aromatics 2
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is electronegativity?
Electronegativity is essentially a measure of how ‘electron attracting’ an element is.
Where are the most and least electronegative elements found in the periodic table?
Most electronegative elements → top right corner
Least electronegative elements → bottom left corner
What is the most electronegative element and which scale is commonly used?
Fluorine is the most electronegative element.
Pauling’s scale is the most common scale used to measure electronegativity.
What happens in covalent molecules formed between atoms of different elements?
There can be a difference in electronegativity between the atoms — for example, in HCl
What is the result of the difference in electronegativity in HCl?
The H–Cl bond becomes polar:
Chlorine gains a small negative charge (δ–)
Hydrogen gains a small positive charge (δ+)
What is the dipole moment (μ) of a molecule?
The permanent electric moment of a molecule is known as its dipole moment (μ).
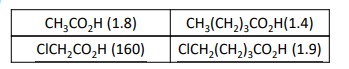
What do the dissociation constants of the given organic acids suggest?
The acids containing a C–Cl bond (like ClCH₂CO₂H) are much stronger acids, showing that the C–Cl dipole has a strong effect on the nearby site.
What is the effect of the C–Cl bond on the molecule?
The dipole of the C–Cl bond exerts a pronounced electrostatic effect on the adjacent carbon,
but this effect decreases with distance.
What is the inductive effect?
the inductive effect is an electrostatic effect caused by the polarization of a sigma bond due to electronegativity differences.
It is given the symbol I and can be –I (electron-withdrawing) or +I (electron-donating).
What are examples of –I (electron-withdrawing) substituents?
The chlorine atom acts as a –I substituent
Other –I substituents include:
CN
NO₂
Halogens
OCH₃
CF₃
+NR₃
These groups withdraw electron density through sigma bonds
What are examples of +I (electron-donating) substituents?
+I groups include:
CH₃
CH₂CH₃
CH(CH₃)₂
C(CH₃)₃
These groups donate electron density through sigma bonds
How does the inductive effect change with distance?
The inductive effect (I) falls off with distance
The further the substituent is from the affected site, the weaker the effect becomes
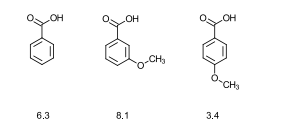
What do the ionization constants of benzoic acids show?
The meta derivative is stronger than benzoic acid.
The para isomer is not stronger.
The reason the para isomer is not stronger is due to resonance.
Why can’t the –I effect alone explain the difference between the meta and para derivatives?
There must be something more than the –I effect in operation.
The effect responsible is the resonance effect (R), also called the mesomeric effect.
What causes the resonance (mesomeric) effect?
It results from conjugation of one of the lone pairs of electrons from the oxygen with the π-system of the ring.
This conjugation alters electron density across the ring.
How can the resonance effect be represented?
It can be represented by drawing resonance structures.
These resonance structures show regions of increased or decreased electron density, helping explain reactivity differences.
• Examples of +R and –R groups include:
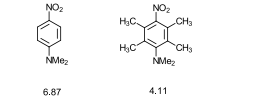
What do the dipole moments in the two examples show?
The compound on the left has a higher dipole moment (6.87).
The compound on the right has a lower dipole moment (4.11).
This difference is due to steric effects affecting resonance.
Why is resonance more effective in the first compound?
In the left example, the groups are coplanar, allowing maximum overlap between π orbitals.
This enables a range of resonance forms to occur, stabilizing the molecule and increasing its dipole moment.
Why is resonance reduced in the second compound?
The methyl groups in the right-hand structure are bulky, forcing the substituents out of coplanarity.
This prevents effective overlap of orbitals, reducing resonance.
When substituents are very bulky, they can prevent resonance from taking place entirely.
What is hyperconjugation?
Hyperconjugation is a mechanism of both electron release and withdrawal.
It involves the interaction of σ-bonds (C–H or C–C) with an adjacent π-system or empty orbital.
What are the two types of hyperconjugation, and which is more important?
Two types: C–H hyperconjugation and C–C hyperconjugation.
C–C hyperconjugation is more important than C–H hyperconjugation.
What is the order of an alkyl group’s ability to stabilize via hyperconjugation?
the more alkyl substituents (CH₃ groups) attached to the positively charged or unsaturated carbon, the more σ bonds are available for hyperconjugation.
So:
tBu > iPr > ethyl > methyl
means tert-butyl provides the greatest stabilization because it has the most C–H bonds capable of participating in hyperconjugation.
How significant is hyperconjugation in electron release for unsaturated systems?
In many unsaturated systems with an alkyl group attached, about 80% of electron release comes from hyperconjugation.
How do alkyl substituents donate electron density?
Alkyl substituents donate electron density through:
The inductive effect (+I)
Hyperconjugation (both C–H and C–C types)
What is the combined effect of +I and hyperconjugation?
The two effects work together
They make alkyl-substituted benzenes more susceptible to electrophilic attack than benzene itself
Why is the product of a Friedel–Crafts alkylation more reactive than the starting material?
The alkyl group added increases electron density in the ring via +I and hyperconjugation.
This makes the product more reactive toward further electrophilic substitution than benzene.
How does the bromination of phenol compare to that of benzene?
Much quicker than benzene.
This is because the OH group activates the ring toward electrophilic substitution.
Why does the bromination of phenol occur so easily?
The OH group donates electrons to the ring through resonance and +I effects.
This increases electron density at the ortho and para positions, making the ring more reactive.
Why is no catalyst needed for bromination of phenol?
The phenol ring is already activated by the OH group, which makes it reactive enough to polarize Br₂ directly — so no FeBr₃ catalyst is required.
Why is the –OH group in phenol ortho-para directing?
The –OH group donates electrons into the benzene ring through resonance, increasing electron density at the ortho (2,6) and para (4) positions.
What happens when phenol reacts with bromine under cold conditions (CS₂, <5 °C)?
Only one bromine atom substitutes at the para position to give 4-bromophenol in 85% yield.
What do the resonance structures under the reaction show?
They show delocalization of electron density from the oxygen atom into the ring, stabilizing the intermediate and highlighting why ortho and para positions are most reactive.
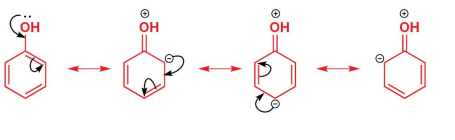
What happens if bromination of phenol continues?
: Multiple substitutions occur at positions 2, 4, and 6, forming 2,4,6-tribromophenol (TCP).