CHEM 1211 Section 3.4 (The Hydrogen Spectrum and the Bohr Model)
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
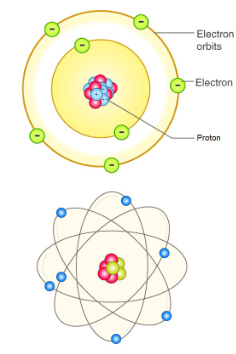
Bohr Model of the Atom
A model for the atom proposed by Niels Bohr where electrons are found at specific distances from the nucleus in orbits, travelling in circular paths with discrete energy levels.
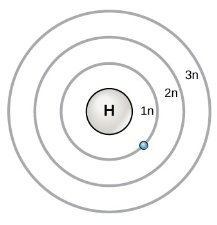
Ground State
the lowest energy level, closest to the nucleus
Excited State
when electrons absorb energy and move to higher levels called excited states
energy
Electrons in excited states will emit ______ to return to the more stable ground state.
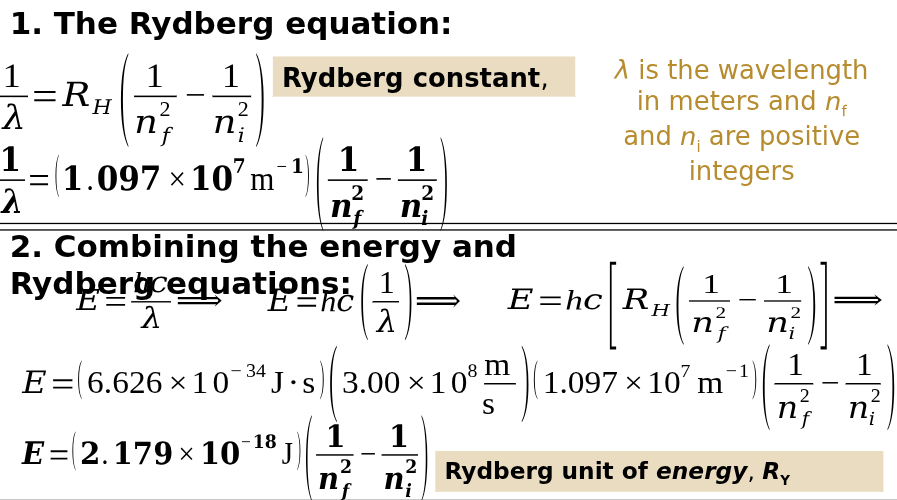
Rydberg Equation
a mathematical equation that predicts the wavelength of light emitted or absorbed when an electron transitions between energy levels
transition
Electrons will __________ between energy levels when absorbing or emitting energy.
Absorption
the process of exciting electrons to higher states using light
Emission
The relaxation of electrons back to lower states
light emission profile
Each element has a unique _____ ________ _______ due to it’s unique electronic levels.
Wave-Particle Duality
Light can be described as both waves and particles