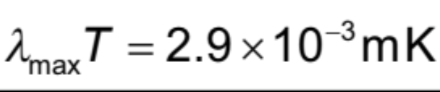B1: Thermal energy transfer
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Thermal equilibrium
Objects at the same temperature
Temperature
a measure of the average kinetic energy of the particles in a substance.
Absolute zero
temperature at which all particle movement ceases, internal energy is zero.
Internal energy
The total kinetic and potential energy of the molecules of a body
Thermal energy
The net amount of energy transferred between two bodies at different temperatures
Thermal capacity
the amount of energy needed to raise an object's temperature by 1 Kelvin
Specific heat capacity
the amount of energy needed to raise the temperature of 1 kg of a substance by 1 Kelvin.
Specific latent heat
the amount of energy per unit mass absorbed or released during a change of phase.
Intensity
Power per unit area
Inverse square law of radiation
The power/intensity of radiation is inversely proportional to the square of the distance from its source (I ∝ 1/r^2)
Stefan-Boltzmann Law
The total power radiated by a black body (per unit area) is proportional to the Kelvin temperature to the fourth power (P α T^4)
Black body
A perfect absorber and emitter of e.m. radiation
Density
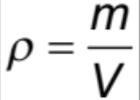
Average random kinetic energy of particles
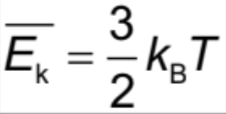
Heat (energy transferred) +Specific
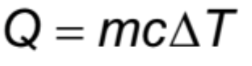
Heat (energy transferred) +Latent
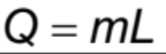
amount of heat (energy) transfer
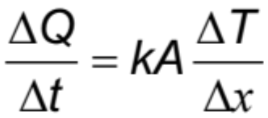
Luminosity (total power output)
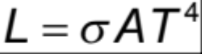
Brightness (intensity)
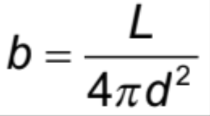
Peak Wavelength