The reactions of acids.
1/6
Earn XP
Description and Tags
https://www.youtube.com/watch?v=QlSsle_jSQ8&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=8
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
What are bases
They are chemicals which can neutralise acids and produce a salt and water
Bases which are soluble in water are also called alkalis.
What happens when acid reacts with base or alkali
We make a salt and water.
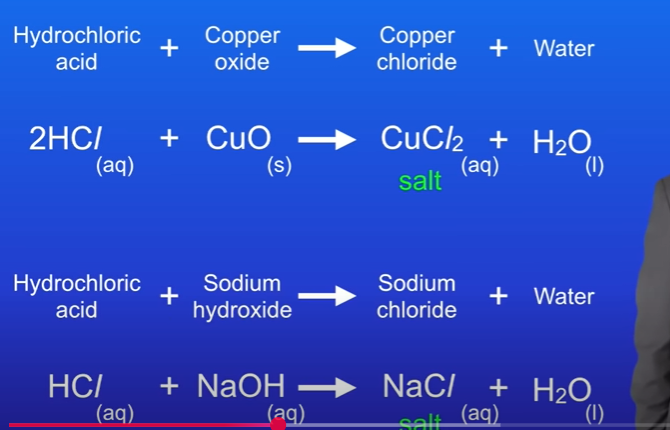
What are salts
Salts contain a positive ion which comes from the base or alkali.
Salts also contain a negative ion which comes from the acid.
Hydrochloric acid → Chlorides
Sulfuric acid → Sulfates
Nitric acid → nitrates.
Predict the products of the following reaction Sulfuric acid + Zinc oxide.
In the base zinc oxide, the positive ion is the metal zinc, and sulfuric acid produces sulfates. This means our products are zinc sulfate + water.
What are three examples of metal carbonates.
Sodium Carbonate
Calcium carbonate
Potassium carbonates

What happens when acid react with metal carbonate
They make salt, water and carbon dioxide.