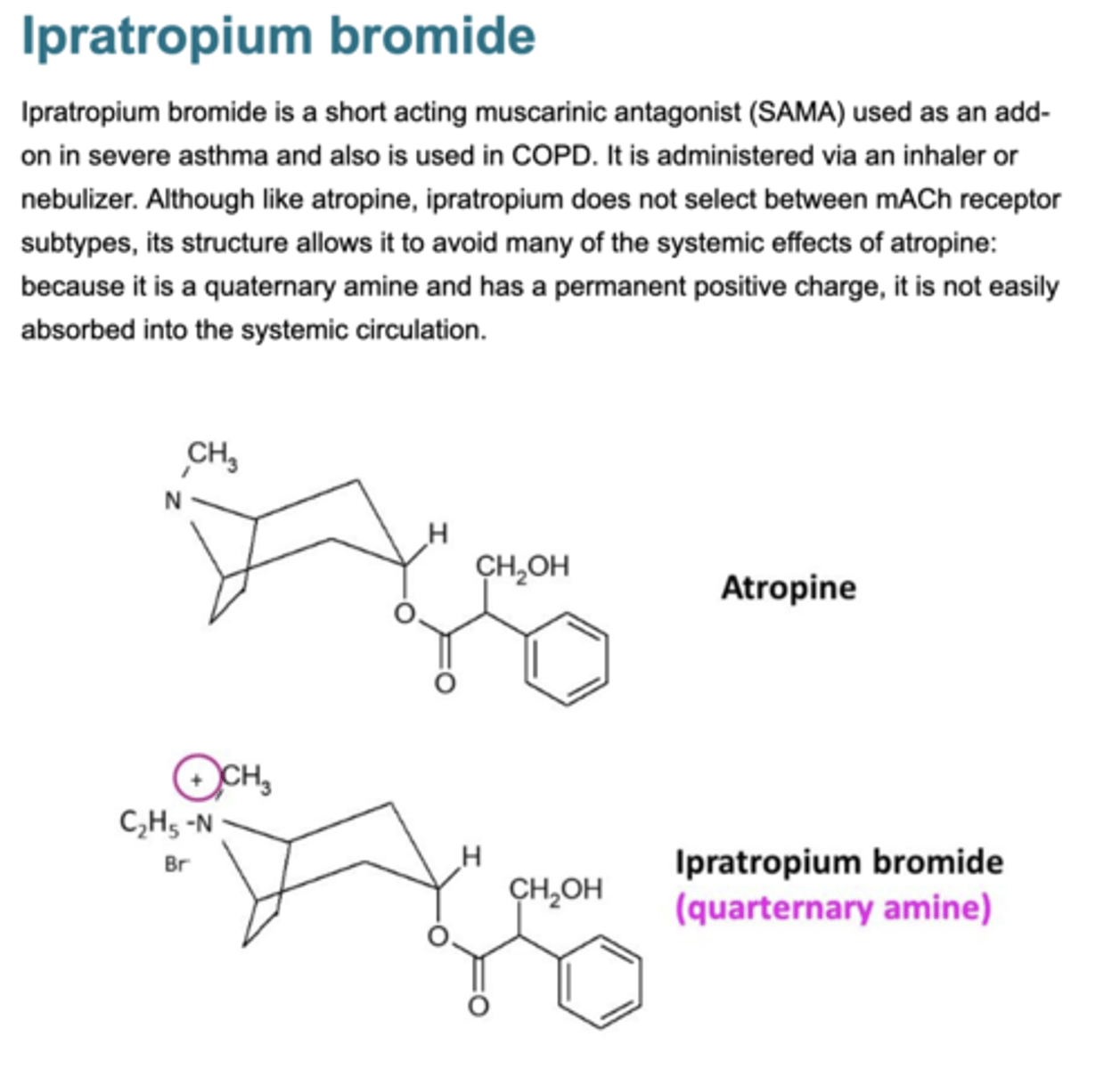
Describe ipratropium
Muscarinic antagonist
Quaternary ammonium compound
Poorly absorbed
No CNS effects
Taken in inhaler form so delivered locally to lung so limits side effects which can occur.
What can ipratropium be used to treat?
- Irritant induced bronchospasm
- Asthma
- Bronchitis
- COPD
What are the drawbacks of ipratropium as a bronchodilator?
It can block M2 receptors as well as M3 receptors so less of negative feedback control of Ach.
This can lead to an increase in Ach.
This can increase airway contraction.
Therefore, not main drug used in asthma - tiotropium is preferred.
ALSO
Ipratropium is a competitive antagonist meaning it can be overcome by increasing [ACh].
![<p>It can block M2 receptors as well as M3 receptors so less of negative feedback control of Ach.</p><p>This can lead to an increase in Ach.</p><p>This can increase airway contraction.</p><p>Therefore, not main drug used in asthma - tiotropium is preferred.</p><p>ALSO</p><p>Ipratropium is a competitive antagonist meaning it can be overcome by increasing [ACh].</p>](https://knowt-user-attachments.s3.amazonaws.com/ea8bc721-35ae-42d9-b6e8-63850755521d.jpg)
Why is tiotropium preferred over ipratropium
Tiotropium has a preference for M3 receptor compared to M2.
It binds to M3 and M2, but has a short action on M2 (fast dissociation) so doesn't block for long.
Therefore, it is better to use tiotropium compared to ipratropium.
Describe the mechanisms if bronchodilation
1. Circulating adrenaline binds to beta-2 adrenergic receptors on smooth muscles
2. Inhibitory non-adrenergic non-cholinergic transmitters (iNANC)
- dilator neuropeptides e.g. CGRP and VIP: activate adenylyl cyclase (AC)
- neuronally-derived NO: acts on soluble guanylate cyclase (GC)
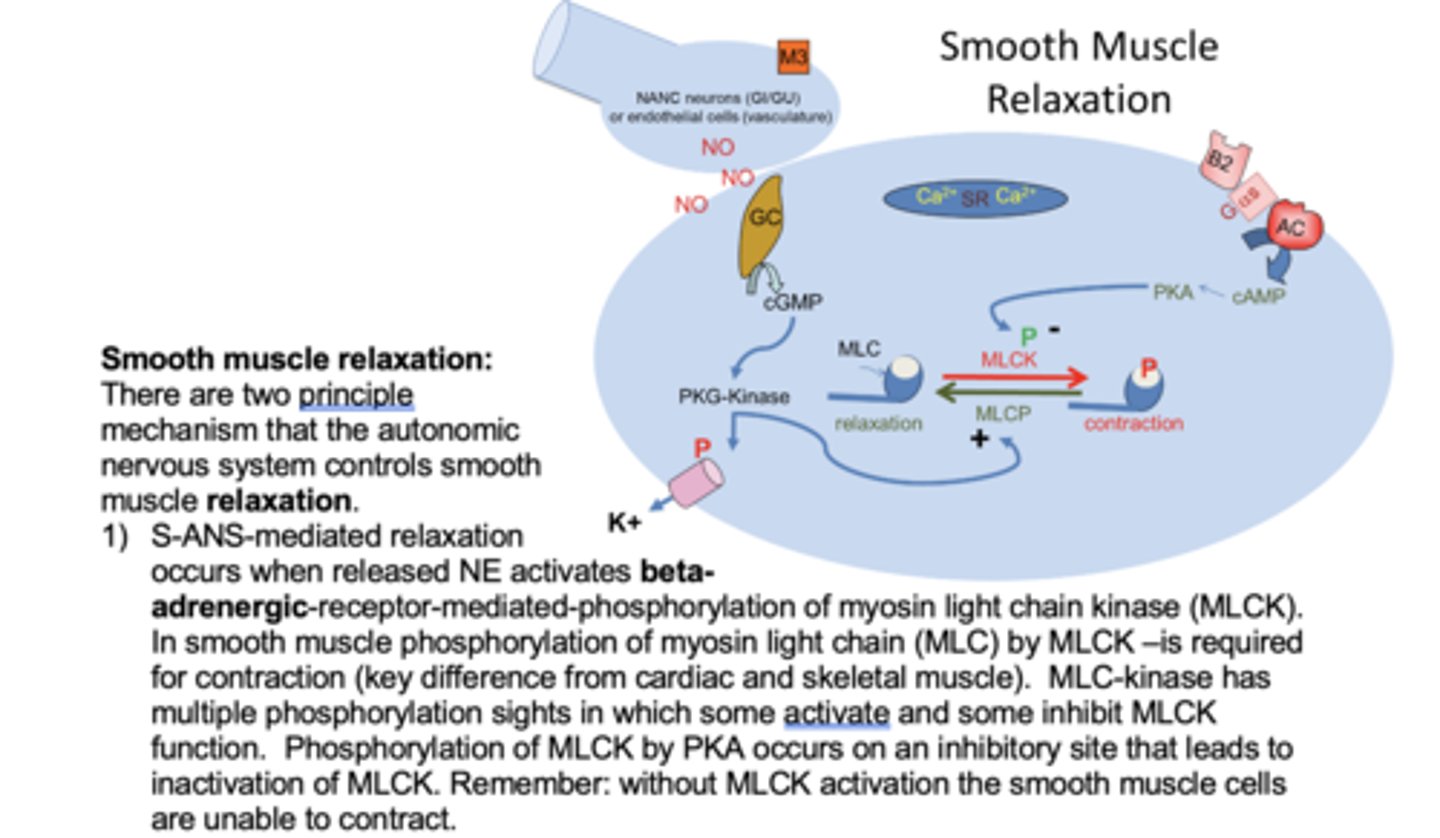
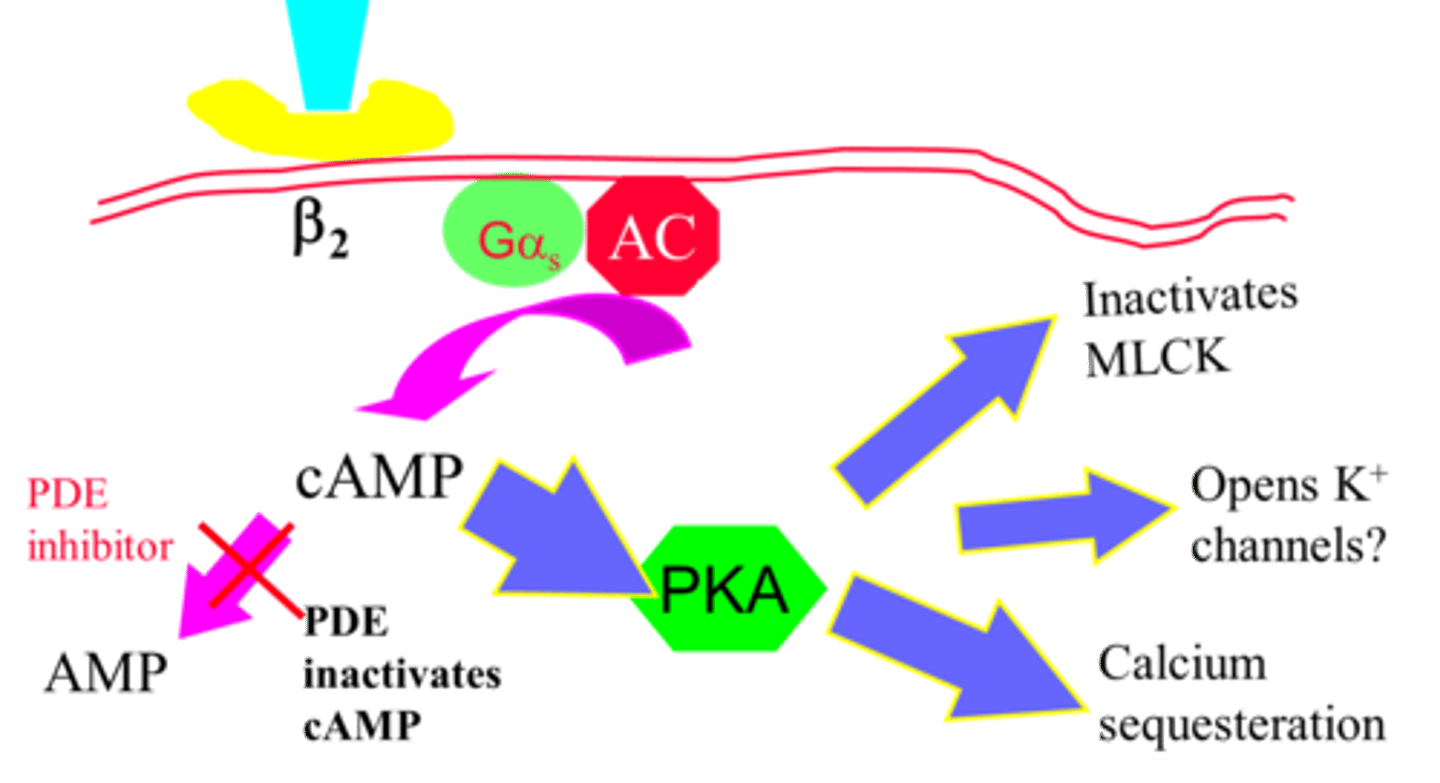
Describe the mechanisms of smooth muscle dilation in the airway via activation of beta-adrenergic receptors
Beta-2 receptor activated
The Gs coupled receptor activated.
AC activated which converts ATP to cAMP. cAMP activates pKa. pKa inactivates MLCK and does calcium sequesteration - keeps it inside SR.
pKa also activates MLC phosphatase which removes phosphate groups from MLC (relaxation).
Describe bronchodilator activity of beta-adrenoreceptor agonists
beta-adrenoreceptor agonists bind to b2 receptors (Gs coupled receptor).
This causes smooth muscle relaxation.
Give example of short-acting beta-adrenoreceptor agonists
Salbutamol (selective beta-2 agonist)
Isoprenaline (non-selective beta-2 agonist) -> NOT USED NOW.
Give example of long-acting beta-adrenoreceptor agonists
Formoterol
Salmeterol
Vilanterol
What beta-2 agonist is used in emergencies?
Adrenaline
What determines whether a beta-adrenoreceptor agonist is short or long-acting
Lipophilicity controls whether the beta-adrenoreceptor agonist is short or long-acting.
Lipophilicity: salmeterol>formoterol>salbutamol
Salbutamol is most hydrophilic so it is only available on the surface. Ithas a short-duration of action and fast onset.
Formoterol forms depot in lipid membrane and 'leaks' out to interact with receptor. Therefore, it is more hydrophobic than salbutamol and has a longer duration of action and fast onset.
Salmeterol is highly lipophilic so it crosses the phospholipid membrane -> induces conformational change. It has a slow onset and long duration
What are the 2 types of bronchodilators?
1. Beta-adrenoreceptor agonists (b2)
2. Phosphodiesterase inhibitors
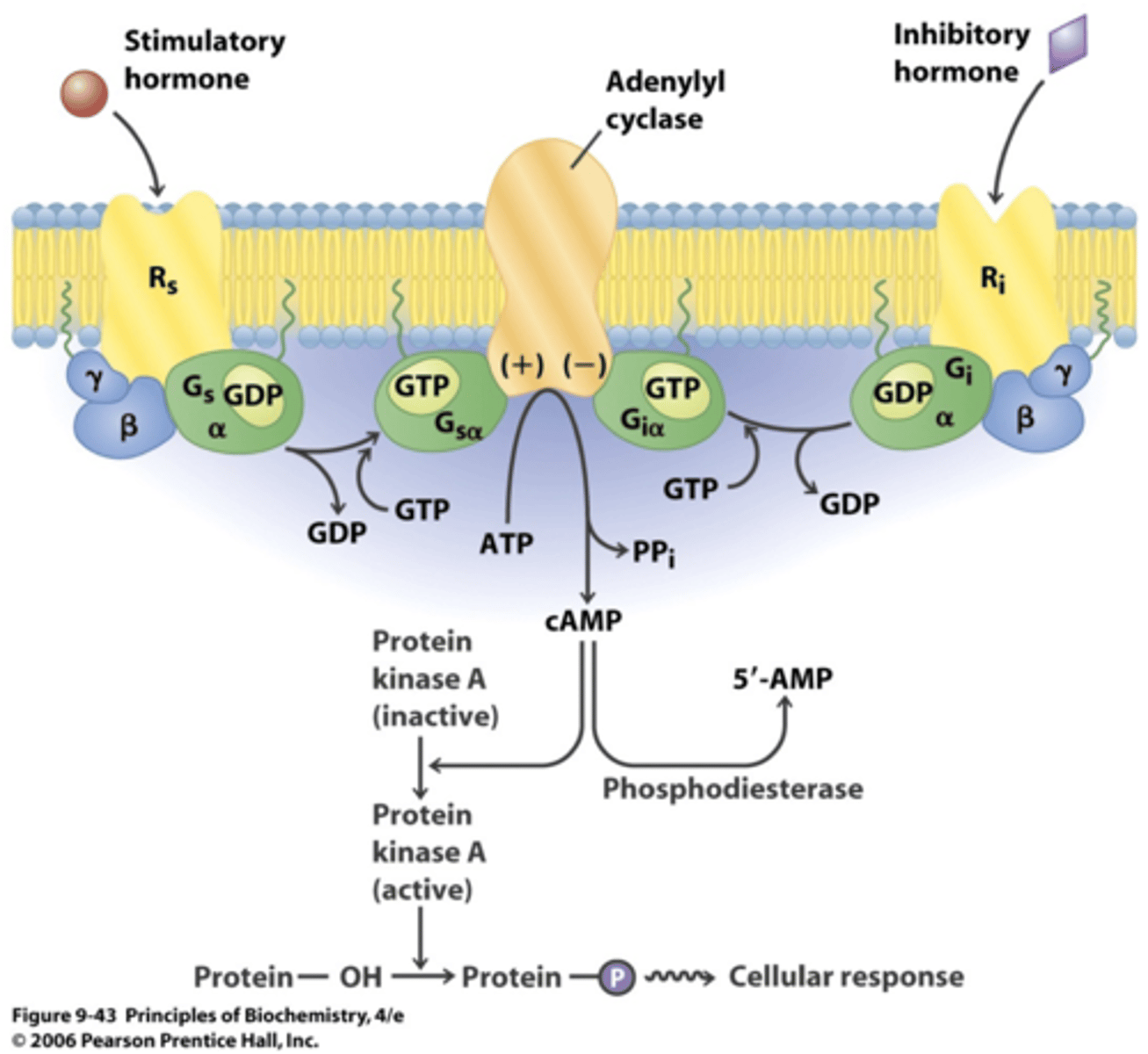
What are the role of phosphodiesterases?
Breaks down cAMP
PDE3 III & IV in airway smooth muscle break down cAMP
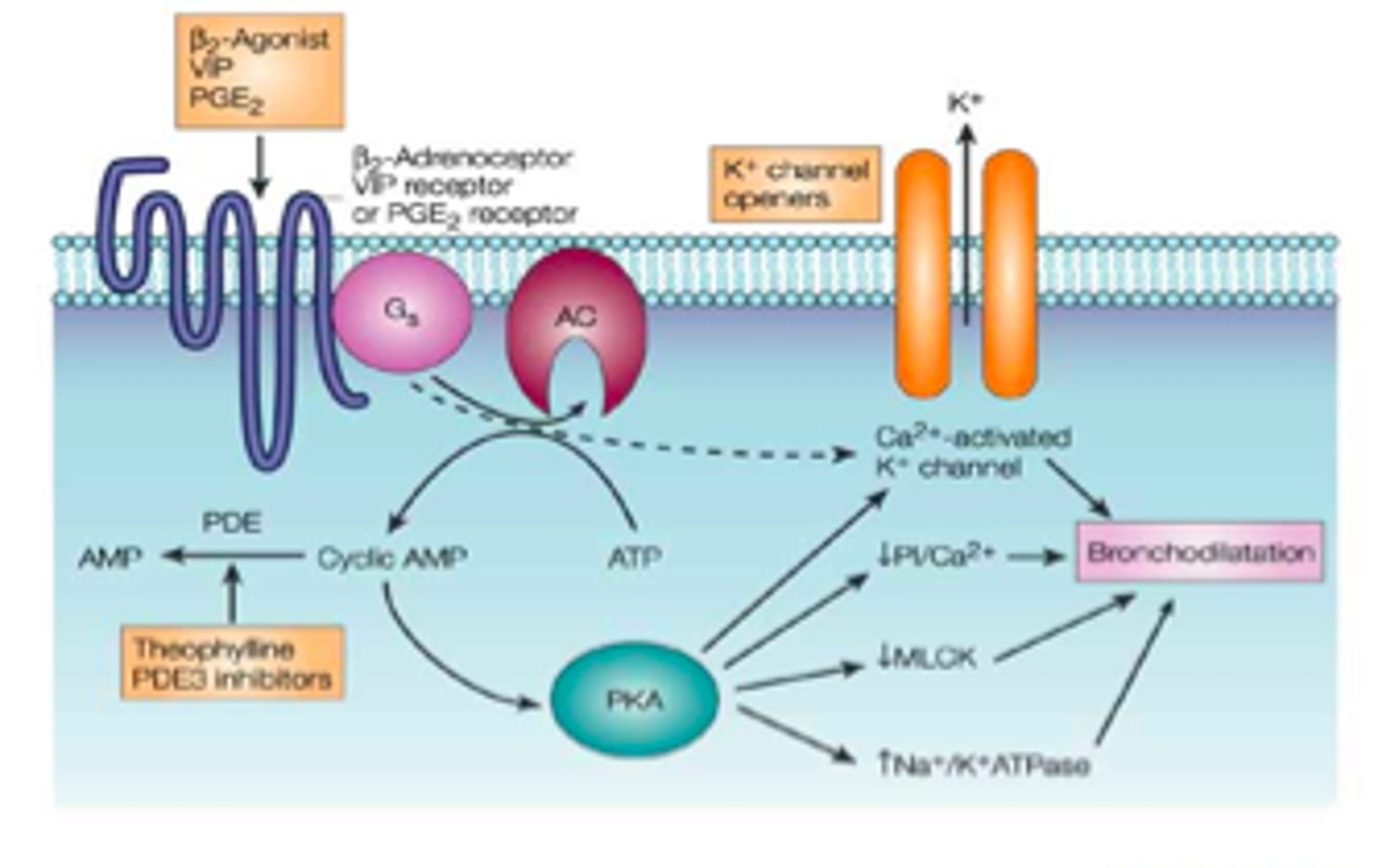
What is the role of phosphodiesterase inhibitors?
Phosphodiesterase inhibitors inhibit the activity of phosphodiesterase. Therefore, more cAMP is available to activate PKA and trigger bronchodilation via
1. Calcium sequestration into SR
2. Inactivate MLCK
3. Open K+ - hyperpolarisation of cells inhibitors Ca2+ voltage dependent channels.
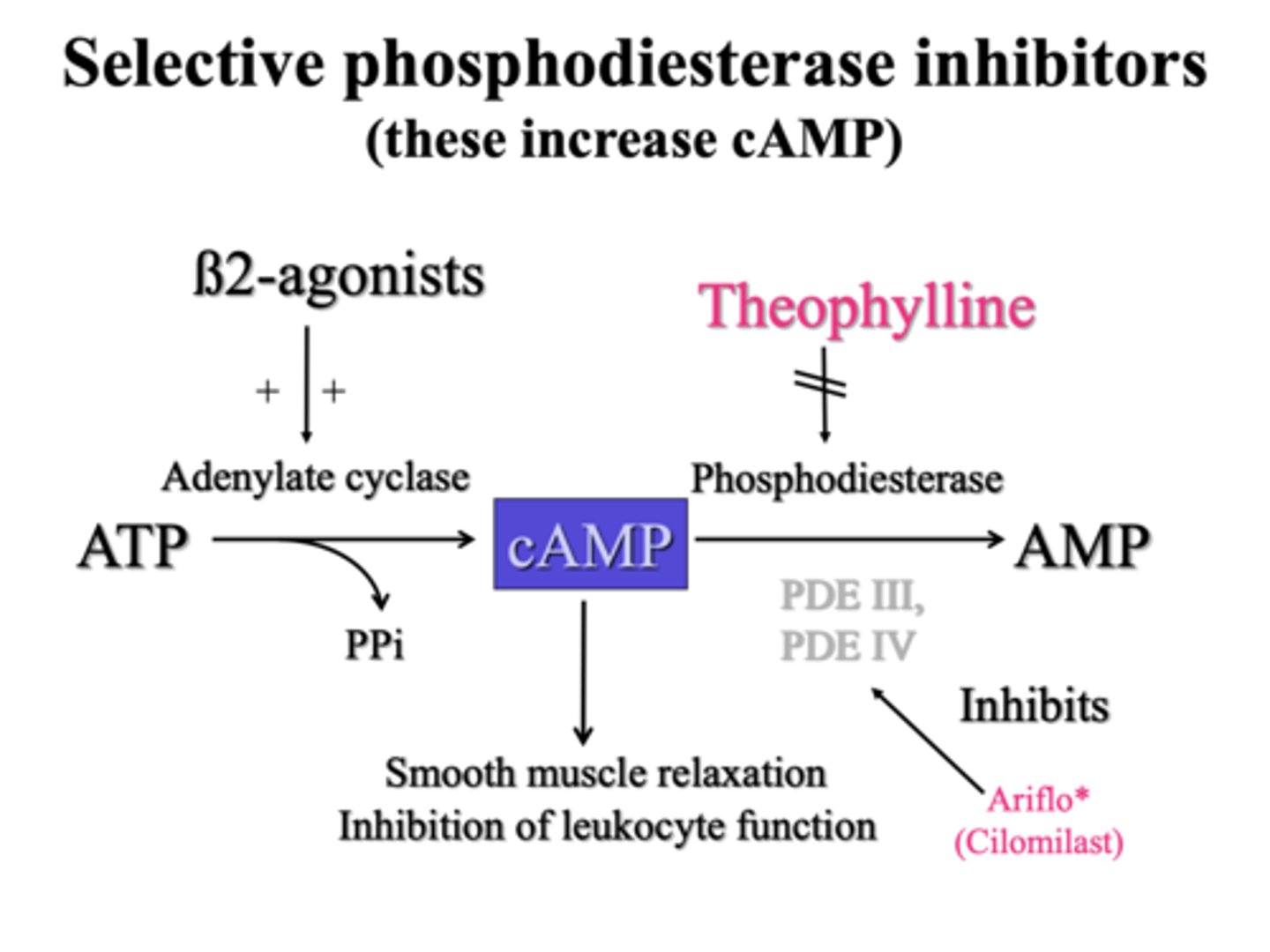
Describe theophylline as a bronchodilator
Theophylline inhibits PDE3 and PDE4 in airway smooth muscle - this increases cAMP by reducing its breakdown.
But it has a narrow therapeutic window (so side effects more likely).
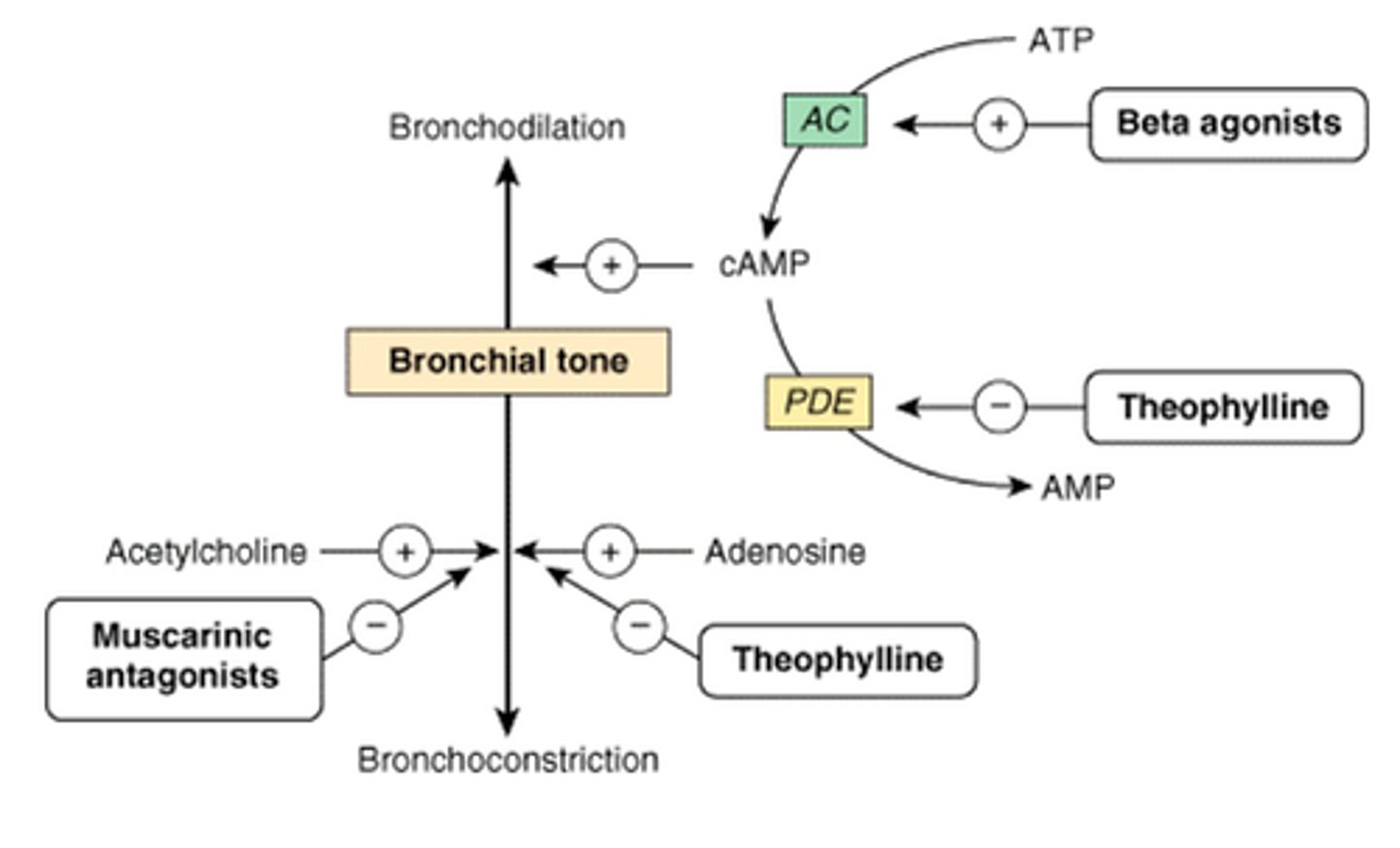
Describe aminophylline as a bronchodilator
Aminophylline is a mixture of theophylline and ethylenediamine.
It is less potent than theophylline and shorter acting.
Give examples of PDE inhibitors
Aminophylline, theophylline
Describe how inflammation acts a mechanism of hyperresponsiveness
Inflammation causes hyperresponsiveness by these 2 mechanisms:
1. Epithelial damage & exposure of sensory nerves
2. Oedema & secretions leading to decreased luminal diameter
Describe how "epithelial damage & exposure of sensory nerves" can lead to the hyperresponsiveness seen in asthma
Epithelial cells are damaged by inflammatory mediums. Epithelial damage exposes sensory nerves. This triggers vagal sensory afferent nerves to send impulses to the CNS. This causes the CNS to send signals via vagal efferent nerves to airway smooth muscles which results in bronchoconstriction.
There is also the antidromic response: (not main response)
- activation of vagal sensory afferent nerves can cause the release of neuropeptides
- this causes vasodilation and increased permeability in the airway
Describe how "oedema & secretions " can lead to the hyperresponsiveness seen in asthma
Oedemas can form and there can be a decrease in the diameter of the lumen.
An increase in secretions e.g. mucous can also block the airway.