Study Design
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
independent variable
Refers to the condition of an experiment that is systematically manipulated by the investigator. It is the presumed cause.
dependent variable
These variables are expected to change as a result of an experimental manipulation of the independent variable(s). It is the presumed effect.
extraneous variables
Variables other than the manipulated variables that affect the results of the experiment
study population
A group of individuals taken from the general population who share a common characteristic, such as HTN, Dyslipidemia, etc.
internal validity
____ validity indicates whether the independent variable was the sole cause of the change in the dependent variable
external validity
_____ validity indicates the extent to which the results of the experiment are applicable to the real world.
randomization
Assignment of subjects and treatment to groups is based on chance
Level I
Which level of evidence is described below?
Evidence from a Meta-Analysis of all relevant Randomized Controlled Trials (RCTs), or systematic reviews of RCTs.
Level II
Which level of evidence is described below?
Evidence obtained from at least one well designed RCT
Level III
Which level of evidence is described below?
Evidence from well-designed cohort studies
Level IV
Which level of evidence is described below?
Evidence from well-designed case-control studies
Level V
Which level of evidence is described below?
Evidence from Case reports, Case reports
Level VI
Which level of evidence is described below?
Evidence from Bench research or expert opinions
Bench research/Expert opinion
Case reports/Case series
Case control studies
Cohort studies
RCTs
Systematic review
Meta-analysis
List the evidence hierarchy in increasing order.
Case report
Case series
Survey
List types of descriptive studies
Observational:
Cross sectional
Case-control
Cohort studies
Experimental:
Quasi (clinical trials)
List the types of analytical studies
Descriptive studies
Studies that often represent the first scientific clue into new areas of scientific inquiry.
- Fundamental element: clear, specific, and measurable information.
A. Disease occurrence
B. Adverse drug reactions
C. New indications (off-label use)
Descriptive studies are often used to evaluate which of the following? (SATA)
A. Disease occurrence
B. Adverse drug reactions
C. New indications (off-label use)
D. Randomized treatment assignment
E. Laboratory bench experiments
Case report
What is the most basic form of medical evidence among descriptive studies?
true
T/F: Case reports are often based on a clinician observing a new disease presentation or an unusual adverse drug reaction.
Case series
An aggregate of individual cases on the same topic in one report.
false; a single case report may not warrant further investigation
T/F: A single case report is usually enough to warrant further investigation.
A. They aggregate multiple similar cases into one report.
B. They can highlight emerging diseases (e.g., AIDS epidemic).
D. Case series may warrant additional investigations.
Which of the following statements about case series are true? (SATA)
A. They aggregate multiple similar cases into one report.
B. They can highlight emerging diseases (e.g., AIDS epidemic).
C. A single case report always warrants further investigation.
D. Case series may warrant additional investigations.
Survey research
The collection of information from a sample of individuals through their responses to questions
Questionnarie
Interview
Data collection methods for surveys
Quantitative
- Ex: Dabigatran use among patients with valvular Afib
Designed to collect numerical or quantitative data that can be used to measure a variable in survey research
Qualitative
- Opinions about the COVID-19 vaccines
Information from survey research that seeks to describe a topic more than measure it. This type of research measures opinions, views, and attributes
Relatively inexpensive
Relatively expeditious; can publish results quickly
Provides very detailed information
Advantages of descriptive studies
Difficult to replicate
May include bias
Cannot be generalized
Disadvantages of descriptive studies
A. Generating hypotheses and associations
B. Describing trends
What are two key roles of descriptive studies in research?
A. Generating hypotheses and associations
B. Describing trends
C. Establishing causality
D. Testing interventions
monitor trends and generate a hypothesis
Descriptive studies are used to monitor _____ and generate a _____.
true
T/F: Descriptive studies lack a comparison and cannot draw conclusions about cause and effect.
observational studies
- In observational studies, the Investigator(s) does not intervene simply observe(s) and collect data of interest
Epidemiological studies involving the observation of the disease, drug use problem, or other phenomenon of interest
False; the investigator only observes and collects data.
T/F: In observational studies, the investigator intervenes directly to test an outcome.
A. Collect data without intervention
B. Establish a causal link between a risk factor and an outcome
D. Assess the strength of exposure-outcome relationships
In observational studies, the investigator attempts to: (SATA)
A. Collect data without intervention
B. Establish a causal link between a risk factor and an outcome
C. Randomize participants into treatment groups
D. Assess the strength of exposure-outcome relationships
The possible effects of a treatment or risks on subjects.
- assignment of treatment or intervention is outside the control of the investigator
What do investigators in observational studies draw inferences about?
Ethical
Several confounders
Rare occurrence
The assignment of treatments/interventions is beyond the control of the investigator for a variety of reasons:
Prevalence
Incidence
Prognosis
Effects of treatment
Characteristics of a disease/Outcomes
Observational studies are used to determine the following:
Relatively fast and inexpensive to conduct
Detect signals about the benefits and risks of certain treatments
Help formulate hypotheses to be tested in subsequent experiments
Strengths of observational studies
Cannot provide definitive evidence of safety or efficacy
Cannot provide causality
Weaknesses of observational studies
false; they cannot prove causality; they may provide associations
T/F: Observational studies are used to show causality
Cross-sectional study
At one point in time, subjects are sampled and assessed to determine whether they were exposed to the relevant agent and whether they have the outcome of interest
- influences the likelihood of a particular diagnosis and the predictive value of a test
- provides estimates for prevalence
Prevalence
What type of measure do cross-sectional studies provide estimates for?
Relative risk; risk ratio
Measures of association for cross-sectional studies
Can measure prevalence
Relatively fast and inexpensive to conduct
No loss to follow up
Associations can be studied
Strengths of cross-sectional studies
Not suitable for rare diseases or side effects
Can not show causality
Weaknesses of cross-sectional studies
case-control
Type of study in which persons with a condition or outcome of interest ("case") are identified; matched to identical people without the condition ("control")
- RETROSPECTIVELY assesses exposures (prospective case-control studies are less common)
odds ratio
Measure of association for case-control studies
- Cannot measure: incidence, prevalence, relative risks
- Very prone to recall bias
Weaknesses of case-control studies
- Rare diseases/outcomes
- Study many exposures
- Fast and inexpensive
- Provides odds ratio
- Can generate a hypothesis
Strengths of case-control studies
Cohort studies
Best method for determining the incidence and natural history of a condition, disease, adverse drug effects, and treatments
- can be prospective or retrospective
true
T/F: Two cohorts' studies can be compared.
Prospective cohort studies
A study that begins with a disease-free population, measures exposures or risk factors, and follows participants over time to see if they develop the outcome of interest.
Retrospective cohort studies
Identification of cohort, measurement of predictor variables, follow-up up and measurement of outcomes have all occurred in the past
- Less costly
- The investigator has minimal control over the study design
Nested case-control study
Case-control study embedded in a cohort study; controls are drawn randomly from the study sample
Double cohort
Type of cohort study that is used to compare two separate cohorts with different levels of exposure to predictor variable (e.g., occupational groups)
- Provides incidence data
- Establishes time sequence for causality
- Eliminates recall bias
- Allows for accurate measurement of exposure and other variables
- Can measure multiple outcomes
- May be able to adjust for confounders
- Can calculate relative risk
Strengths of cohort studies
- Expensive
- Exposure may change over time
- Disease may have a long preclinical phase
- Attrition of the study population
Weaknesses of cohort studies
1. Biases: information and selection bias
2. Confounders
Limitations of observational studies
Study bias
Any trend in the collection, analysis, interpretation, publication, or review of data that can lead to conclusions that are systematically different from the truth.
false; bias relates to internal validity; generalizability relates to external validity
T/F: Lack of generalizability is considered a form of bias.
- Precise case and exposure definitions
- Clear definition of study population
- Classification of exposed and non-exposed without knowing disease status
- Aim for high response and follow-up (check on non-responders, loss to follow-up)
How to minimize selection bias
- Increase inclusion and exclusion criteria
- Matching subjects according to confounding factors
- Randomization (Only in clinical trials)
- Nonrandomized studies are prone to confounding factors
How to minimize confounding factors in study design
- Stratification
- Balance groups with respect to confounding factors
- Use of the correct statistical test, Multivariate analysis
How to minimize confounding factors in data analysis
- Looks for cause and effect relationships
- Highly controlled, objective, and systematic operations
- Involves accurate measurement, analysis, and interpretation of the dependent and independent variables
- Can state a hypothesis
- A researcher can test the hypothesis Can draw a conclusion
Elements of experimental research
Manipulation
What is a key feature of experimental research?
control
- physical, selective, and statistical
Experimental ____ attempts to predict events that will occur in the experimental setting by neutralizing the effects of other factors
Randomization
A process by which study participants have an equal chance of being assigned to the treatment arm; eliminates selection and accidental bias
- produces comparable study groups
Simple randomization
randomization that is computer-generated or a coin is tossed for each participant
A. Age group
B. Severity of condition
C. Risk factors
D. Comorbidities
Which factors are commonly used for stratified randomization? (SATA)
A. Age group
B. Severity of condition
C. Risk factors
D. Comorbidities
E. Favorite color
To restrict imbalances and ensure treatment groups are as alike as possible.
What is the purpose of stratified randomization?
Pilot studies and dose-ranging studies
Open-label studies are useful for:
- Toxicity over-/under-reporting
- Efficacy overestimation
In open-label study applications may be substantially biased by knowledge of the treatment given and may result in:
When it would be ethically unacceptable to give an appropriate placebo treatment to a patient, and in such a case, the assessor (not the patient) should be the one blinded to the treatment
In single-blind studies, the patient is blinded to treatment. When would you use single blinding?
Double-blind studies
Serves as the standard by which all studies are judged; minimizes both potential patients' and investigators' bias
Experimental study design
Best design for determining the strongest evidence between exposure and outcome
- can be controlled or non-controlled
- described as prospective or longitudinal
- Best measure of casual relationships
- Best design for controlling bias
- Can measure multiple outcomes
Strengths of experimental study designs
- Expensive
- Ethical issues may be a problem
- Compliance
- Issues with external validity
Weaknesses of experimental study designs
RCTs
In ____, patients are randomly assigned to one of two, three, or more groups that receive a drug or intervention and are followed over time for the outcome of interest. Some patients may fall under the control group, which serves as the comparison group. The group may be given a placebo drug (inactive drug, aka "sugar pill"), or a comparison drug may be given. Both groups are compared in terms of outcomes, and treatment effectiveness is determined.
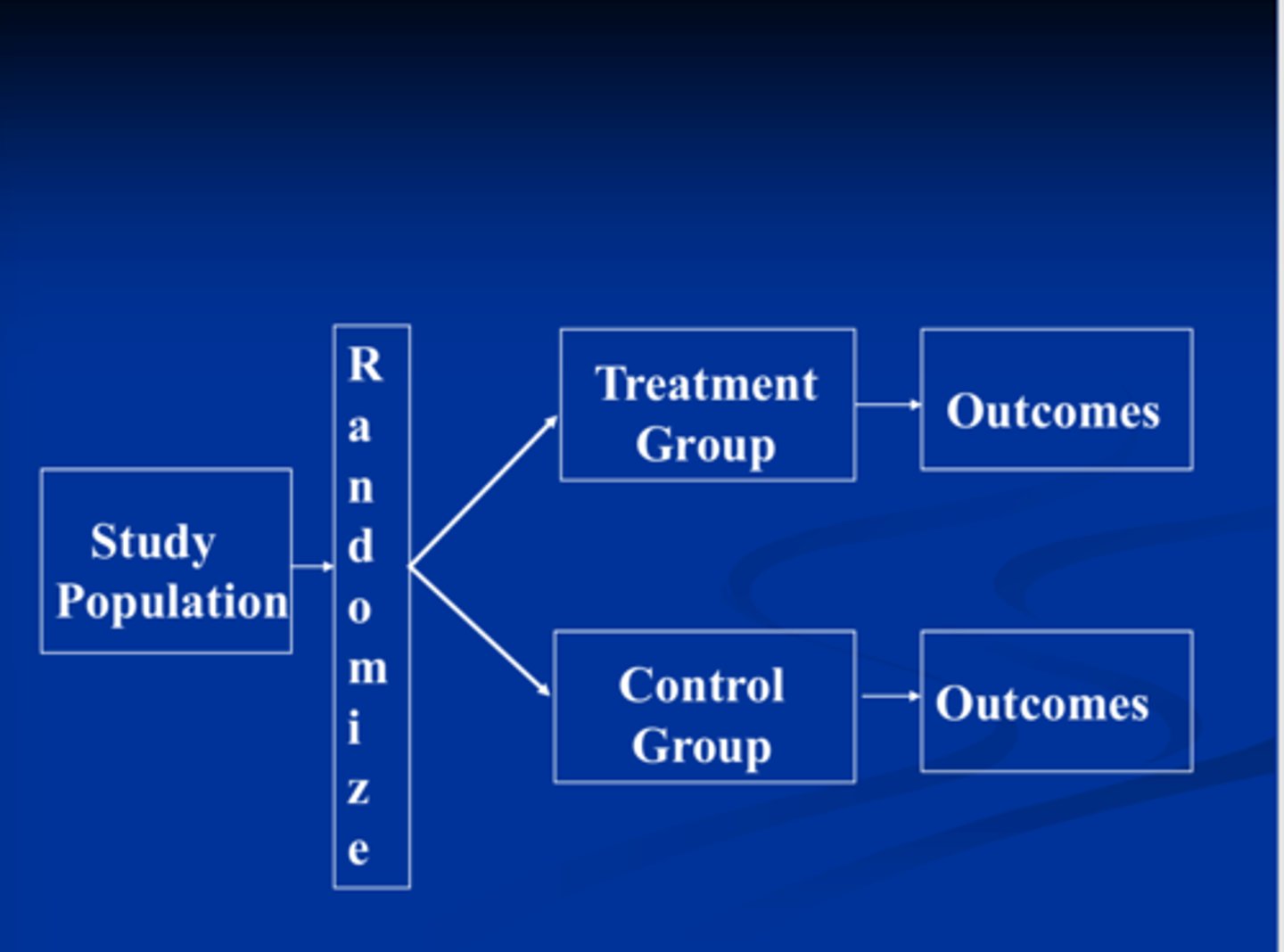
- Provides the most robust evidence for determining drug efficacy
- Employs a control group (ideal for comparisons)
- Bias is minimized
- Extraneous factors are minimized
- Most reliable trial for evaluating therapies
- Trials are reproducible and can be replicated
Advantages of RCTs
- Studies can be odious and time-consuming
- Expensive: Most expensive study design
- Patient enrollment can be difficult
- The dropout rate is high
- Ethical problems in testing new drugs in humans and in certain populations
Disadvantages of RCTs
exposure and outcomes
RCTs are considered the best design for providing evidence for ____ and _____
The ability of the investigator to actively intervene
What is the major difference between a clinical trial and an observational study?
Non-controlled experimental study
Type of study in which there is no control group; the drug or procedure to be evaluated is administered to a single group of patients, and outcomes of interest are measured and analyzed
Parallel design
Design that includes independent study groups, and each group receives a different treatment regimen or intervention
- more useful for studying conditions that are prone to change over time (pain, acute exacerbations of disease, remissions)
Parallel
RCTs are often ____ design
Parallel
Which design is useful for studying conditions that are prone to change over time?
Crossover design
In a ____ design, both groups receive treatments or interventions (drug and placebo); the groups are later switched
- Statistically sensitive and efficient; uses fewer patients
Fewer patients can lead to a more homogenous group with less variability in measurement.
- Less variability between groups implies a measured difference is more likely to be due to treatment effects instead of interpatient variability.
What is a benefit of using fewer patients, like in a crossover design?
1. A smaller number of patients is required since the same patient groups receive both treatments
2. Relatively less expensive
3. The ability to analyze patients both within groups as well as between groups.
- Within groups: baseline factors (age, gender differences) that could influence the results are eliminated because patients serve as their own control group.
Advantages of cross-over study design
- Time involved for a crossover design is longer than other design types
- More dropouts because of time involved.
- Study design is very sensitive to drop outs since small number of patients involved.
- Period effects
- Sequence effects
- Carry over effects (need a washout period)
Disadvantages of cross-over study design
washout period
Crossover study designs require a ____ period.
Bioavailability studies
- interactions would be less likely since the patients serve as their own controls
Cross-over designs are useful for: