basic atomic structure
1/22
Earn XP
Description and Tags
w/ test qs added
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
what number do we give to shells? how does this work?
principal quantum number
the further away the shell from the nucleus, the higher the principal quantum number
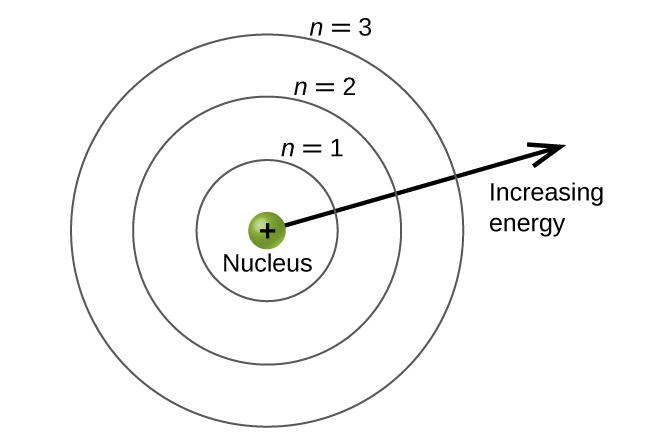
what can shells be broken down into?
subshells
what are subshells made up of?
orbitals
what are the type of subshell and what exactly are they made up of?
s = one orbital: 2 e-
p = three orbitals: 6 e-
d = five orbitals: 10 e-
f = seven orbitals: 14 e-
what is an orbital?
a region of space containing e-
each orbital can hold 2 e-
what subshell does the first shell have? how many electrons can it hold?
1s
holds 2 e-
what subshells does the second shell have? how many electrons can it hold?
2s, 2p
holds 8 e-
what subshells does the third shell have? how many electrons can it hold?
3s, 3p, 3d
holds 18 e-
what is the order in which you fill up the orbitals when working out e- configurations?
how do you write an electron configuration in the noble gas configuration notation?
find the noble gas before (periodically/horizontally) the atom and write it in [], then the rest of the configuration
e.g. silicon: [Ne]3s²3p² (1s²2s²2p63s²3p²)
how can we classify elements?
can be in s, d, p or f block
elements are classified into blocks depending on the highest orbital energy level it contains
e.g. Na - 1s²2s²2p63s1 ∴ s block
what are the exceptions to the block classifications of elements?
He is in group 0 due to its properties but is an s block element
H is an s block element but has different properties to its group 1 counterparts
what are the transition metal exceptions to normal electron configuration?
Cr: [Ar]4s13d5
Cu: [Ar]4s13d10
what is the rule for electron configurations for transition metal ions?
transition metals lose all 4s e- before losing any 3d e-
(e.g. Fe: 1s²2s²2p63s²3p64s²3d6
Fe2+: 1s²2s²2p63s²3p64s13d10
Fe3+: 1s²2s²2p63s²3p63d9)
in an atom, which electrons will be easiest to lose?
electrons in the orbital furthest from nucleus
this is because they have the weakest attraction to nucleus and positive charge of nucleus is shielded by inner
in an atom, which electrons will be most difficult to lose?
electrons in orbital closest to nucleus
this is because they have the strongest attraction to nucleus
what is ionisation energy (IE)?
the energy required to removes 1 mole of e- from 1 mole of atoms in the gaseous state
what is ionisation energy (IE) measured in?
kJ mol-1
what is the ‘general equation’ for ionisation energy (IE)?
X(g) → X+(g) + e-
what is it called when a 1+ ion loses another e- to become a 2+ ion?
2nd ionisation energy
(naming is self explanatory and continues on the number scale)
why is the second ionisation energy always greater than the first?
because the e- is being removed from a +1 ion and there is an attraction between the positively charged ion and the negative nucleus
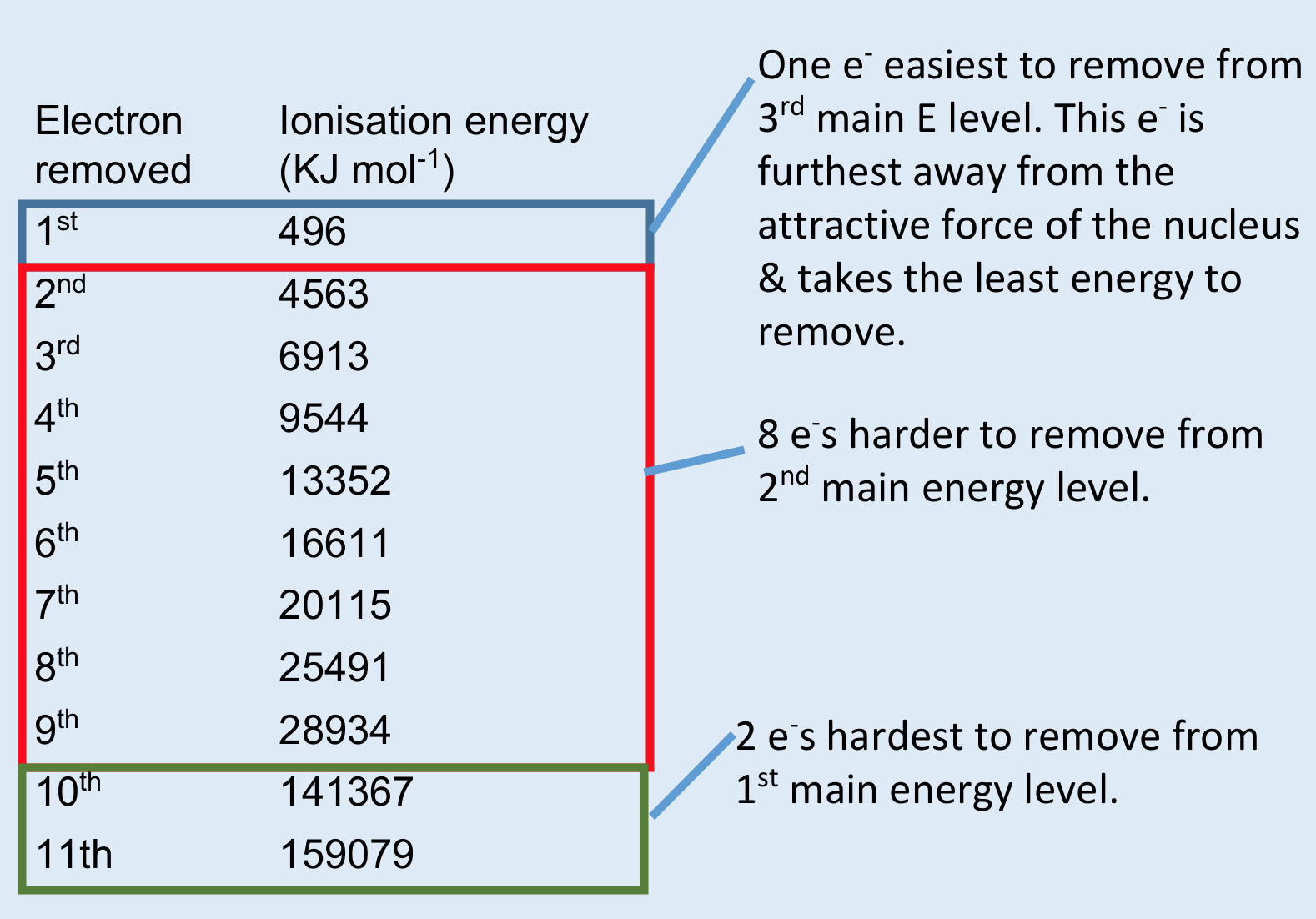
how do successive ionisation energies for an element provide evidence for an element’s electron configuration?
where there are the greatest jumps in energies, the shells are distinguishable
this means that where the greatest jump in energy is allows you to distinguish which group an element is in
e.g. if the first four energies have small gaps, then the fifth has a big jump, we can conclude that the element has 4 e- in its outer shell and is in group 4
X and Zn are different elements.
explain why the chemical properties of 70X and 70Zn are different (1)
they have different numbers of electrons