chem 215 exam 1
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
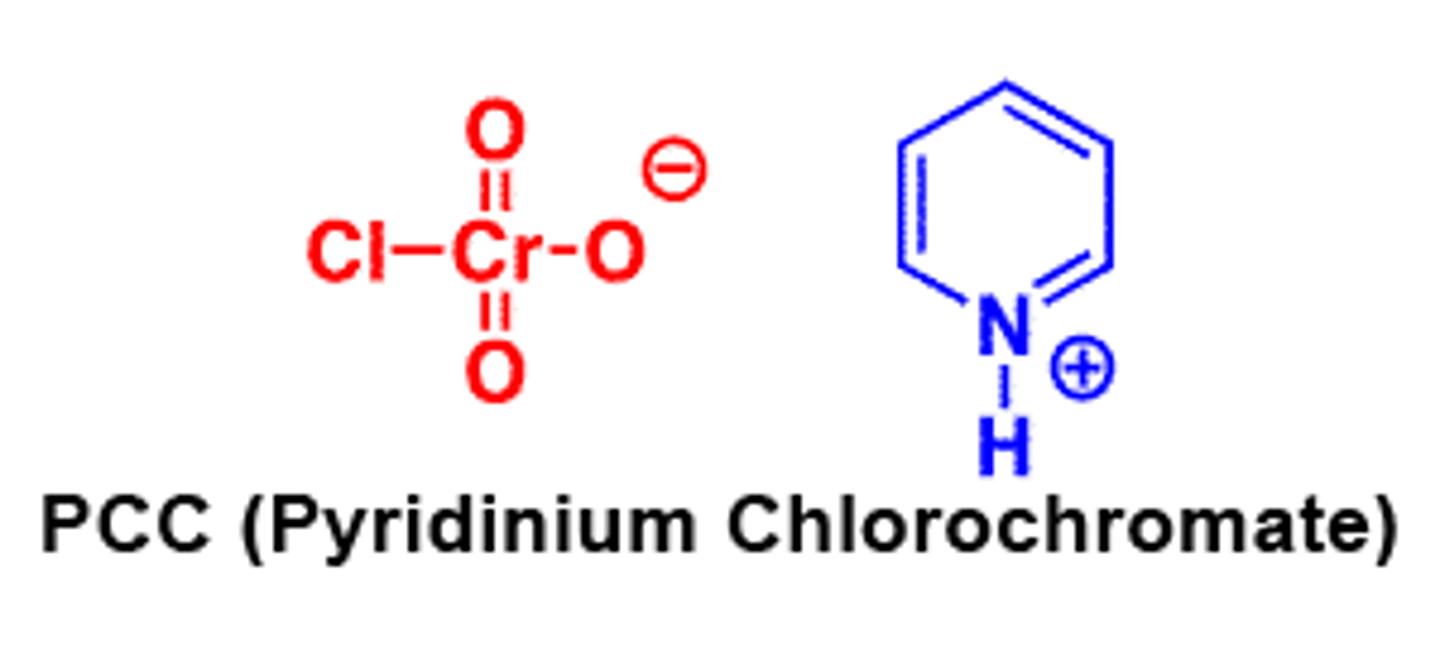
PCC reagents
CrO3/pyridine/HCl
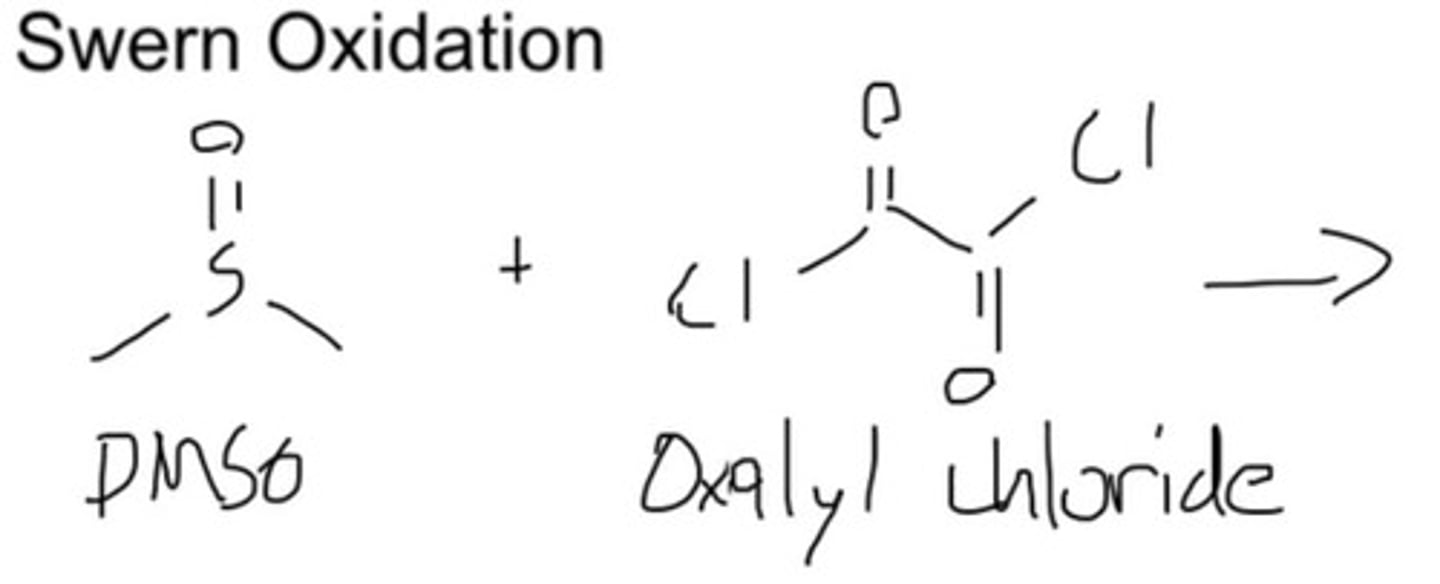
Swern reagents
1) DMSO/oxalyl chloride 2) triethalmine
reactive species in Swern
chloro dimethyl sulfonium (Ch3)2-S+-
Cl
Jones reagents
CrO3/H2SO4/H2O
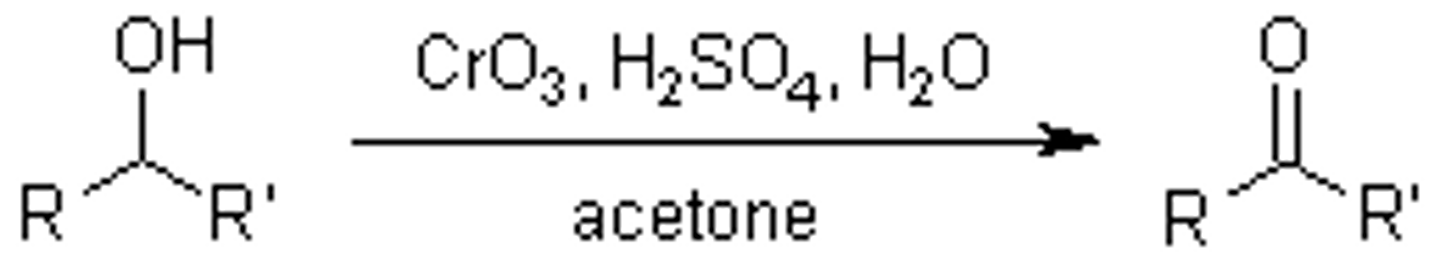
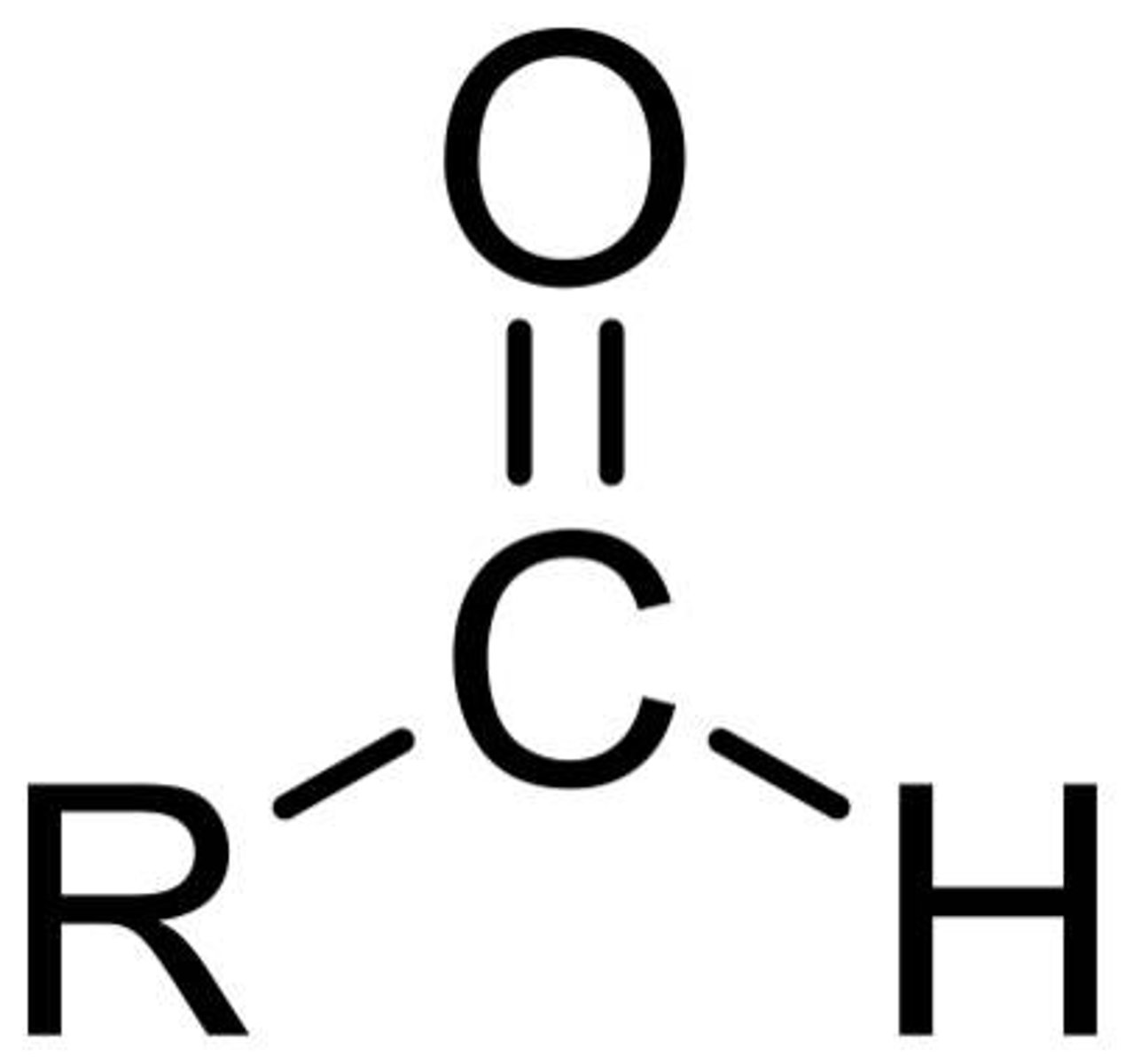
aldehyde
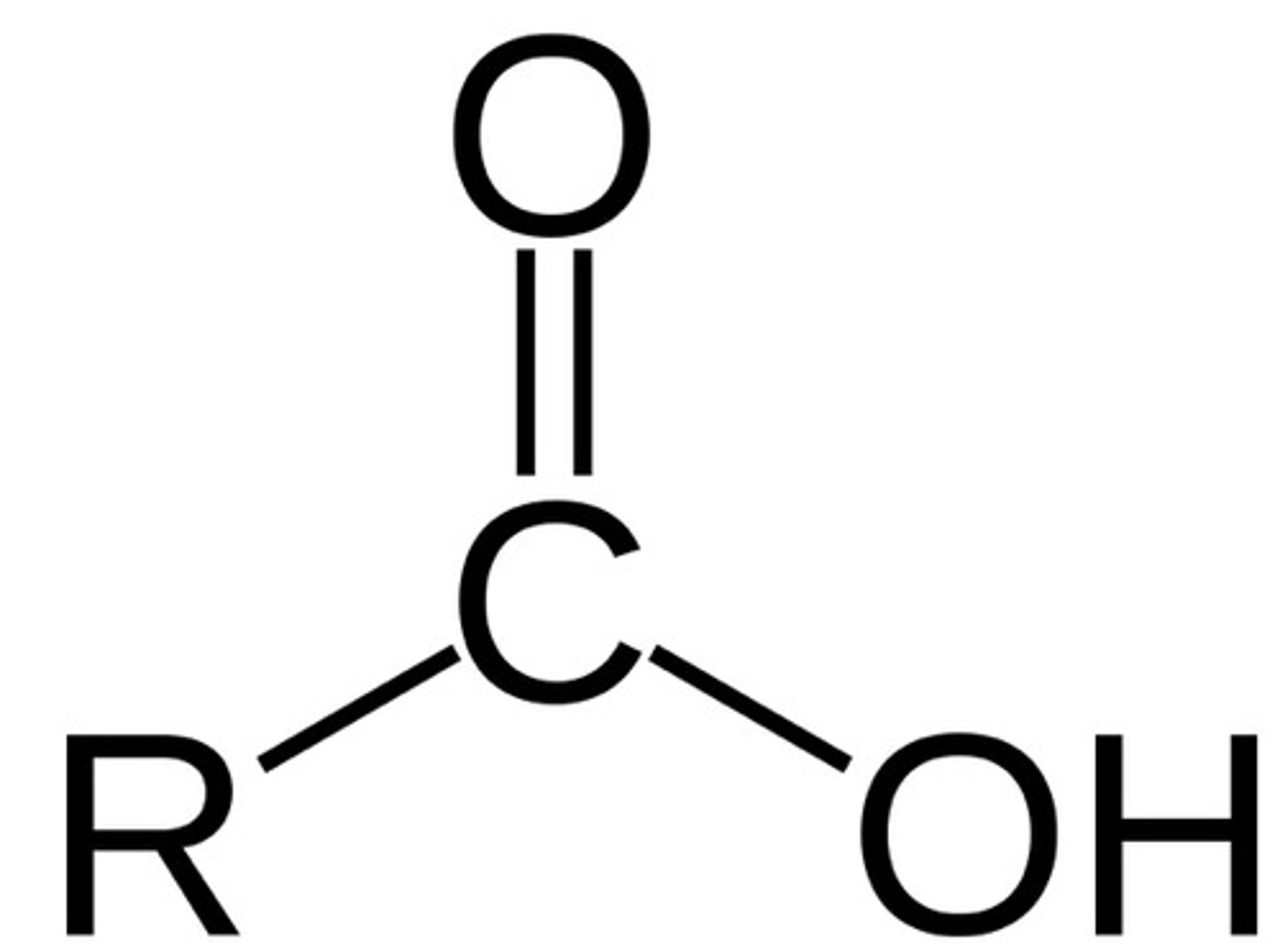
carboxylic acid
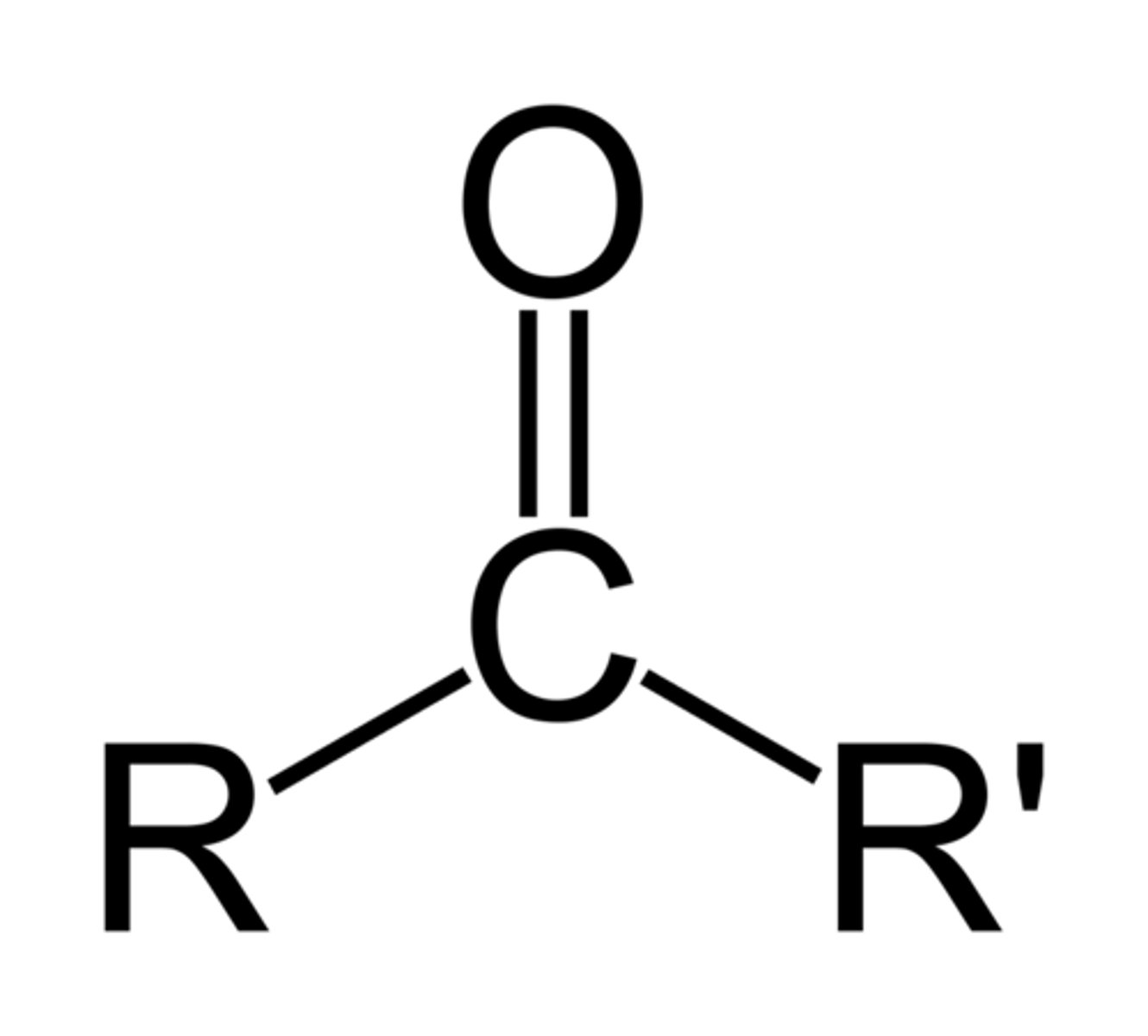
ketone
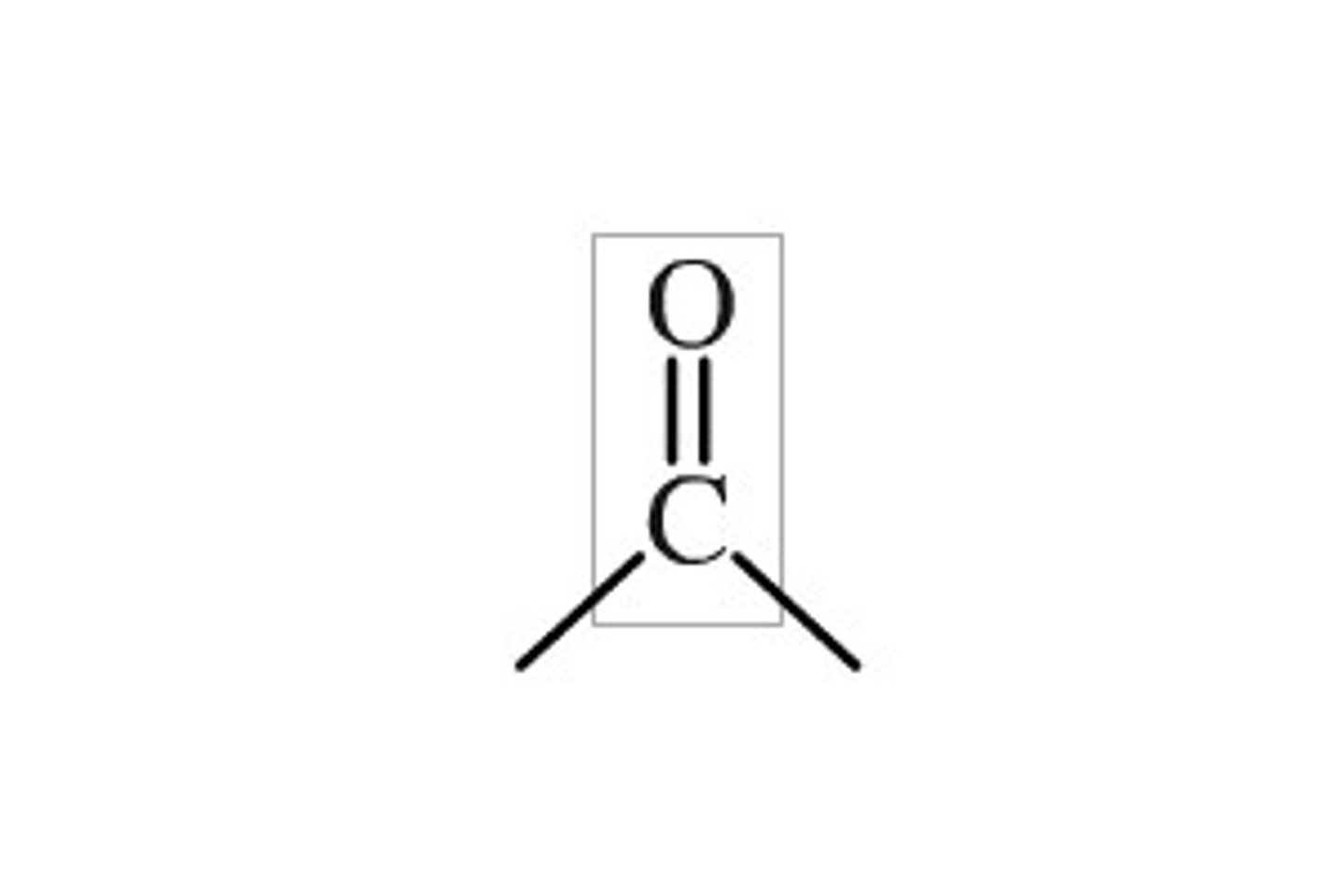
carbonyl
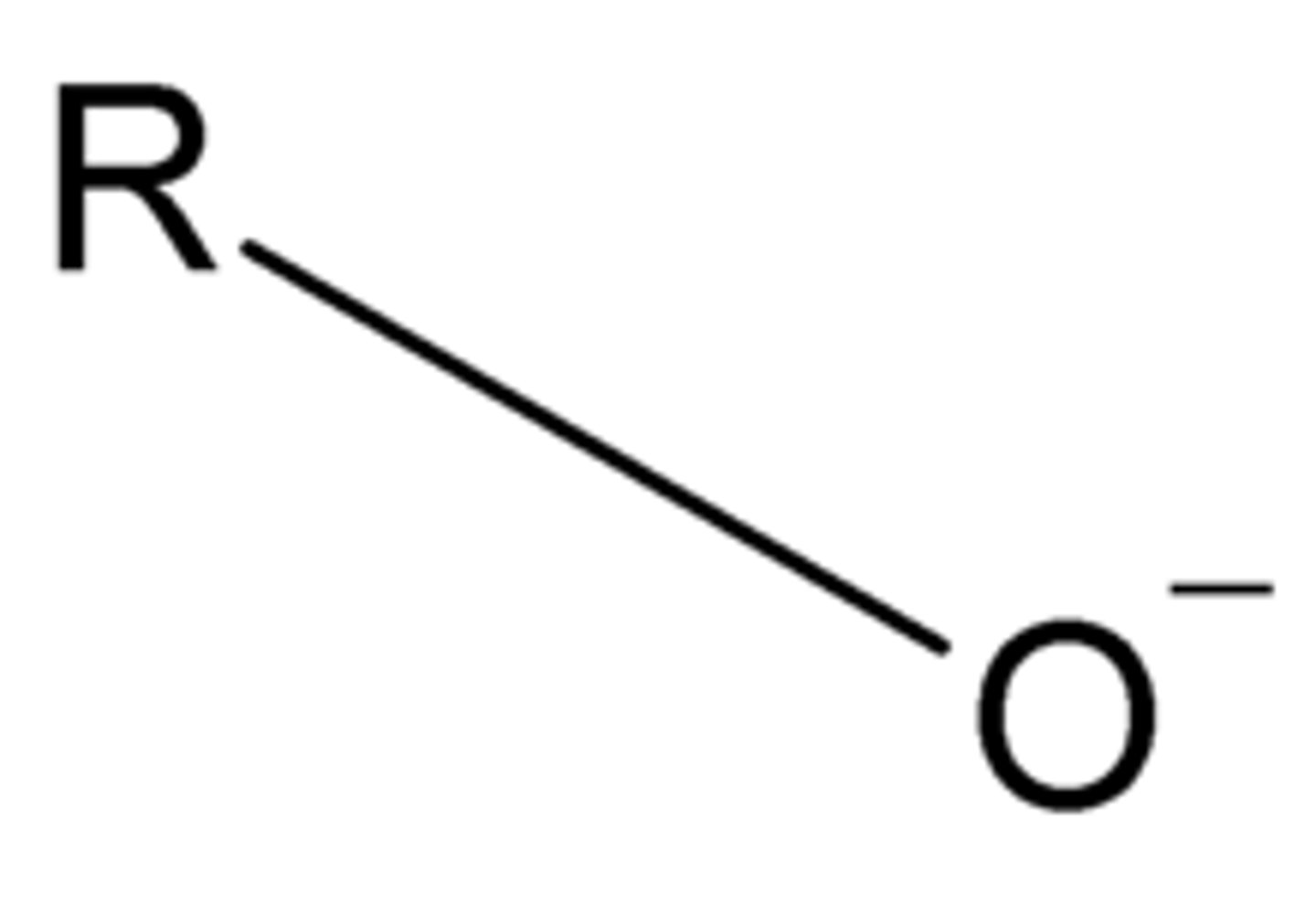
alkoxide
base just deprontonates
1° alcohol w/ CrO3/pyridine/HCl
PCC, aldehyde
2° alcohol w/ CrO3/pyridine/HCl
PCC, ketone
1° alcohol w/ 1) DMSO/oxalyl chloride 2) triethalmine
Swern/ aldehyde
2° alcohol w/ 1) DMSO/oxalyl chloride 2) triethalmine
Swern, ketone
1° alcohol w/ CrO3/H2SO4/H2O
Jones, carboxylic acid
2° alcohol w/ CrO3/H2SO4/H2O
Jones, ketone
2° alcohol w/ ANYTHING
ketone
1° alcohol will make?
aldehyde with PCC or Swern, carboxylic acid with Jones
hemiacetal
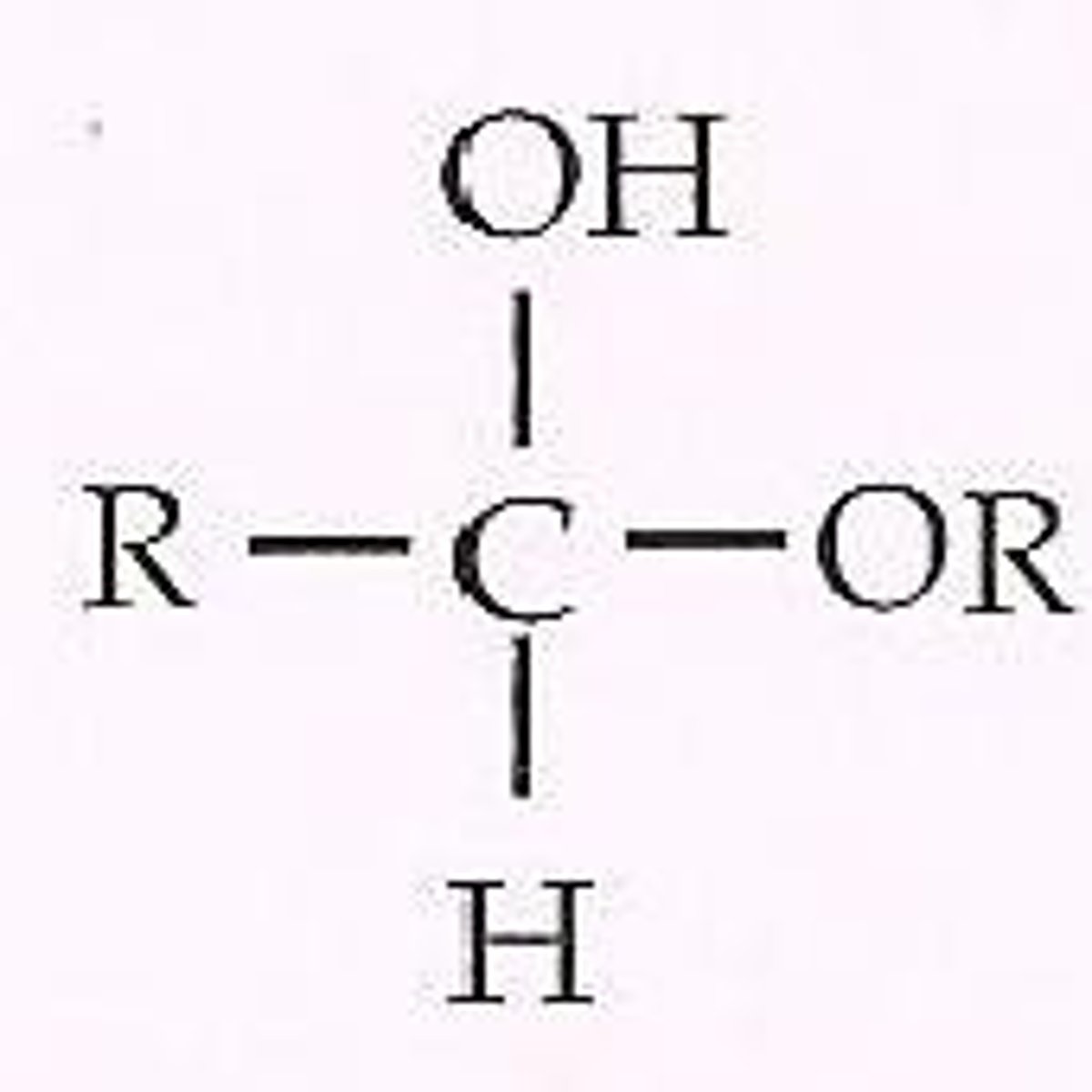
acetal
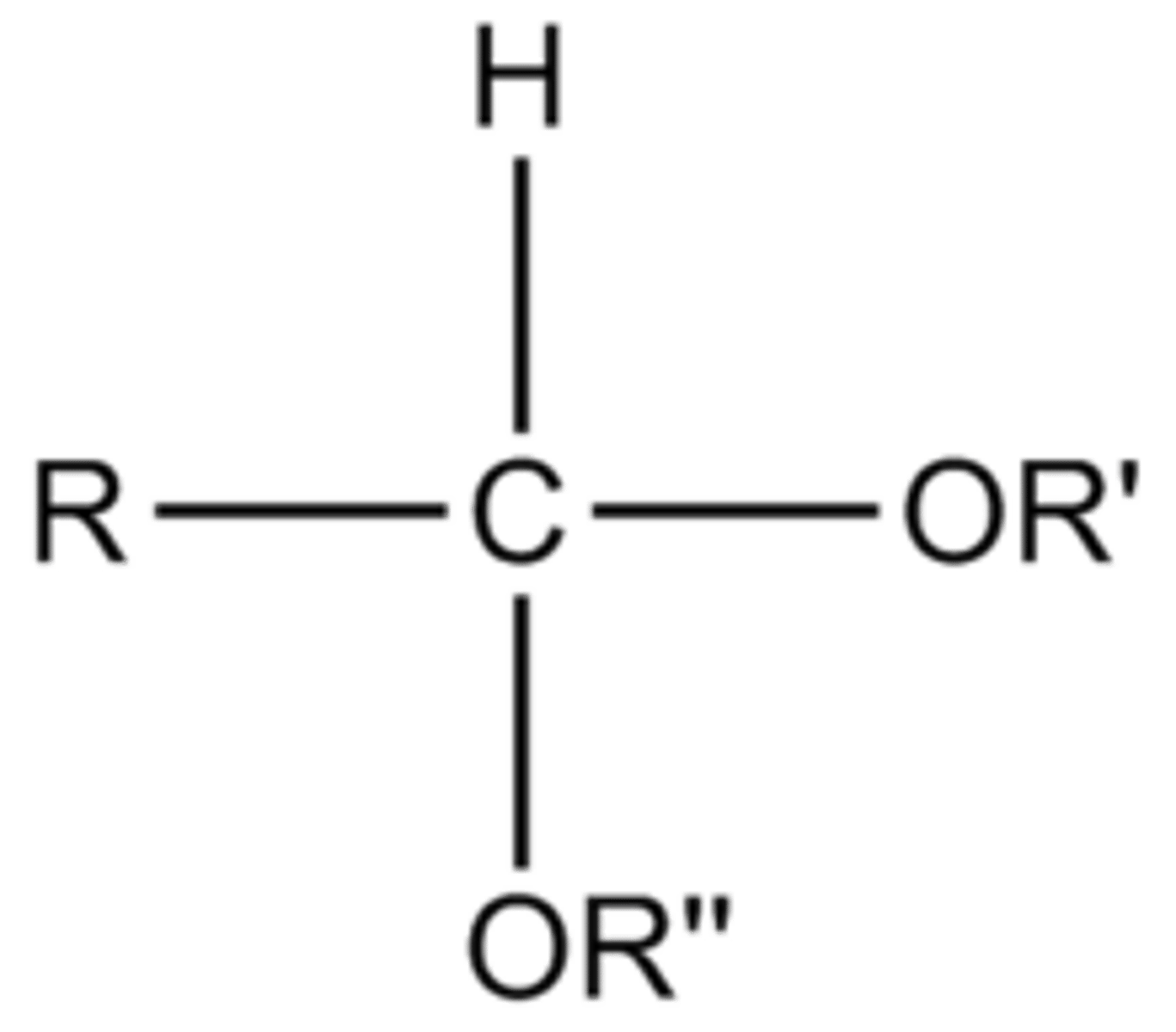
hemiketal
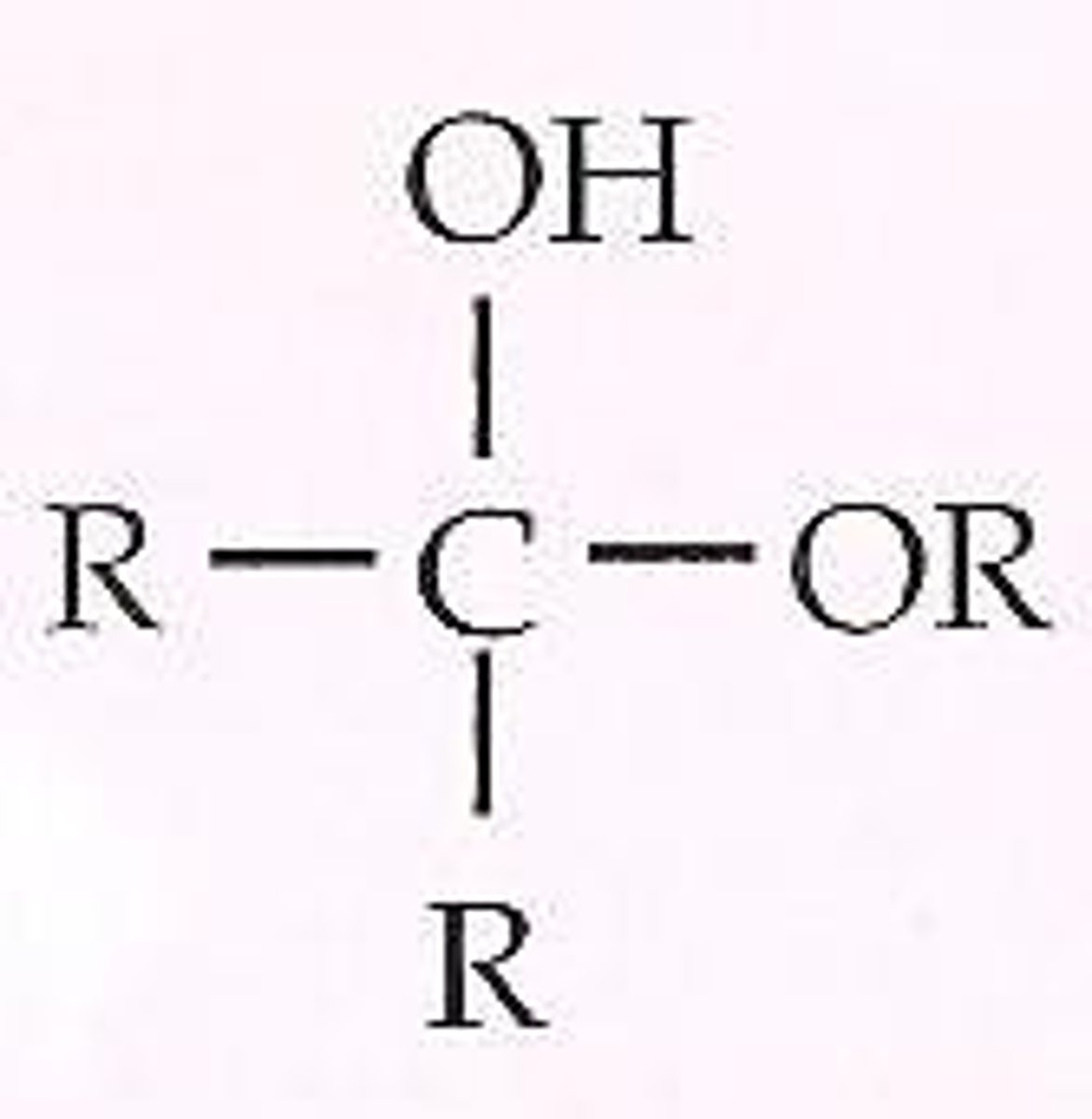
ketal

N group attaching on a carbonyl will form an _______
amine
epoxide ring opening in basic conditions
attack the least substituted carbon
epoxide ring opening in acid conditions
first protonate O, then attack most substituted carbon
H- additions to carbonyls: reagents
Li+ AlH4-, Na+ BH4-
CN- (cyanide) addition to carbonyl
:C-≡N: Na+
:CH3- addition to carbonyl
CH3 MgBr
imine formation
N replaces the O in the carbonyl, use catalytic amount of weak acid, N is the starting nucleophile
-one suffix
=O
-thiol suffix
-S-H
carboxy- prefix
COOH-
methane
CH4
ethane
CH3-CH3
under basic conditions...
DEPROTONATE your NUCLEOPHILE
under acidic conditions...
PROTONATE your ELECTROPHILE
oxidation number
just look at the first things the atom is attached to, the same atom attached counts as 0, more electronegative atom takes the negative charge towards it
/\/ OH w/reagent HCl or HBr or 1)TsCl, pyridine 2) NaCl
makes /\/ X (X is Cl or Br)
/\/ OH w/reagent Cl-Cl-S=O, pyridine
makes /\/ X (X is Cl)
/\/ OH w/reagent PBr3 or PCl3
makes /\/ X (X is Br or Cl)
needed trajectory/stereochem for alkoxides to form epoxides
in chair formations of rings you want the O- and the leaving group to be either BOTH equatorial or BOTH axial: if one away on a ring stereochem should be opposite (1 wedge and 1 dash), if two away on a ring stereochem should be same (2 wedges or 2 dashes); if two away on a ring and stereochem is opposite you do E2 and form a double bond