Inorganic ACS
1/189
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
190 Terms
Nuclide
Type of atom with atomic number Z, equal to number of protons in nucleus (and electrons, since atom is neutral)
Mass number
A = the number of protons and neutrons in nucleus
Number of neutrons in an atom
A (mass number) - Z (atomic number)
Isotope
atom of an element that differ only by the number of neutrons (mass)
Monotopic
one natural nuclide
Relative atomic mass from abundance (Ar)
= (( %/100 ) * (accurate mass)) + ““…
Isotopes of an element have the same BLANK, but different BLANK
atomic number Z, atomic masses
ΔE = (in terms of frequency)
hv
Units of E
J, Joules
Units of v (frequency)
s-1 or Hz
c (speed of light)
= λv
units of λ
m (meters)
ΔE = (in terms of wavelength)
hc/λ
ṽ = (in terms of wavelength)
1/λ
ṽ = (in terms of n)
R((1/n2 )-(1/n’ 2 ))
R (Rydberg constant) for Hydrogen
= 1.097e7 m-1 or 1.097e5 cm-1
n (principal quantum #) =
mvr/(h/2pi) (where m = mass of electron, v = velocity of electron, r = radius of orbit)
rn (electron orbit radius)
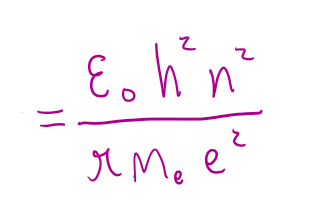
ε0 (permittivity of a vacuum)
= 8.854e-12 Fm-1
e (elementary charge on an electron)
= 1.602e-19 C
Ionization in terms of n
n=1 → n = ∞
Units of IE (ionization energy)
per mol of atoms
IE =
E∞ - E1 = hc/λ
Ionization energy of Hydrogen
1312 kJ mol-1 or 13.6 eV
λ (in terms of particles)
= h/mv (m = mass of particle, v = velocity of particle)
1eV
= 96.4853 kJ mol-1
possible values of principal quantum number
1<=n<=∞
l (orbital quantum number)
determines shape of orbital and angular momentum
possible values of l
0,1,2…(n-1)
ml (magnetic quantum number)
determines orientation of orbital
possible values of ml
between +l and -l
For a 1s orbital, what is n
1
For a 1s orbital what is l
0
For a 1s orbital what is ml
0
for a 1s orbital what is the radial part of the wavefunction R(r)
2e-r
for a 1s orbital what is the angular part of the wavefunction A(θ,Φ)
1/(2sq(pi))
for a 2s orbital what is n
2
for a 2s orbital what is l
0
for a 2s orbital what is ml
0
for a 2s orbital what is the radial part of the wavefunction R(r)
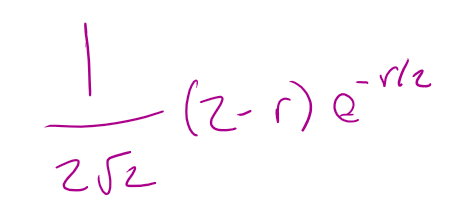
for a 2s orbital what is the angular part of the wavefunction A(θ,Φ)
1/(2sq(pi))
for a 2px orbital what is n
2
for a 2px orbital what is l
1
for a 2px orbital what is ml
+1
for a 2px orbital what is the radial part of the wavefunction R(r)
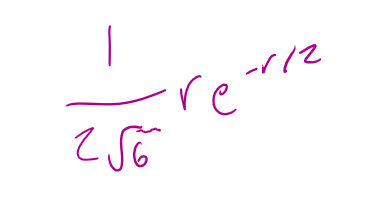
for a 2px orbital what is the angular part of the wavefunction A(θ,Φ)
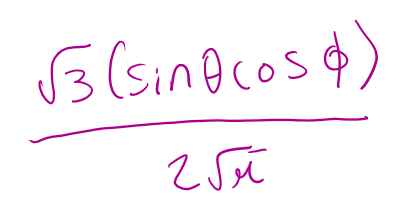
for a 2pz orbital what is n
2
for a 2pz orbital what is l
1
for a 2pz orbital what is ml
0
for a 2pz orbital what is the radial part of the wavefunction R(r)
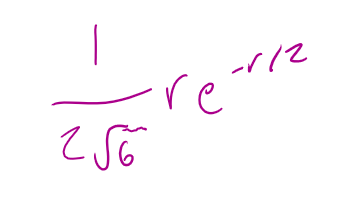
for a 2pz orbital what is the angular part of the wavefunction A(θ,Φ)
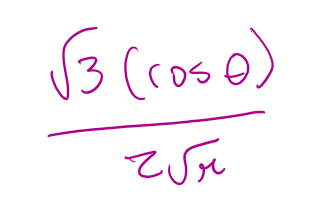
for a 2py orbital what is n
2
for a 2py orbital what is l
1
for a 2py orbital what is ml
-1
for a 2py orbital what is the radial part of the wavefunction R(r)
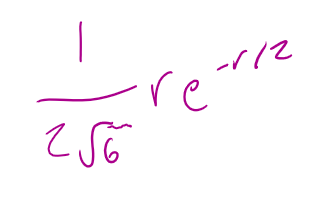
for a 2py orbital what is the angular part of the wavefunction A(θ,Φ)
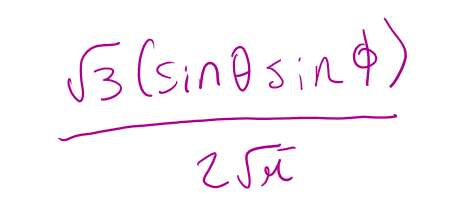
degenerate orbitals possess the same
energy
For a given value of n (n greater than or equal to 1) there is
1 s atomic orbital
For a given value of n (n greater than or equal to 2) there is
3 p atomic orbitals
For a given value of n (n greater than or equal to 3) there is
5 d atomic orbitals
For a given value of n (n greater than or equal to 4) there is
7 f atomic orbitals
likelihood of an electron being further from the nucleus
increases as n increases (not linearly)
s atomic orbitals have a finite value of
R(r) at the nucleus
for all orbitals other than s
R(r) = 0 at the nucleus
for the 1s orbital
R(r) is always positive
for the first orbital of all other orbital types besides 1s (ie. 2p, 3d, 4f)
R(r) is positive everywhere except at the origin
for the second orbital of a given type (ie. 2s, 3p, 4d, 5f)
R(r) may be positive or negative but the wavefunction has only one sign change
the point at which R(r)=0 (not including the origin) is called
a radial node
for the third orbital of a given type (ie. 3s, 4p, 5d, 6f)
R(r) has two sign changes (it possesses two radial nodes)
ns orbitals have BLANK radial nodes
(n-1)
np orbitals have BLANK radial nodes
(n-2)
nd orbitals have BLANK radial nodes
(n-3)
nf orbitals have BLANK radial nodes
(n-4)
Radial distribution
=4pir2R(r)2
Using the radial distribution function, disregarding r=0, what points correspond to radial nodes?
Anywhere 4pir2R(r)2=0
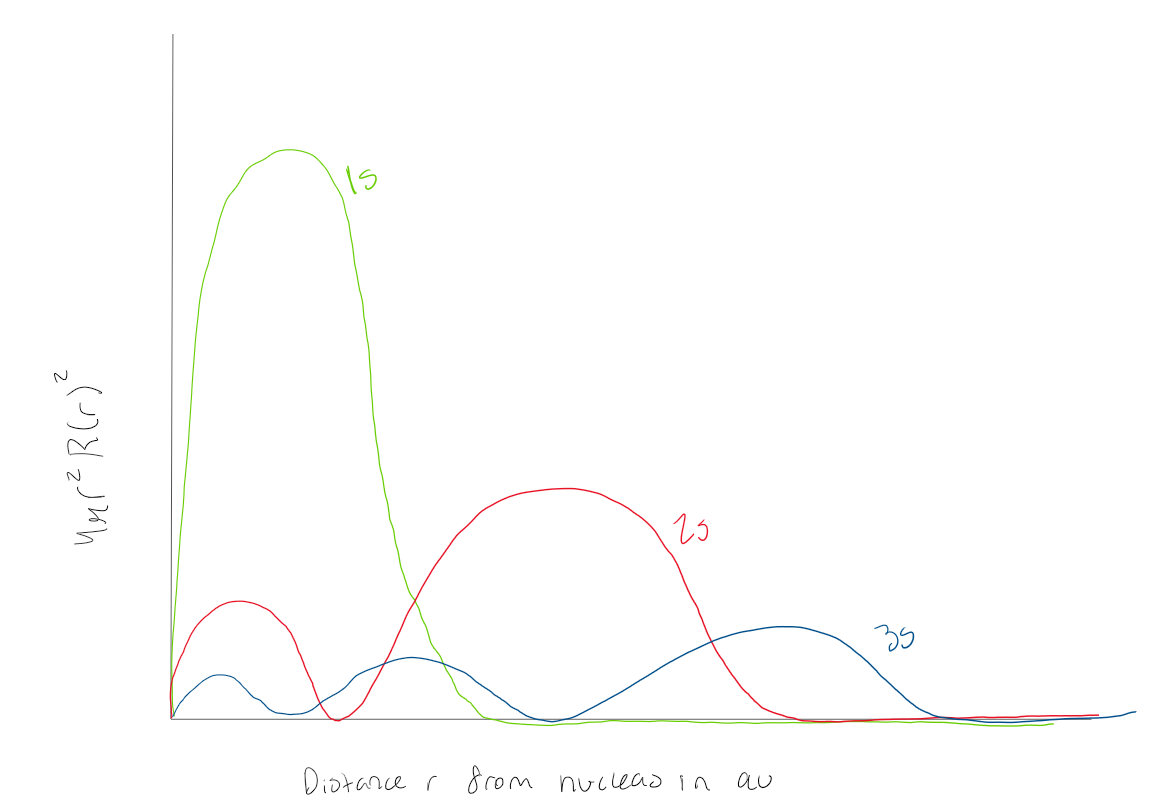
Using the graph, how many radial nodes does a 1s orbital have
0 radial nodes
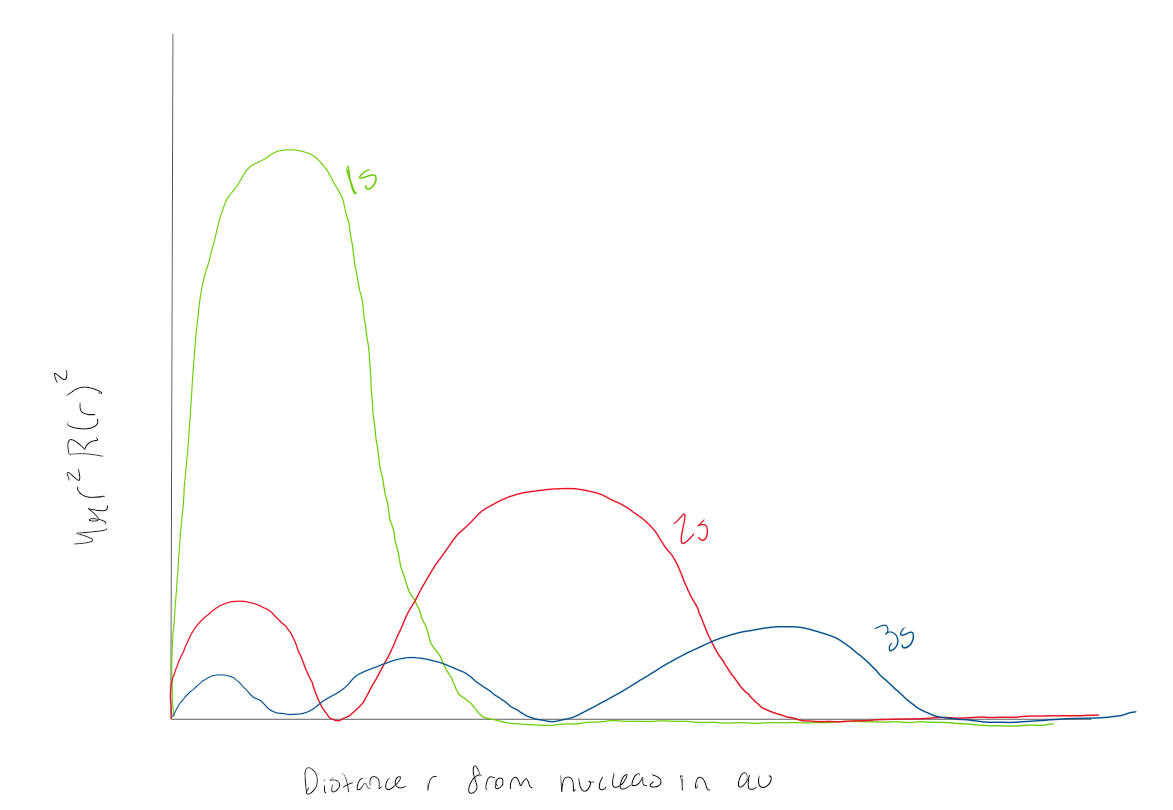
Using the graph, how many radial nodes does a 2s orbital have
1 radial node
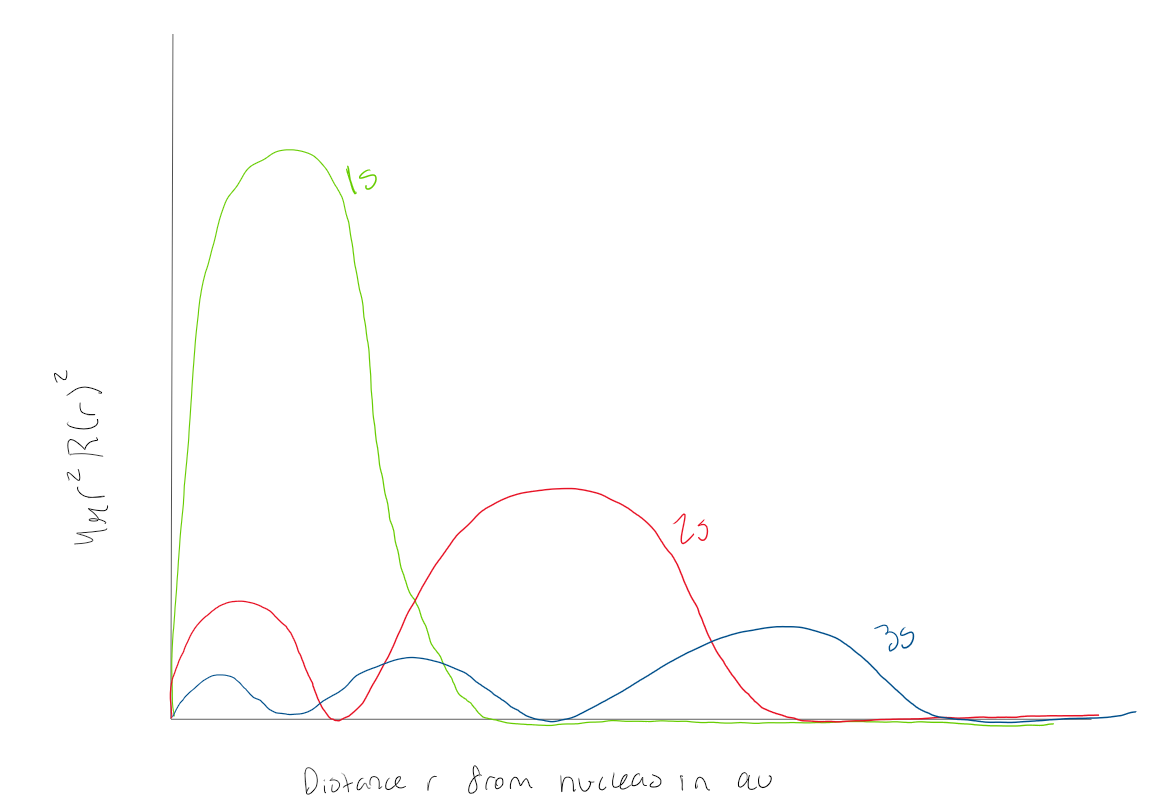
Using the graph how many radial nodes does a 3s atomic orbital have?
2 radial nodes
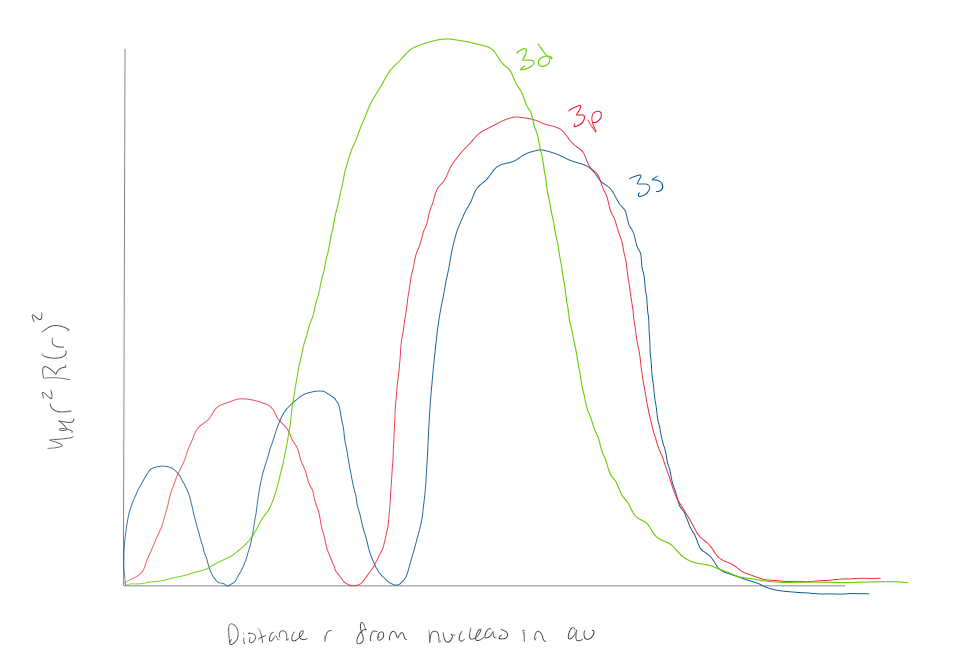
Using the graph how many radial nodes does a 3d orbital have?
0 radial nodes
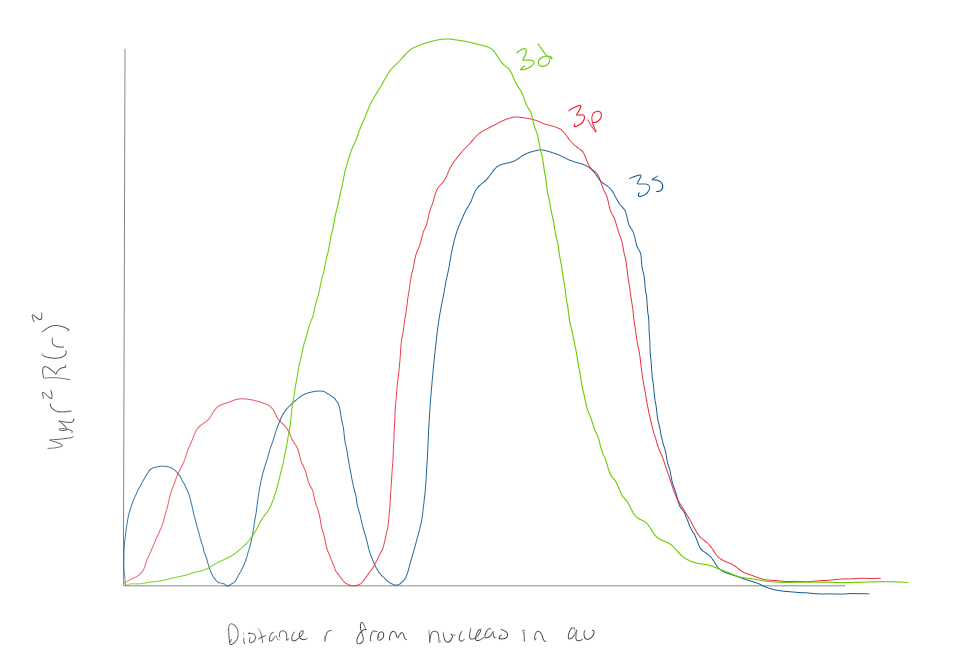
Using the graph, how many radial nodes does a 3p orbital have?
1 radial node
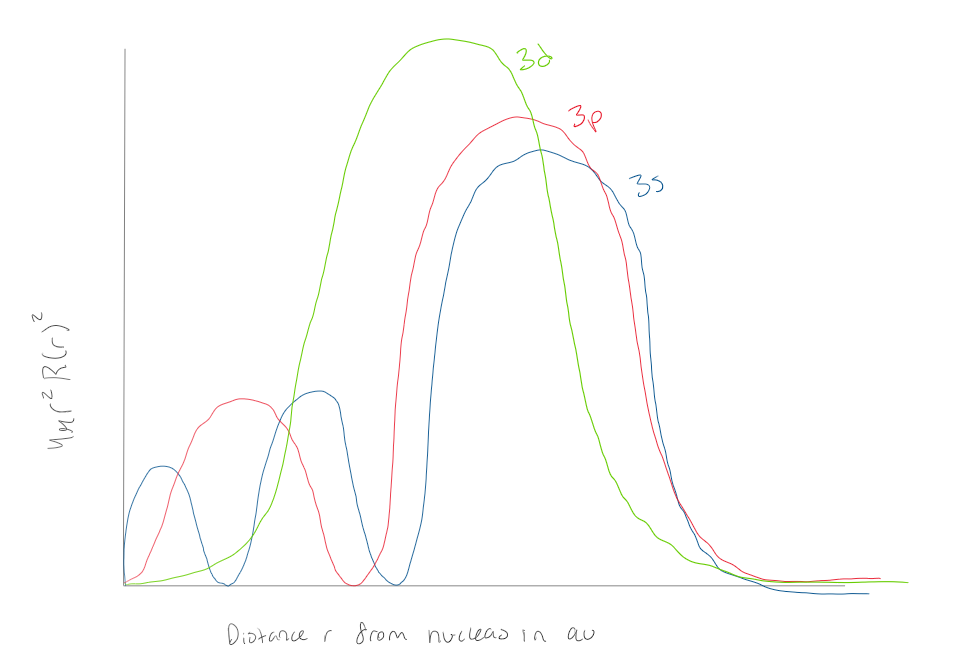
Using the graph how many radial nodes does a 3s orbital have?
2
The angular part of the wavefunction A(θ,Φ) is independent of
n
For s orbitals, A(θ,Φ) is independent of
θ and Φ
E (orbital energies) =
-(kZ2)/n2 (where Z = atomic number and k = hcR = 1.312e3 kJ mol-1)
Orbital energy levels get closer as
n increases
The larger the n the larger the
size of the orbital
And increase in orbital size corresponds to an increase in
an orbital being more diffuse
S (spin quantum number) =
1/2
ms (magnetic spin quantum number) =
± 1/2
Orbital angular momentum =
Sqrt(l(l+1))*(h/2pi)
spin angular momentum =
Sqrt(s(s+1))*(h/2pi)
total angular momentum =
Sqrt(j(j+1))*(h/2pi)
j (inner quantum number) =
|l+s| or |l-s|
In a multi-electron atom, orbitals with the same value of n but different values of l are
NOT degenerate
The IE of noble gases are
high
IE of group one elements are
low
IE increases
across a period
IE is similar for
a given row of d block elements
EA =
-ΔU (at 0K)
Adding an electron to an atom is typically
exothermic