6.2.3 Polyesters and polyamides
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What is condensation polymerisation?
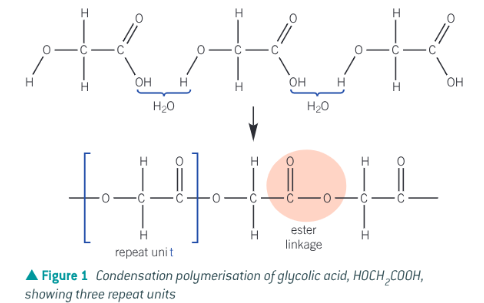
The joining of two monomers to make a polymer with the removal of a small molecule (i.e. water or HCl)
How are polyesters made?
By joining together monomer units by ester linkages to form a long chain via a condensation reaction since water is removed
What types of reactants are used to make polyesters?
Any of:
One monomer containing both carboxylic acid and alcohol group
One monomer containing two alcohol groups with one monomer containing two carboxylic acid groups
Using glycolic acid (HOCH2COOH) as an example, show how polyesters are made from one monomer containing two functional groups
Show the product of lactic acid (HOCH(CH3)COOH) undergoing condensation polymerisation to form poly(lactic acid) (PLA)
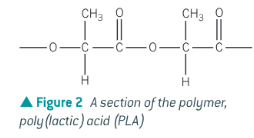
Why is lactic acid better than other polymers?
It is developed from maize so its production is more sustainable than polymers derived from fossil fuels
Terylene is a condensation polymer made from the reaction between the two monomers listed below. Show the formation of terylene (PET).
Benzene-1,4,-carboxylic acid (HOOCC6H4COOH)
Ethane-1,2-diol (HO(CH2)2OH)
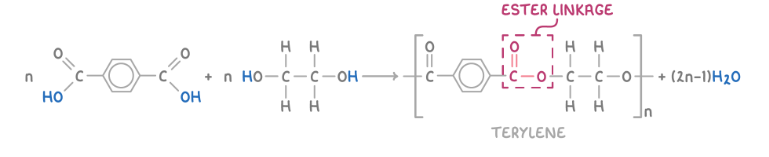
Give 2 applications of terylene
Plastic bottles and containers
Fibres for clothing and fabrics
What are poylamides?
Polyamides are condensation polymers formed when monomers are joined together by amide linkages in a long chain to form the polymer
What types of reactants are used to make polyamides?
One monomer containing both a carboxylic acid (or acyl chloride) and an amine group
Two monomers, one containing two carboxylic acid groups (or acyl chlorides) and the other containing two amine groups
What are polypeptides?
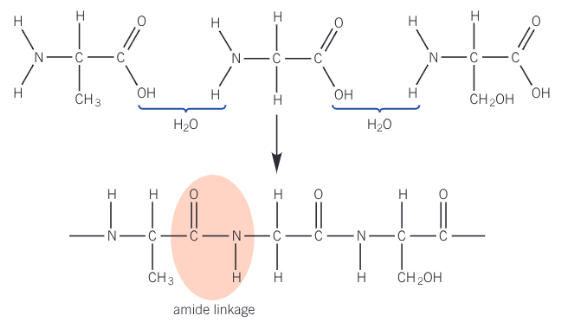
Polypeptides (proteins) - formed when many amino acids are linked together by amide bonds via condensation polymerisation
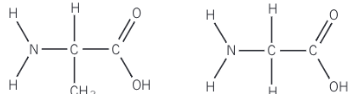
Show the polypeptide formed from these monomers
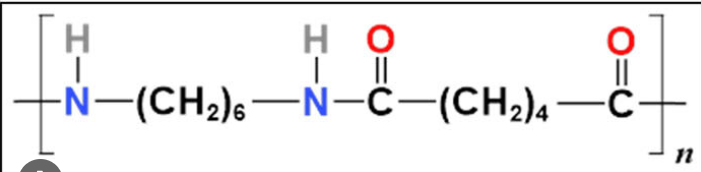
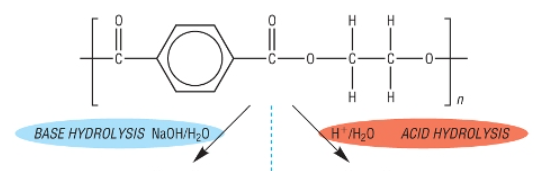
What are the 2 monomers of nylon-6-6?
Hexanedioic acid
1,6-diaminohexane
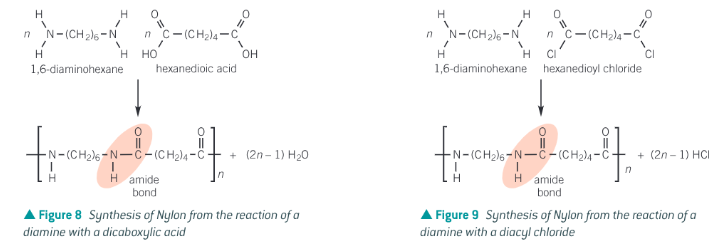
Show the 2 ways of forming nylon-6-6
Diamine + dicarboxylic acid
Diamine + diacyl chloride
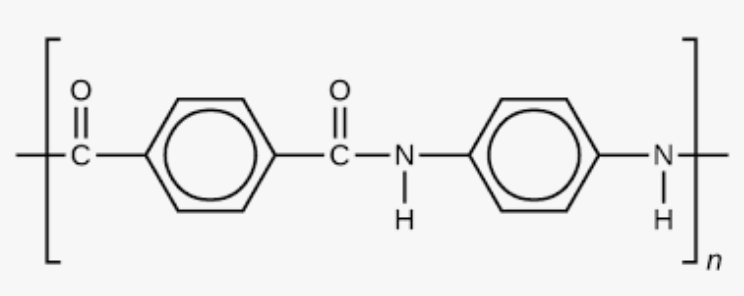
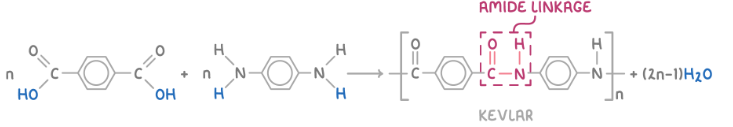
What are the monomers used to form kevlar?
1,4-dicarboxylic acid
1,4-diaminobenzene
Show the formation of kevlar
Define hydrolysis
The breaking of a bond with a water molecule
What is hydrolysis like in polyesters and polyamides?
Ester bonds (polyesters) or amide bonds (polyamides) are broken
What conditions are polyesters and polyamides hydrolysed under?
Hot aqueous acidic or hot aqueous alkaline conditions
What are the conditions for hydrolysis always?
Aqueous acid or aqueous alkali in the presence of heat
Why is aqueous conditions needed for hydrolysis?
Provides water needed for the hydrolysis
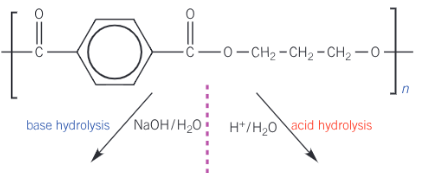
Complete the flow diagram for Poly(Trimethylene Terephthlate) to show the two types of polyester hydrolysis
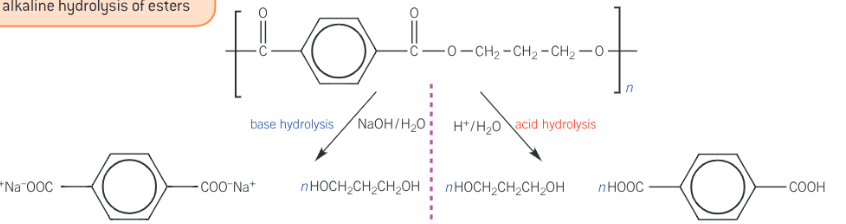
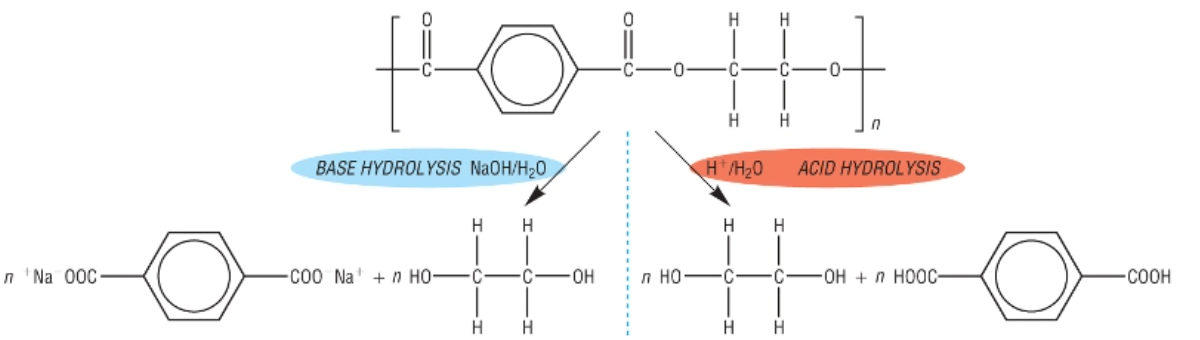
Show the acid and alkali hydrolysis of terylene
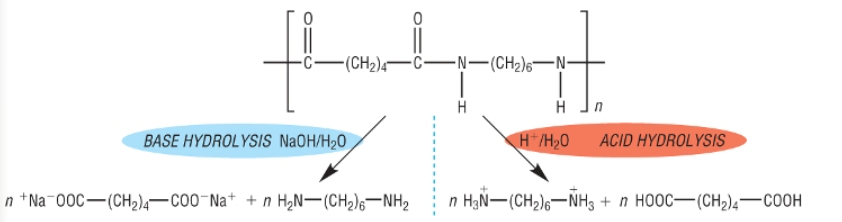
Show the acid and alkali hydrolysis of nylon-6,6
Define a biodegradable polymer
A polymer that breaks down completely into carbon dioxide and water
Define a degradable polymer
A polymer that breaks down into smaller fragments when exposed to heat, light or moisture
Define a photodegradable polymer
A condensation polymer where the C=O bond absorbs radiation that has sufficient energy to facilitate the decomposition of the polymer