CHS1440 - Dixon Final Exam Review
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
Which of the following represents a physical change?
1. When the element reacts with the base, a gas is given off
2. The metal rusts when exposed to air and moisture
3. A block of metal has a volume of 7.3 cm^3 and a mass of 18.5g
4. The element melts when heated gently
a) 2 only
b) 1 only
c) 4 only
d) 3 and 4
e) all 1, 2, 3, and 4
c) 4 only
The name of the compound is copper(II) chloride. What is the charge of the chloride ion?
a) the charge is 2+.
b) the charge is 2-
c) the charge is 1+
d) the charge is 0
e) the charge is 1-
e) the charge is 1-
Select the substance in the current state that will exhibit metallic bonding.
a) NaCI
b) Na
c) F2
d) HCOOH
e) BaO
b) Na
Give the name of the following compound: N205
a) pentanitrogen dioxide
b) nitrogen oxide
c) dinitrogen pentoxide
d) nitric oxide
e) nitrate
c) dinitrogen pentoxide
Which of the following properties of a typical metal like Al, are chemical properties?
1. It is thin II
2. It floats on water III
3. It forms a basic oxide IV
4. It is very shiny.
a) 1 and 2
b) 2 and 3
c) 3 only
d) 1 and 4
e) all of 1 to 4
c) 3 only
Calculate the following to the correct number of significant figures.
[(28.7 x 10^5) ÷ 48.533] + 144.99
a) 1.18x10^2
b) 1.18x10^3
c) 5.93 x 10^4
d) 6.46 x 10^4
e) 5940
c) 5.93 x 10^4
The compound has a boiling point of -162 °C. What is this temperature in Kelvin?
a) 223 °K
b) -12 °K
c) 55 °K
d)111 °K
e) 340 °K
d)111 °K
Which of the following statements is/are correct?
1. Electrons and protons have identical masses and charges.
2. Most of an atom's mass is concentrated in a large, neutral nucleus.
3. All the atoms of an element have the same number of neutrons.
a) 1 only
b) 2 only
c) 1 and 3
d) 1, 2, 3 are incorrect
e) 1 and 2
d) 1, 2, 3 are incorrect
The density of iron is 7.86 g/cm^3, and the volume of a cube is the edge length cubed, or l^3. What is the edge length of a 155-g iron cube?
a) 11.9 cm
b) 1.99 cm
c) 2.70 cm
d) 1.22 cm
e) 5.37 cm
c) 2.70 cm
The group 17 (or group 7A) elements are commonly known as the family of
a) halogens
b) alkali metals
c) noble gases
d) transition metals
e) alkaline earth metals
a) halogens
Which of the following is an example of a molecular compound?
a) CaCl2
b) Na2CO3
c) C2H5COOH
d) LiCN
e) KNO3
c) C2H5COOH
Select the incorrect statement from the set below.
a) Accuracy indicates how near the measured value is compared to the 'true' value.
b) A series of precise measurements have values which are not close together.
c) A net of measurements can be both inaccurate and imprecise.
d) A set of measurements can be both accurate and precise.
e) A set of measurements can be precise but inaccurate.
b) A series of precise measurements have values which are not close together.
How many significant figures are in the following reported number: 312,000 mg?
a) 6
b) 4
c) 3
d) 1
e) unlimited
c) 3
An element has three naturally occurring isotopes with the following masses and natural
abundances:
- Isotope A
mass: 23.9850
Abundance (%): 78.99
- Isotope B
mass: 24.9858
Abundance (%): 10.00
- Isotope C
mass: 25.9826
Abundance (%): 11.01
What is the identity of the element above?
a) Al
b) P
c) K
d) Ne
e) Mg
e) Mg
Which pair of elements belong to the set of alkaline earth metals?
a) Ti, Sn
b) Co, Bi
c) Cu, Ce
d) Mg, Ba
e) Ar, Se
d) Mg, Ba
15. If a vehicle is traveling 30. m/s, what is its velocity in miles per hour, mi/hr? (0.62 miles = 1.00 km; 1000 m = I km; 60 s = I minute, 60 minutes = 1 hr, etc....)
a) 2.7 mi/hr
b) 9.1 mi/hr
c) 98 mi/hr
d) 67 mi/hr
e) 290 mi/hr
d) 67 mi/hr
The cations from the alkali metals have a charge of what?
a) 1+
b) 2+
c) 3+
d) 1-
e) 0
a) 1+
How many atoms of oxygen are in the following hydrated ionic compound:
Fe3(PO4)^2 ⋅ 8H20?
a) 7
b) 70
c) 4
d) 16
e) 10
d) 16
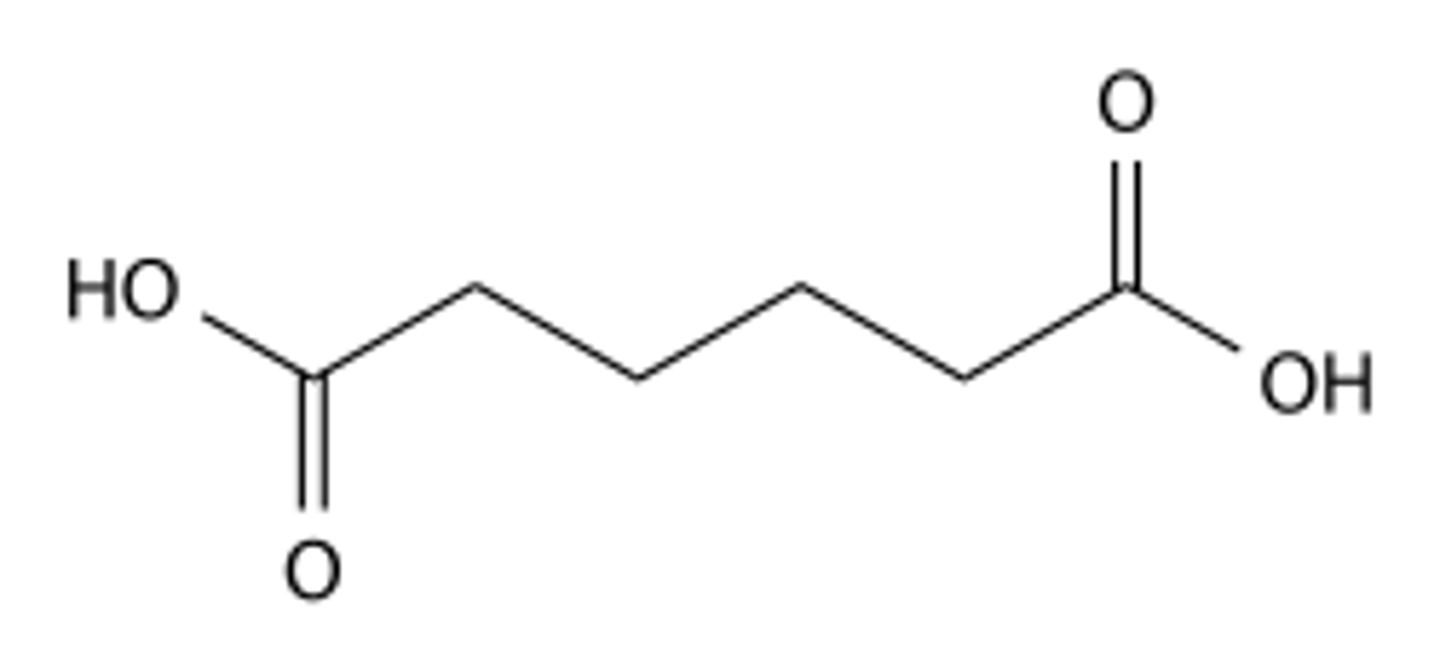
What is the molecular formula of the following compound? (see image)
a) C5H2O4
b) C10H7O5
c) C6H10O4
d) CsF11204
e) CsH402
c) C6H10O4
Which of the following is most likely an empirical formula?
a) Si4H10
b) CH4
c) C6H6
d) S4N4
e) C6H12O6
b) CH4
With respect to covalent bonding, what is the best description of the region between the molecules?
H2C=CH2
a) a lone pair of electrons
b) a double bond
c) a single bond
d) a triple bond
e) the electron 'sea'
b) a double bond
How many valence electrons are present in Selenium?
a) 3
b) 7
c) 8
d) 1
e) 6
e) 6
Hund's rule states that a set of p or d orbitals in an atom:
a) has a filled valence shell of electrons
b) has two electrons per orbital, each with the same spin
c) has ml values greater than or equal to zero
d) has the maximum number of unpaired electrons, all with the same spin
e) can accommodate two electrons, and each electron must have opposing spin
d) has the maximum number of unpaired electrons, all with the same spin
Which d-block element has the ground state electron configuration: [Ar]4s^1 3d^10
a) V
b) Zn
c) Cu
d) Fe
e) Ru
c) Cu
Mandelic acid is an organic acid composed of 63.15% carbon, 5.30% hydrogen, and 31.55% oxygen. What is the empirical formula for the acid?
a) C3H6O3
b) C8H8O3
c) C7H12O2
d) C8H5O
e) C4H4O
b) C8H8O3
Select the element that is expected to have the largest first ionization energy.
a) K
b) Ar
c) Cl
d) Be
e) As
b) Ar
The products of the complete combustion of a hydrocarbon are carbon dioxide and water. Write a balanced chemical equation for the combustion of pentane, C5H12.
a) C5H12(g) + 8O2 = 5CO2(g) + 6H20(g)
b) C5H12(g) = 5 C(s) + 6H2(g)
c) C5H12(g) + 9O2(g) = 4CO2(g) + 5H2O(g)
d) C5H12(g) + 11O2(g) = C5O10(g) + 6H2O(g)
e) C5H12(g) + 11O2(g) = 5CO2 + 12H2O(g)
a) C5H12(g) + 8O2 = 5CO2(g) + 6H20(g)
Write the ground state electron configuration for bromine.
a) [Ar]4s^3 3d^14
b) [Ar]4s^2 3d^10 4p^5
c) [Ar]4s^1 3d^6 4p^10
d) 1s^2 2s^2 2p^7 3s^2 3p^6 3d^10 4s^2 4p^5
e) [Ar]3d^5 4s^2
b) [Ar]4s^2 3d^10 4p^5
What is the correct polyatomic ion in the folowing hydrated ionic compound: Fe3(PO4)2 ⋅ 8H2O ?
a) Fe^3+, iron(III)
b) H2O, water
c) OH^-, hydroxide
d) PO4^-, phosphite
e) PO4^3-, phosphate
e) PO4^3-, phosphate
Which of the following compounds is considered insoluble in water?
a) NaF
b) AgNO3
c) PbS
d) KBr
e) (NH4)2SO4
c) PbS
In a laboratory experiment, a student heats 42.0 g of NaHCO3 and determines that 22.3 g of the Na2CO3 is formed. What is the percentage yield of this reaction?
a) 63.1%
b) 42.0%
c) 22.3%
d) 84.2%
e) 53.0%
d) 84.2%
Place the following atoms in increasing atomic radii: Si,Sr, Ne and Cl.
a) Ne, Cl, Si, Sr
b) Si, Sr, Ne, Cl
c) Si, Sr, Cl, Ne
d) Ne, Sr, Cl, Si
e) Sr, Si, Cl, Ne
a) Ne, Cl, Si, Sr
What is the molarity of a solution of nitric acid, if a 0.216 g sample of barium hydroxide is required to neutralize a 20.0 mL sample of nitric acid? Molar mass Ba(OH)2 = 171.34 g.
2HNO3(aq) + Ba(OH)2(aq) = Ba(NO3)2(aq) + 2H2O
a) 0.252 M
b) 0.126 M
c) 0.0630 M
d) 1.17 M
e) 0.218 M
b) 0.126 M
Choose the compound below that is considered a strong base and also a strong electrolyte.
a) HNO3
b) H3PO3
c) CH3OH
d) KOH
e) CH3CO2H
d) KOH
How many electrons are represented by the following quantum numbers?
n=4, l=1, ml = -1, ms= -1/2
a) 1
b) 2
c) 6
d) 10
e) 14
a) 1
The laser in most supermarket barcode scanners operates at a wavelength of 632.8 nm. What is the energy of one mole of these photons?
a) 189 kJ
b) 1.60x10^22 kJ
c) 7.60x10^22 kJ
d) 121 kJ
e) 1.54 kJ
a) 189 kJ
Combustion of C2H3Cl(liq) yeilds carbon dioxide, steam, and hydrogen chloride gas. If 25.00 g of C2H3Cl(liq) react, what mas of oxygen gas, O2, react? Molar mass C2H3Cl = 62.49 g/mol.
2C2H3Cl(liq) + 5O2 = 4CO2(g) + 2H2O(g) + 2HCl(g)
a) 20.01 g
b) 82.51 g
c) 16.00 g
d) 64.10 g
e) 32.00 g
e) 32.00 g
Which of these represents the electron affinity of S?
a) S(g) + e^- = S^-(g)
b) S^-(g) +e^- = S^2-(g)
c) S(g) = S^+(g) + e^-
d) S^-(g) = S(g) + e^-
e) S^2-(g) = S^-(g) + e^-
a) S(g) + e^- = S^-(g)
Which is the net ionic equation for the reaction between aqueous potassium hydroxide and aqueous sulfuric acid?
a) H^+(aq) + KOH(aq) = H2O(l) + K^+(aq)
b) H^+(aq) + OH^-(aq) = H2O(l)
c) H2SO4(aq) + 2KOH(aq) = 2H2O(l) + K2SO4(aq)
d) 2K^+(aq) + SO4^2-(aq) = K2SO4(aq)
e) KOH(aq) + H2O(l) = H^+(aq) + K(OH)2(s)
b) H^+(aq) + OH^-(aq) = H2O(l)
Which of the following is a valid set of quantum numbers?
a) n=3, l=3, ml=0
b) n=2, l=1, ml=+1
c) n=6, l=5, ml=-6
d) n=3, l=1, ml=-2
e) n=2, l=3, ml=-3
b) n=2, l=1, ml=+1
Determine the initial volume of methanol, in L, needed to generate 10.0 L of a 0.900 M solution from a 3.00 M solution of methanol.
a) 1.35 L
b) 3.00 L
c) 4.55 L
d) 10.5 L
e) 7.50 L
b) 3.00 L
Select the molecule that is most likely to be a liquid at ordinary temperature and pressure.
a) F2
b) HOC2H4OH
c) N2
d) CH4
e) CO2
b) HOC2H4OH
Select the element that is most likely to have the largest band gap.
a) Zn
b) S
c) Cu
d) Ag
e) Si
b) S
In relation to ions and ionic bonding, select the compound having the weakest ionic bonds.
a) LiF
b) KI
c) CaO
d) MgO
e) Al2O3
b) KI
If 19.6 L of H2S at 722 torr and 17 °C reacts with excess SO2, what mass of solid sulfur could be produced?
2H2S(g) + SO2(g) = 3S(s) + 2H2(g)
a) 2.55 g
b) 37.6 g
c) 52.0 g
d) 25.1 g
e) 12.8 g
b) 37.6 g
Choose the substance that corresponds to a p-type semiconductor.
a) Ge doped with As
b) Si doped with Al
c) Si doped with As
d) Ge doped with P
e) Si doped with P
b) Si doped with Al
Identify the bond with the highest bond energy
a) C≡O
b) C=C
c) C=N
d) N-N
e) C-O
a) C≡O
Which carbon containing substance could be used in making pencils and lubricants?
a) CO2
b) Diamond
c) Fullerene
d) Graphite
e) Na2CO3
d) Graphite
Place the following elements in order of increasing electronegativity:
Ba, C, Sc
a) Ba < Sc < C
b) Sc < C < Ba
c) Ba < C < Sc
d) C < Ba < Sc
e) C < Sc < Ba
a) Ba < Sc < C
Which molecule has the largest dispersion forces?
a) H2
b) I2
c) Cl2
d) Br2
e) F2
b) I2
Dipole-dipole forces are present in which of the following?
1. CH4
2. F2
3. O2
a) 1 only
b) 2 only
c) 3 only
d) all of them
e) none
e) none
Which of the following gases occupies the smallest volume at STP?
a) CH4
b) O2
c) N2
d) CO2
e) F2
d) CO2
How many lone pairs and bond pairs are present on the central atom in the H2S molecule?
a) 3 bond pairs; 0 lone pairs
b) 4 bond pairs; 2 lone pairs
c) 2 bond pairs; 2 lone pairs
d) 3 bond pairs; 2 lone pairs
e) 5 bond pairs; 0 lone pairs
c) 2 bond pairs; 2 lone pairs
Predict the three dimensional or molecular shape of SF4.
a) Tetrahedral
b) See-Saw
c) Square Pyramidal
d) Octahedral
e) Bent
b) See-Saw
Select the molecule that has 2 resonance structures
a) H2O
b) CH4
c) SF4
d) XeF2
e) SO2
e) SO2
At a constant temperature and pressure STP what volume of NH3(g) in L will be produced if 3.6 L of H2(g) react?
N2(g) + 3H2(g) = 2NH3(g)
a) 3.6 L
b) 2.4 L
c) 4.2 L
d) 1.2 L
e) 0.36 L
b) 2.4 L
How many electrons, per chromium atom, are delocalized in the sea of electrons?
a) 8
b) 18
c) 6
d) 5
e) 2
c) 6
Which one of the following is expected to form inter-molecular hydrogen bonds in the liquid state?
a) CH3OH
b) CHCl3
c) Br2
d) CH4
e) CH3OCH3
a) CH3OH
Which of the following is not generally true of gases?
a) Gases have lower densities than solids or liquids
b) Gases expand to fill the volume of a container
c) Gases have variable densities depending on conditions
d) Some gases tend not to mix readily or thoroughly with other gases
e) Gas volume can change with changing temperature
d) Some gases tend not to mix readily or thoroughly with other gases
Select the correct statement when comparing the properties of ethane, C2H6, with those of water, H20.
a) Water and ethane have the same vapor pressure
b) Water has a higher vapor pressure
c) Water has a lower boiling point
d) Ethane has a lower boiling point, but a higher vapor pressure
e) Ethane has a lower vapor pressure, because it has a larger molar mass
d) Ethane has a lower boiling point, but a higher vapor pressure
Select the correct bonding information for the diatomic nitrogen molecule.
a) N2 has 3 sigma bonds
b) N2 has a triple bond, but one is a sigma bond
c) N2 has 3 pi-bonds
d) N2 has 2 sigma bonds and a pi-bond
e) N2 has 2 sigma bonds and 2 pi-bonds
b) N2 has a triple bond, but one is a sigma bond
What is the electron configuration for iron(II) ion, Fe^2+ ?
a) [Ar] 3d^8
b) [Ar] 4s^2 3d^6
c) [Ar] 3d^4
d) [Ar] 4s^1 3d^5
e) [Ar] 3d^6
e) [Ar] 3d^6
Use the information provided to determine the heat of reaction, ΔHrxn^0, for the reaction:
Fe3O4(s) = 3Fe(s) + 2O2(g) ΔHrxn = ?
ΔH1^0 Fe3O4(s) = -1118 kJ/mol
a) +2236 J
b)+1118 kJ
c) +155 kJ
d) -2300 kJ
e) -1118 kJ
b)+1118 kJ
Non-ideal behavior for a gas is most likely to be observed under conditions of:
a) high temperature and low pressure
b) high temperature and high pressure
c) low temperature and low pressure
d) standard temperature and pressure
e) low temperature and high pressure
e) low temperature and high pressure
Calculate ΔHf for sulfur dioxide, SO2(g),
S(s) + O2(g) = SO2(g) ΔHf=?
Given the 2 thermochemical equations below.
2S(s) + 3O2(g) = 2SO3(g) ΔH=-791.5
2SO2(g) + O2(g) = 2 SO3(g) ΔH=-197.9
a) -296.8 kJ/mol
b) -395.7 kJ/mol
c) -494.7 kJ/mol
d) -593.6 kJ/mol
e) -989.4 kJ/mol
a) -296.8 kJ/mol
Which of the following linear chain alcohols is likely to have the highest standard entropy in the gaseous state?
a) CH3OH
b) CH3CH2OH
c) CH3CH2CH2OH
d) CH3CH2CH2CH2OH
e) CH3CH2CH2CH2CH2OH
e) CH3CH2CH2CH2CH2OH
In the first 15.0 s of the reaction, 0.015 mol of C is produced in a reaction vessel with a volume of 0.50 L. What is the rate of this reaction in this time interval?
2A = 2B + C
a) 5.0x10^-4 M/s
b) 3.5x10^-3 M/s
c) 2.0x10^-3 M/s
d) 1.0x 10^-2 M/s
e) 1.5x10^-5 M/s
c) 2.0x10^-3 M/s
If the enthalpy of fusion, ΔHfusion, of a substance is +43.4 kJ/mol, what is its
enthalpy of freezing?
a) 8.314 x ΔHfusion
b) -43.4 kJ/mol
c) +43.4 kJ/mol
d) +43.4 kJ/mol divided by the molar mass of the substance.
e) 43.4 kJ/mol x mol of substance.
b) -43.4 kJ/mol
Which reaction is likely to have a negative change in entropy?
a) 2 HNO3(l) + NO(g) = 3NO2(g) + H20(l)
b) FeCl2(s) + H2(g) = Fe(s) + 2HCl(g)
c) 2H20(g) = 2H2(g) + 02(g)
d)2CO(g) = 2C(s) + O2(g)
e) CH3OH(l) = 2H2(g) + CO2(g)
d)2CO(g) = 2C(s) + O2(g)
For which of the following substances is ΔGf=0?
a) Fe2O3(s)
b) H2O(g)
c) C4H10(g)
d) C(s; graphite)
e) CO2(g)
d) C(s; graphite)
Which statement is true regarding the evaporation of liquid water?
a) ΔH is positive, ΔS is positive, and ΔG is positive at low temperatures and negative at high temperatures
b) ΔH is negative, ΔS is negative, and ΔG is negative at low temperatures and positive at high temperatures
c) ΔH is negative, ΔS is positive, and ΔG negative at all temperatures
d) ΔH is positive, ΔS is negative, and ΔG is positive at all temperatures
e) The signs of ΔH and ΔS cannot be determined
a) ΔH is positive, ΔS is positive, and ΔG is positive at low temperatures and negative at high temperatures
The sign of ΔHrxn and ΔSrxn for several reactions are given. In which case is the reaction spontaneous at low temperatures, but not at high temperatures?
a) ΔHrxn > 0 ;ΔSrxn < 0
b) ΔHrxn < 0 ;ΔSrxn > 0
c) ΔHrxn > 0 ;ΔSrxn < 0
d) ΔHrxn > 0 ; ΔSrxn > 0
e) ΔHrxn = ΔSrxn
a) ΔHrxn > 0 ;ΔSrxn < 0
At what temperatures will a reaction be spontaneous if ΔH= +158 kJ and ΔS = +411 J/K?
a) All temperatures below 384 K
b) Temperatures between 158 K and 411 K
c) All temperatures above 384 K
d) The reaction will be spontaneous at any temperature
e) The reaction will never be spontaneous.
c) All temperatures above 384 K
12. Calculate the ΔGrxn using the following information.
2H2S(g) + 3O2(g) = 2S02(g) + 2H2O(g) ΔGrxn=?
ΔGf (kJ/mol):
2H2S(g) = -33.4
3O2(g) = 0
2S02(g) = -300.1
2H2O(g) = -228.6
a)+112.4 kJ
b) -495.3 kJ
c) -528.7 kJ
d) +66.8 kJ
e) -990.6 kJ
e) -990.6 kJ
Calculate ΔE for the system in which a gas absorbs 31 J of her heat and does 18 J of work on the surroundings?
a) -49 J
b) -13 J
c) +13 J
d) +31 J
e) +49 J
c) +13 J
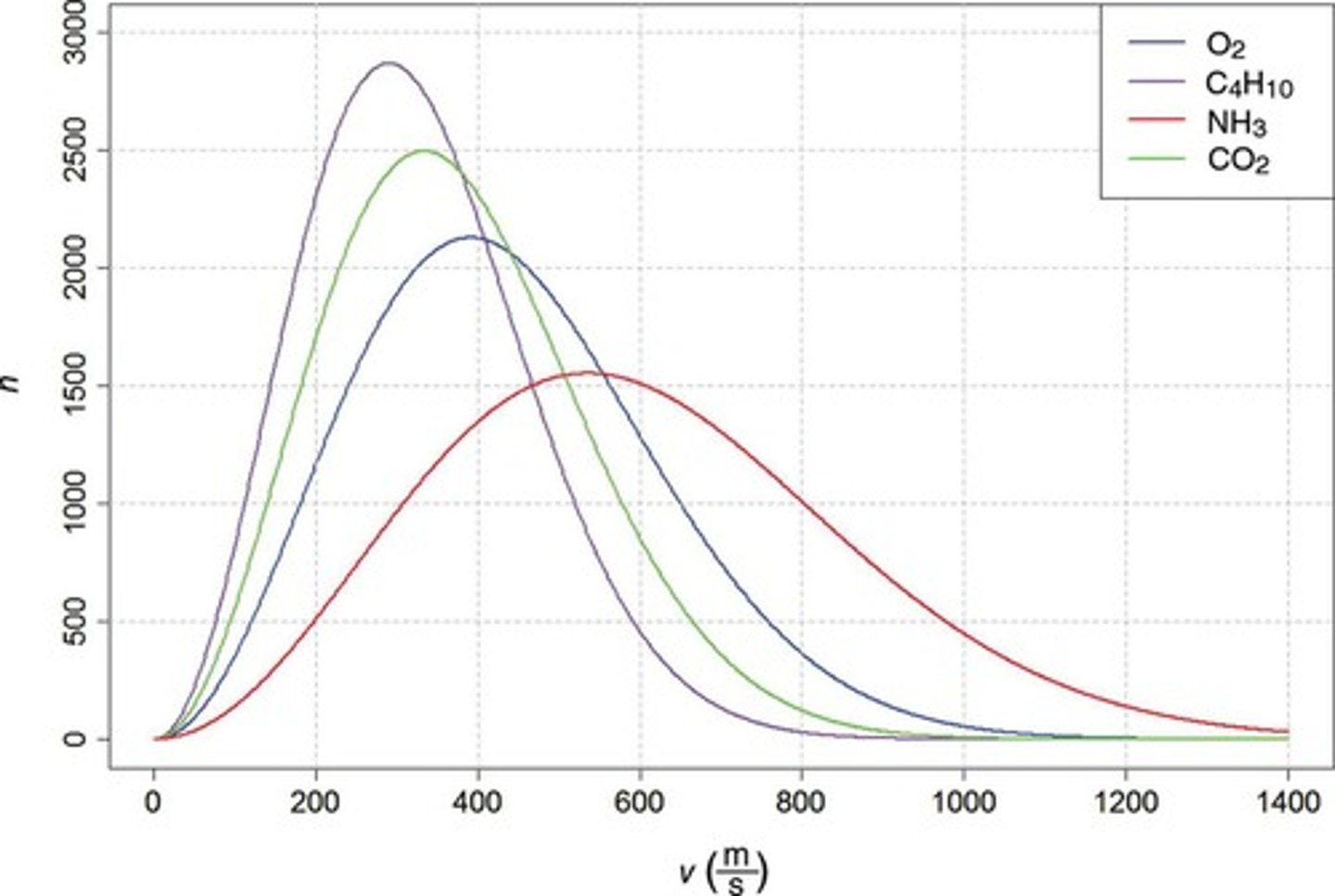
At the same temperature and pressure, which of the gases in the graph below has the largest molar mass? (see image)
a) C4H10
b) CO2
c) O2
d) NH3
e) They are all the same
a) C4H10
Which of the following chemical equations corresponds to the standard molar enthalpy
of formation, ΔHf, for N2O gas?
Ne) NO(g) Si Nds) -• RIO® ,h) NS(g) + k 040 -• f4d0lB) knit) + OB0 -• NAB)
`tioli(g) O(g) -• N20(g) c12 Ns) — 2 ts130(1)
a) NO(g) + 1/2N2(g) = N2O(g)
b) N2(g) + 1/2O2(g) = N2O(g)
c) 2N(g) + O(g) = N2O(g)
d) N2(g) + O(g) = N2O(g)
e) 2N2(g) + O2(g) = 2N2O(g)
b) N2(g) + 1/2O2(g) = N2O(g)
What is the second law of thermodynamics?
a) The standard Gibbs free energy change, ΔG, can be calculated from Gibbs Free
energies of formation, ΔGf
b) The entropy of a perfect crystal of any pure substance approaches zero, as the
temperature approaches absolute zero (0 K)
c) The entropy change for a reaction, ΔS, can be calculated from the standard molar entropies of the reactants and products.
d) ΔEuniverse = ΔEsystem + ΔEsurroundings = 0
e) ΔSuniverse = ΔSsystem + ΔSsurroundings > 0
e) ΔSuniverse = ΔSsystem + ΔSsurroundings > 0
A mixture of He and O2 is placed in a 4.00 L flask at 32 °C. The partial pressure of the He is 2.7 atm and the partial pressure of the O2 is 1.4 atm. What is the mole fraction of?
a) 0.224
b) 0.341
c) 0.481
d) 0.518
e) 0.659
b) 0.341
How much energy is required to decompose 765 g of PCl3, according to the reaction below? The molar mass of PCl3 is 137.32 g/mol.
4PCl3(g) = P4(s) + 6Cl2(g) ΔHrxn = +1207 kJ
a) 2.31 x 10^3 kJ
b) 4.33 x 10^3 kJ
c) 6.72 x 10^3 kJ
d) 1.68 x 10^3 kJ
e) x 10^3 kJ
d) 1.68 x 10^3 kJ
In order to determine the amount of heat (in kJ) required to vaporize 1.55 kg of water at
its boiling point, which information can be used?
a)
b)
c)
d)
e)
c
a)
b)
c)
d)
e)
d
a)
b)
c)
d)
e)
c