ch1. lasers and fibres
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
characteristics of laser (4)
- highly monochromatic
- highly coherent
- highly directional
- sharply focused
(not found in ordinary night)
types of emmision
spontaneous emmision, stimulated emmision
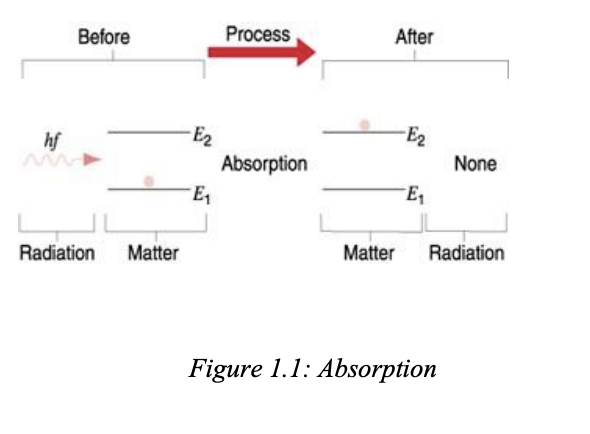
absorption
when radiation (photon) of a frequency hits a atom and basically it goes to a higher energy level from e1 to e2
ok so the wiggly line is the photon and the ball is an atom
"Absorption of a photon of frequency f takes place when the energy difference E2 – E1 of the allowed energy states of the atomic system equals the energy hf of the photon. Then the photon disappears and the atomic system moves to upper energy state E2.”
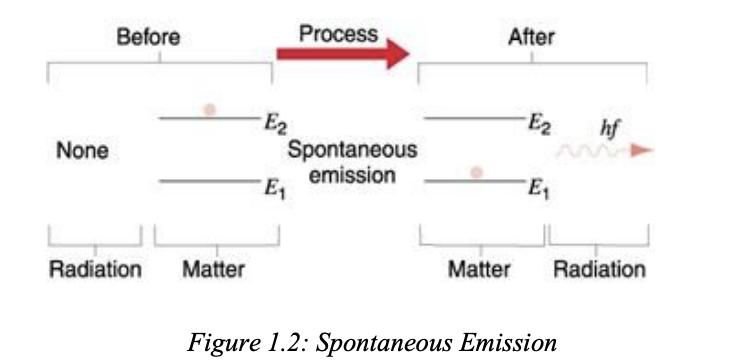
spontaneous emmision
okok so basically like the photon is already at e2 right. and so the lifetime here is 10-8 sec so it cant stay there longer than that. so when it comes back down, it releases this ray thingy of radiation, but its like super scattered and random which cant be used as a laser,
“Spontaneous emission is the emission of a photon when a system transits from a higher energy state to a lower energy state without the aid of any external agency.
The average life time of the atomic system in the excited state is of the order of 10-8 s.
After the life time of the atomic system in the excited state, it comes back to the state of lower energy on its own accord by emitting a photon of energy hf = E2– E1 .”
time of atomic system in excited state
The average life time of the atomic system in the excited state is of the order of 10-8 s.
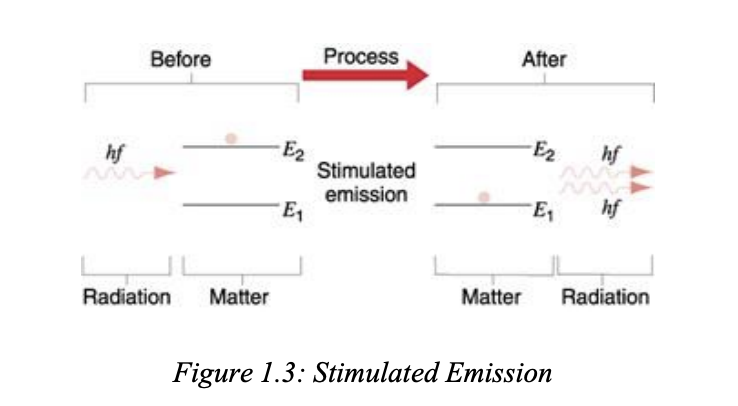
stimulated emmision
so like the atom is already at e2 right, but the thing is if it comes down by itself the radiation will be random and scattered and not laser. if theres an additional photon/ radiaiton sent then it will come back down at 10-3 seconds (metastable state), so it will come down faster
and like the radiaiton coming out is same frequency, state of polarisation, so it causes laser.
“When a photon (called stimulating photon) of suitable frequency interacts with an excited atomic system, the latter comes down to ground state before its life time.
In stimulated emission, both the stimulating photon and the stimulated photon are of same frequency, same phase and are in same state of polarization.
These photons are emitted in the same direction.”
Stimulated emission occurs when an excited atomic system emits a photon after being triggered by another photon of the same frequency, resulting in two coherent photons moving in the same direction.
polarization
light is like a bunch of tiny jump ropes and polarization means theyre moving in the same direction
They wiggle at the same speed (same frequency),
They wiggle together (same phase),
And they wiggle in the same direction (same polarization).
Polarization in stimulated emission means that the electric fields of both the stimulating and the emitted photons oscillate in the same direction. This makes them more coherent and is one of the reasons laser light is so uniform and powerful.
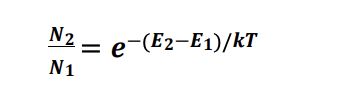
population inversion
usually there are like more atoms in e1 than e2 but for laser basically like we need stimulated emmision right but we cant do that when theres soo less atoms in e2, so population inversion bascially just brings the ones at the bottom to the top through metastable state
k= boltzman constant
N1= density of atoms with energy E1
N2= density of atoms with energy E2
Population inversion is the condition where a greater number of atoms or molecules are in an excited state than in a lower energy state, allowing for stimulated emission to dominate over spontaneous emission.
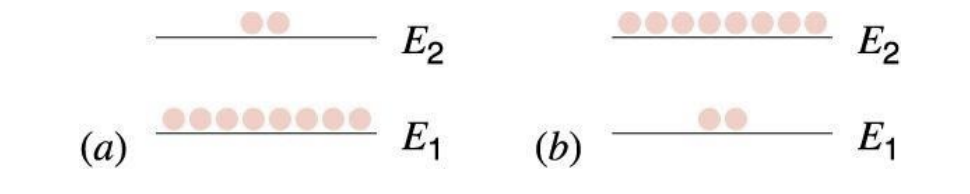
metastable state
a place higher than e2 (10-3 sec) so that atoms can undergo stimulated emmision
einstein’s coefficients (3 cases)
how likely it is for atom to fall or rise to different energy levels.
(i) absorption
(ii)
(iii)
(i)
Einstein's coefficients are parameters that describe the probability of stimulated emission, spontaneous emission, and absorption processes in atomic systems, relating the transition rates between energy levels
[stuff]
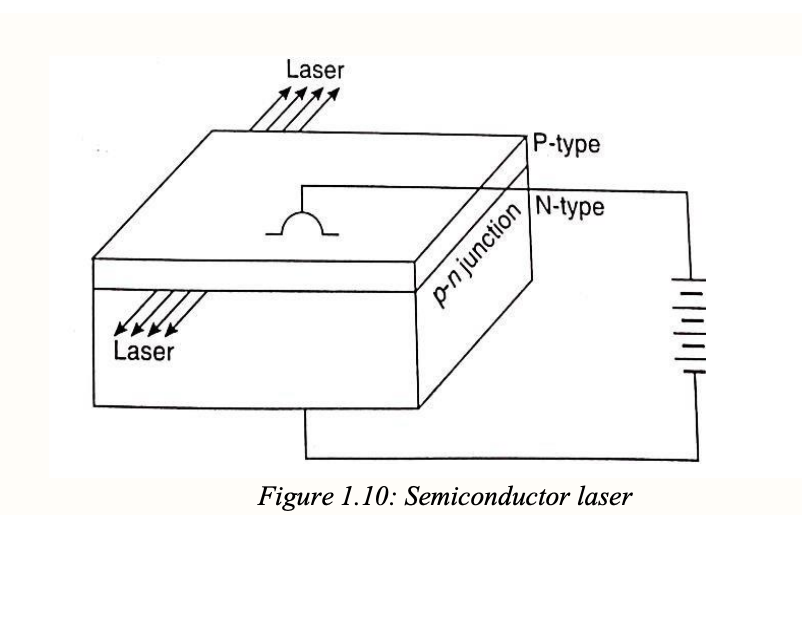
semiconductor laser
so basically like the holes and electrons right so like when an electron enters a hole it releases the light and theres like a window in the semiconductor so like there is a way for the light to come out
Semiconductor laser explanation using analogy:
Applying voltage (toll) lets electrons (cars) cross and recombine with holes (parking spots).
Recombination releases photons (honks).
Photons bounce between mirrors, stimulating more honking — this is stimulated emission.
A small window in one mirror lets out a coherent, directional beam of light — the laser.
applications of laser
barcode scanner
laser printer
laser cooling
optical fibers
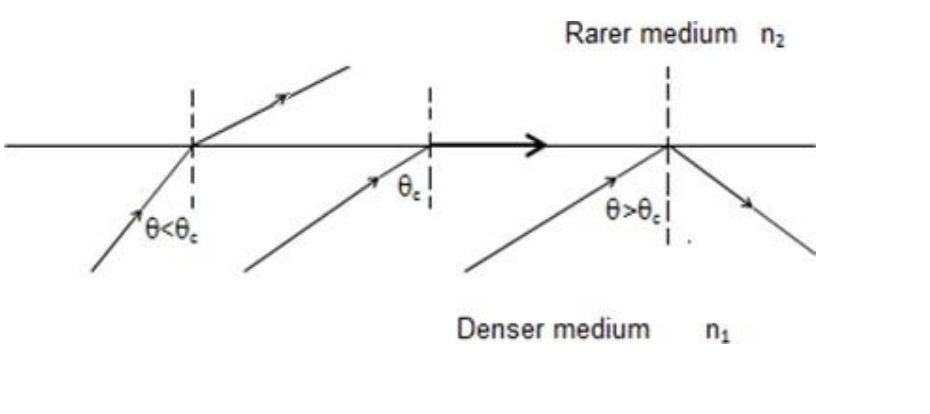
total internal reflection
most superior type of reflection (slay)
almost the entire energy is returned to the first medium through relection without
optical fiber
used to guide ir and visible light waved through a curved path
basically like its special bc the outer cladding thing has a higher refractive index compared to the inside thing so the light can go through total internal reflection inside it
numerical aperture