Types and Properties of Matter with Changes Quiz
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
What is matter?
Anything that has mass and takes up space (has volume)
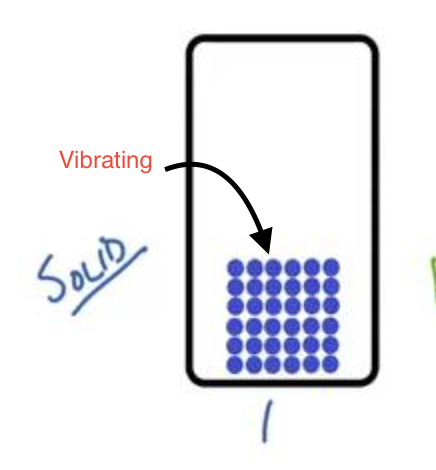
What is a solid?
Matter that has a definite shape (cannot flow), a definite volume, particles are tightly packed, and it is not compressible.
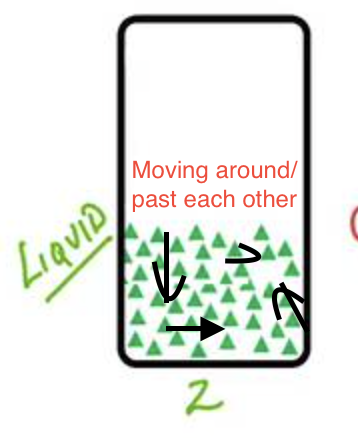
What is a liquid?
Matter that has a definite volume, but takes the shape of it’s container (flows), particles are less tightly packed, and it is not really compressible.
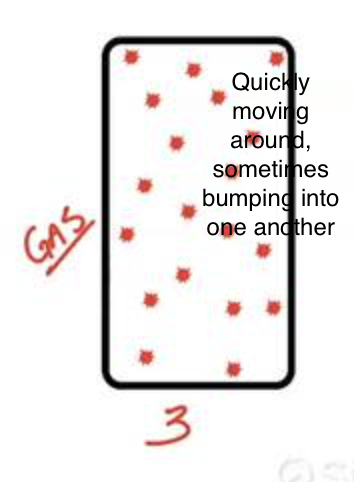
What is a gas?
Matter that takes the shape of it’s container and fills the entire volume of it’s container, particles are far apart, and it is very compressible.
Law of conservation of mass
Mass is neither created nor destroyed during ordinary chemical reactions or physical changes
Law of conservation of energy
Energy cannot be created or destroyed
What is an atom?
Smallest unit of an element that still has the same chemical identity as the element
What is an element?
Pure substance that cannot be broken down into simpler stable substances; made up of 1 type of atom.
What is a compound?
Pure substance made of three or more atoms of different elements joined by chemical bonds
What is a heterogenous mixture?
Mixture that does not blend smoothly throughout, and in which the individual substances stay distinct
What is a homogenous mixture/solution?
Mixture that has constant composition throughout; always has a constant phase
What is a pure substance?
Substance with a uniform and unchanging composition, with only one type of particle
What is an intensive property, and examples?
A characteristic that does not depend on the amount of substance (e.g, density, color, and boiling point, melting point, conductivity, etc)
What is an extensive property and examples?
A characteristic that depends on the amount of matter (e.g mass, volume, amount of energy, concentration, etc)
What is a physical property and examples?
Characteristic that can be observed or measured without changing the sample’s composition, so the substance stays the same (e.g, color, density, odor, hardness, melting point, boiling point, etc)
What is a chemical property and examples?
Characteristic of a substance’s ability to go through changes that will produce a different substance (e.g, rusting, oxidation, flammability, reactivity, toxicity, etc.)
What is a physical change and an example?
Alters substance without changing chemical composition (ex: shattering glass)
What is a chemical change?
Process involving one or more substances being changed into a new one (AKA chemical reaction), always changes properties
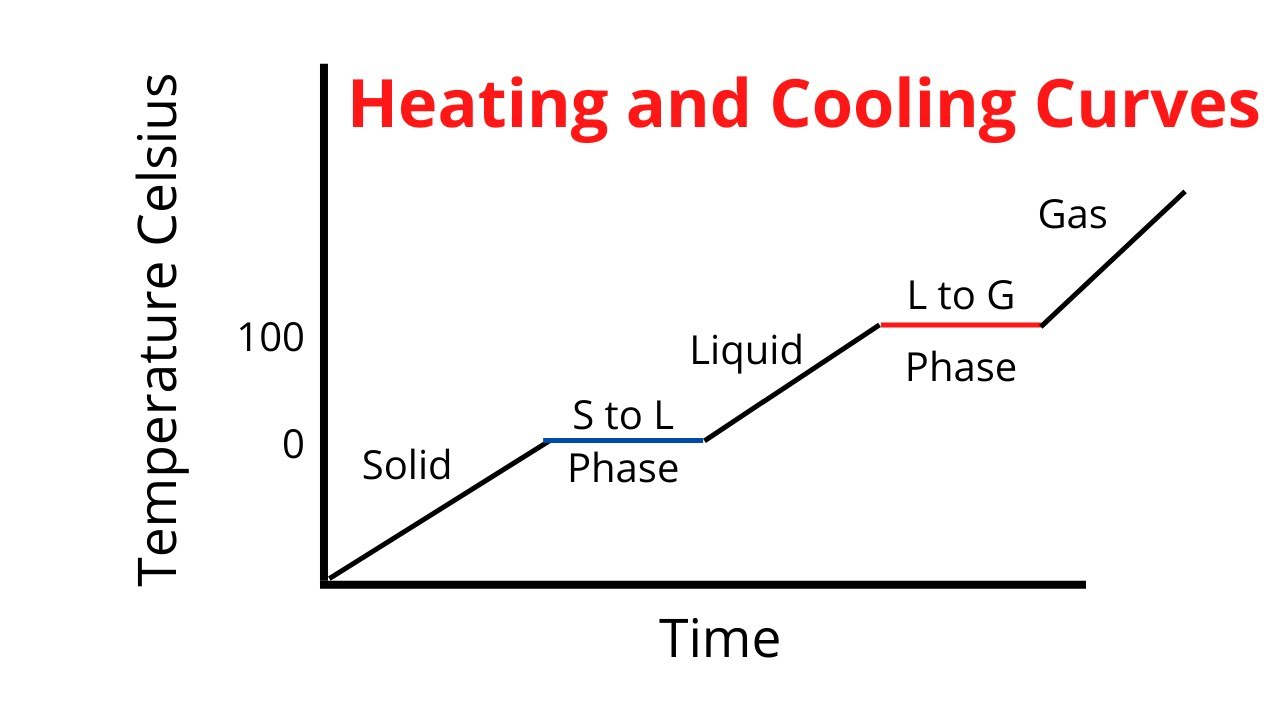
What is a phase change and examples?
A type of physical change where matter transitions from one state to another (e.g melting, freezing, etc.)
Solid to liquid
Melting
Liquid to solid
Freezing
Solid to gas
Sublimation
Gas to solid
Deposition
Gas to liquid
Condensation
Liquid to gas
Vaporization
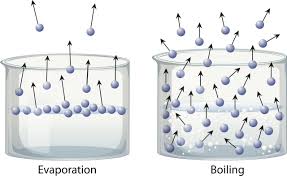
Types of vaporization
Evaporation - Sun adds kinetic energy to molecules at the top of water, causing them to move faster, break attractive forces, and become a gas
Boiling - Heat is added directly. Gas forms within the liquid, then escapes
What is a phase?
Any part of a system that has uniform properties and composition
What is a heat curve diagram?
Graph showing how a substance changes as it is heated
What is a phase diagram?
A graph of pressure vs. temperature that shows the conditions under which the phases of a substance exist
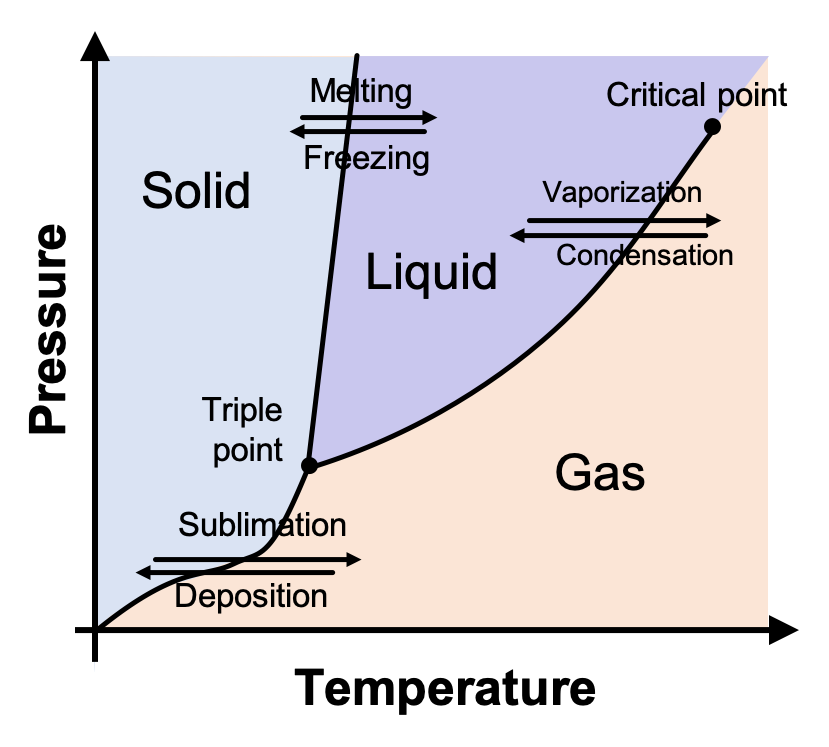
What is a triple point?
Temperature and pressure conditions where solid, liquid, and vapor can exist at equilibrium
What is a critical point?
Shows the temperature and pressure where a substance can no longer exist as a liquid
4 signs of a chemical change
Transfer of energy
Color change*
Gas production (besides boiling)
Precipitate formation (liquid + liquid = solid, think milk + vinegar)
Law of conservation of mass/energy in a candle
Mass: Wax (hydrocarbons) + O₂ → CO₂ + H₂O
The candle looks smaller, but atoms aren’t lost — they turn into gases that float away.
Energy: Chemical energy in wax → light + heat energy
Energy isn’t destroyed, just transformed.
Law of conservation of mass/energy in chromatography
Mass: The ink/pigment mixture doesn’t disappear — molecules just separate as they move with the solvent. Each pigment’s atoms are still present, just spread out on the paper.
Energy: No energy is destroyed — it’s mainly the solvent’s kinetic energy (movement of molecules) that carries pigments along.